Comprehensive Epitope Analysis of Monoclonal Antibodies Binding to Hen Egg Ovalbumin Using a Peptide Array
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Production of Anti-Hen Egg Ovalbumin Monoclonal Antibody
2.3. Selection of Antibody via Direct ELISA
2.4. Epitope Analysis of 65F2 and 962H2 Monoclonal Antibodies Using a Peptide Array
2.5. Epitope Analysis of 65F2 Monoclonal Antibody by Competitive ELISA
2.6. Cross-Reactivity Test of 65F2 Antibody Using Peptides with Sequences Similar to the Epitope
2.7. Production of Lateral Flow Immunoassay (LFI) Kit Using 65F2 and 962H2 Antibodies
2.8. Evaluation of LFI Sensitivity in Detecting Allergens in Sample Solutions
2.9. Evaluation of LFI Sensitivity in Detecting Allergens in Model Foods
2.10. Cross-Reactivity of the LFI Kit
2.11. Validation of the Practicality of the LFI Kit Using Commercially Available Food Products
3. Results and Discussion
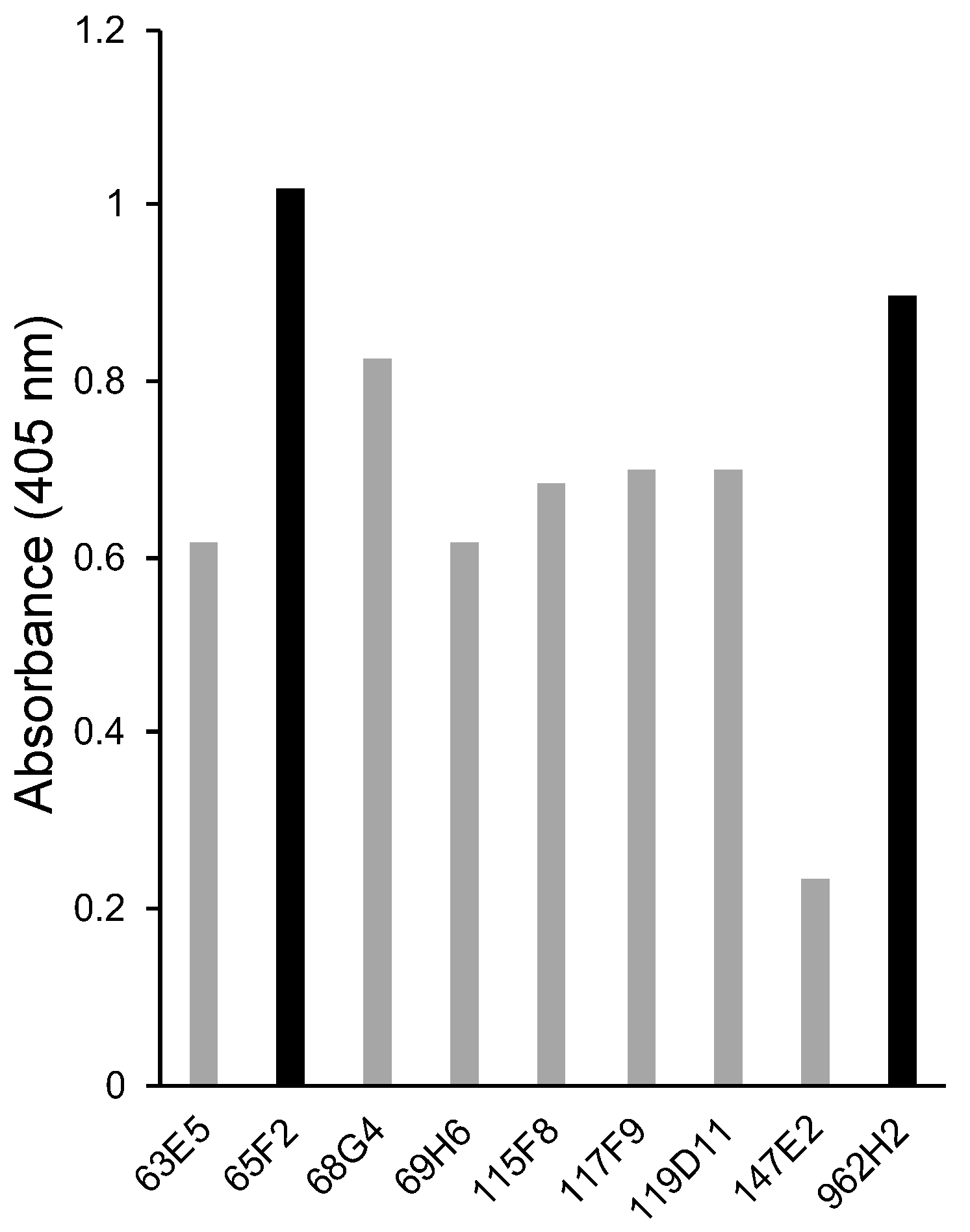
3.1. Selection of Monoclonal Antibodies against Hen Egg Ovalbumin Using Direct ELISA
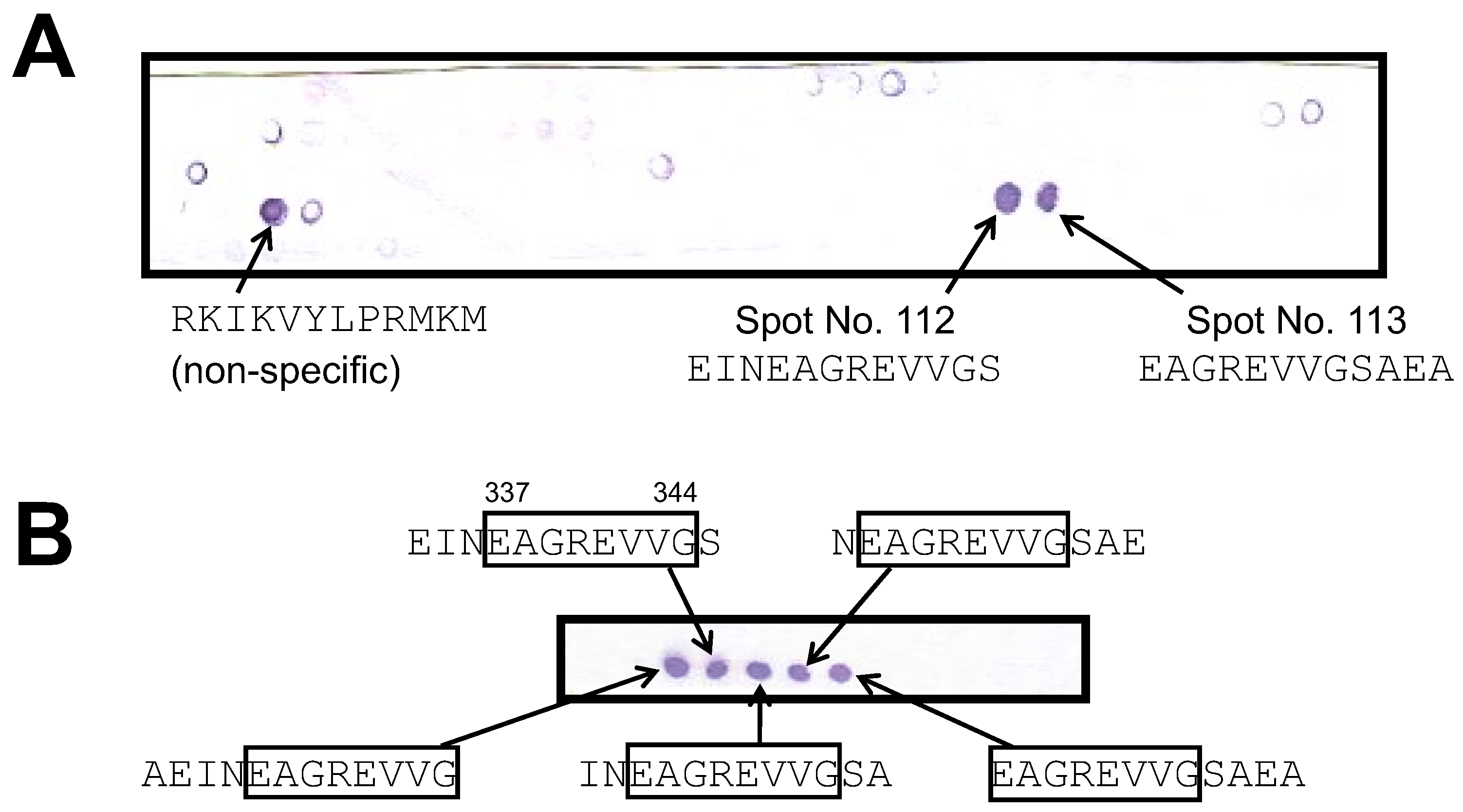
3.2. Epitope Analysis of Monoclonal Antibodies against Hen Egg Ovalbumin Using a Peptide Array
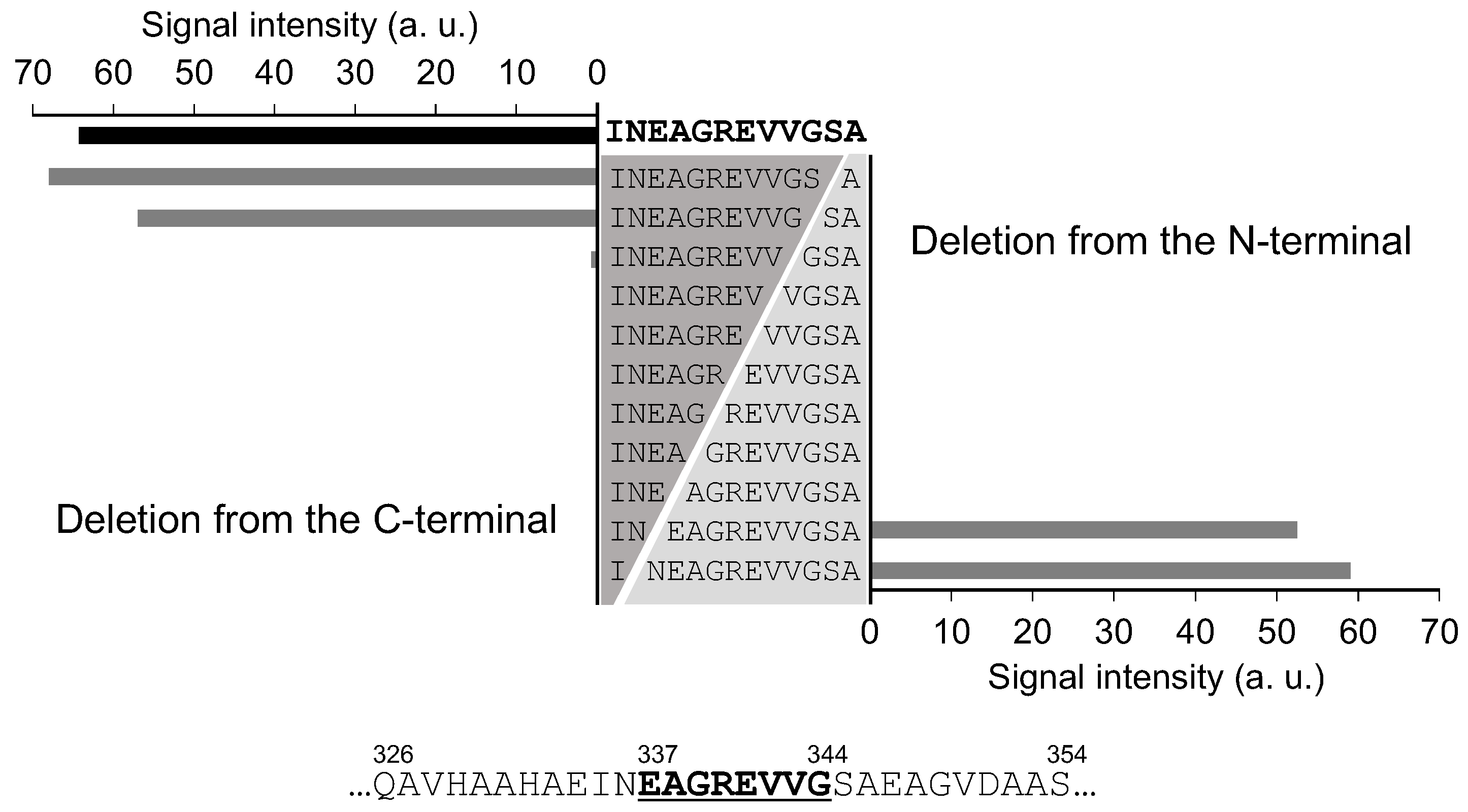
3.3. Precise Epitope Analysis via Deletion Analysis
3.4. Epitope Analysis by Competitive ELISA and Validation of Peptide Array Analysis Method
3.5. Analysis of Epitope-Constituting Amino Acids Critical for Antibody Binding by Alanine Scanning
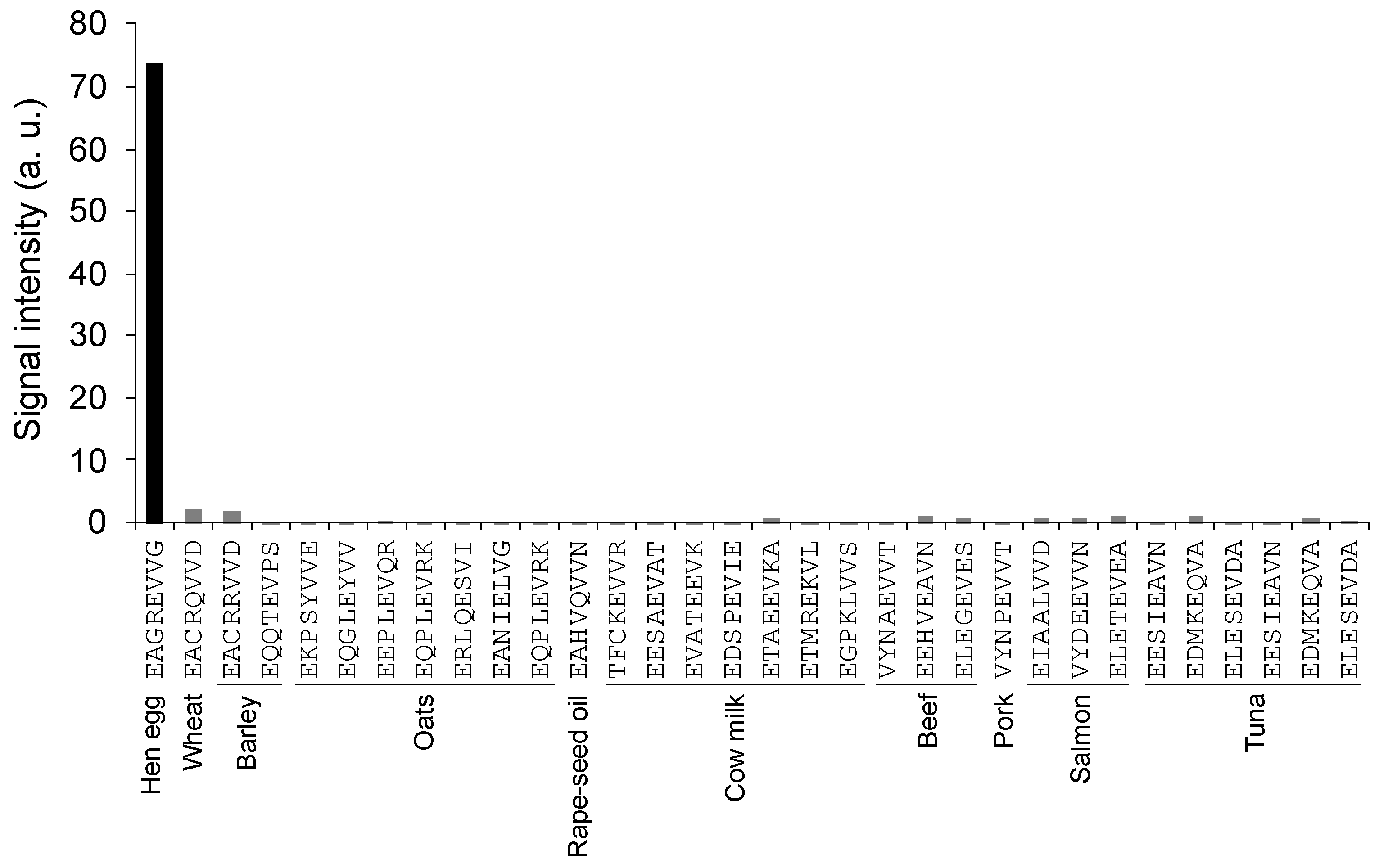
3.6. Cross-Reactivity Test of 65F2 Antibody Using Peptide Array
3.7. Lateral Flow Immunoassay Using the 65F2 and 962H2 Antibodies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. New Perspectives in Food Allergy. Int. J. Mol. Sci. 2020, 21, 1474. [Google Scholar] [CrossRef] [PubMed]
- Elghoudi, A.; Narchi, H. Food allergy in children-the current status and the way forward. World J. Clin. Pediatr. 2022, 11, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Eng, L.; Chang, C. Food Allergy Labeling Laws: International Guidelines for Residents and Travelers. Clin. Rev. Allergy Immunol. 2023, 65, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Vriesekoop, F. Beer and Allergens. Beverages 2021, 7, 79. [Google Scholar] [CrossRef]
- Sena-Torralba, A.; Pallás-Tamarit, Y.; Morais, S.; Maquieira, Á. Recent advances and challenges in food-borne allergen detection. TrAC Trends Anal. Chem. 2020, 132, 116050. [Google Scholar] [CrossRef]
- Ebisawa, M.; Ito, K.; Fujisawa, T. Committee for Japanese Pediatric Guideline for Food Allergy, The Japanese Society of Pediatric Allergy and Clinical Immunology; Japanese Society of Allergology. Japanese guidelines for food allergy 2020. Allergol. Int. 2020, 69, 370–386. [Google Scholar] [CrossRef]
- Akiyama, H.; Adachi, R. Japanese Food Allergy-Labeling System and Comparison with the International Experience; Detection and Thresholds. Food Saf. 2021, 9, 101–116. [Google Scholar] [CrossRef]
- Lee, N.A.; Lopata, A.L.; Colgrave, M.L. Analytical Methods for Allergen Control in Food Processing. Foods 2023, 12, 1439. [Google Scholar] [CrossRef]
- Xing, G.; Sun, X.; Li, N.; Li, X.; Wu, T.; Wang, F. New Advances in Lateral Flow Immunoassay (LFI) Technology for Food Safety Detection. Molecules 2022, 27, 6596. [Google Scholar] [CrossRef]
- Segura, V.; Siglez, M.A.; Ruiz-Carnicer, A.; Martin-Cabrejas, I.; van der Hofstadt, M.; Mellado, E.; Comino, I.; Sousa, C. A Highly Sensitive Method for the Detection of Hydrolyzed Gluten in Beer Samples Using LFIA. Foods 2022, 12, 160. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, S.; Lai, X.; Peng, J.; Lai, W. Developmental trend of immunoassays for monitoring hazards in food samples: A review. Trends Food Sci. Technol. 2021, 111, 68–88. [Google Scholar] [CrossRef]
- Zhou, F.; He, S.; Sun, H.; Wang, Y.; Zhang, Y. Advances in epitope mapping technologies for food protein allergens: A review. Trends Food Sci. Technol. 2021, 107, 226–239. [Google Scholar] [CrossRef]
- Frank, R. Spot-synthesis: An easy technique for the positionally addressable, parallel chemical synthesis on a membrane support. Tetrahedron 1992, 48, 9217–9232. [Google Scholar] [CrossRef]
- Frank, R. The SPOT-synthesis technique. Synthetic peptide arrays on membrane supports--principles and applications. J. Immunol. Methods 2002, 267, 13–26. [Google Scholar] [CrossRef]
- De-Simone, S.G.; Gomes, L.R.; Napoleao-Pego, P.; Lechuga, G.C.; de Pina, J.S.; da Silva, F.R. Epitope Mapping of the Diphtheria Toxin and Development of an ELISA-Specific Diagnostic Assay. Vaccines 2021, 9, 313. [Google Scholar] [CrossRef]
- Szymczak, L.C.; Mrksich, M. Using Peptide Arrays To Discover the Sequence-Specific Acetylation of the Histidine-Tyrosine Dyad. Biochemistry 2019, 58, 1810–1817. [Google Scholar] [CrossRef]
- Ito, K.; Koike, M.; Kuroda, Y.; Yamazaki-Ito, T.; Terada, Y.; Ishii, T.; Nakamura, Y.; Watanabe, T.; Kawarasaki, Y. Bitterness-masking peptides for epigallocatechin gallate identified through peptide array analysis. Food Sci. Technol. Res. 2021, 27, 221–228. [Google Scholar] [CrossRef]
- Crestfield, A.M.; Moore, S.; Stein, W.H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J. Biol. Chem. 1963, 238, 622–627. [Google Scholar] [CrossRef]
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. 1975. J. Immunol. 2005, 174, 2453–2455. [Google Scholar]
- Jones, S.L.; Cox, J.C.; Pearson, J.E. Increased monoclonal antibody ascites production in mice primed with Freund’s incomplete adjuvant. J. Immunol. Methods 1990, 129, 227–231. [Google Scholar] [CrossRef]
- Standards Relating to the Care and Keeping and Reducing Pain of Laboratory Animals (Notice of the Ministry of the Environment No. 88 of 2006); Ministry of the Environment: Tokyo, Japan, 2016.
- Clement, C.C.; Nanaware, P.P.; Yamazaki, T.; Negroni, M.P.; Ramesh, K.; Morozova, K.; Thangaswamy, S.; Graves, A.; Kim, H.J.; Li, T.W.; et al. Pleiotropic consequences of metabolic stress for the major histocompatibility complex class II molecule antigen processing and presentation machinery. Immunity 2021, 54, 721–736.e10. [Google Scholar] [CrossRef]
- Suprun, M.; Sicherer, S.H.; Wood, R.A.; Jones, S.M.; Leung, D.Y.M.; Burks, A.W.; Dunkin, D.; Witmer, M.; Grishina, G.; Getts, R.; et al. Mapping Sequential IgE-Binding Epitopes on Major and Minor Egg Allergens. Int. Arch. Allergy Immunol. 2022, 183, 249–261. [Google Scholar] [CrossRef]
- Jiang, X.; Rao, Q. Effect of Processing on Fish Protein Antigenicity and Allergenicity. Foods 2021, 10, 969. [Google Scholar] [CrossRef]
- Mine, Y.; Yang, M. Epitope characterization of ovalbumin in BALB/c mice using different entry routes. Biochim. Biophys. Acta BBA Proteins Proteom. 2007, 1774, 200–212. [Google Scholar] [CrossRef]
- Mine, Y.; Rupa, P. Fine mapping and structural analysis of immunodominant IgE allergenic epitopes in chicken egg ovalbumin. Protein Eng. 2003, 16, 747–752. [Google Scholar] [CrossRef]
- Morinaga Institute of Biological Science. Rapid Test ProII for Egg. Cat. M2261. Available online: https://www.miobs-e.com/product/rapid_test_pro2/egg/index.html#link03 (accessed on 22 December 2023).
- NH Foods Ltd. FASTKIT SLIM EGG <<Instruction Manual>>. Available online: https://www.rdc.nipponham.co.jp/kit_eng/images/fk_slim_egg.pdf (accessed on 22 December 2023).
- Affidia s.r.l. SB. Fastkit Slim Egg. Available online: https://foodtestcompass.com/fastkit-slim-egg (accessed on 22 December 2023).
- Szymczak, L.C.; Kuo, H.Y.; Mrksich, M. Peptide Arrays: Development and Application. Anal. Chem. 2018, 90, 266–282. [Google Scholar] [CrossRef]

| Method | 65F2 |
|---|---|
| Peptide array | EAGREVVG |
| Competitive ELISA | NEAGREVVG |
| Detection Method | Ovalbumin Concentration (µg/g) | ||||||
|---|---|---|---|---|---|---|---|
| 5 | 4 | 2.5 | 1.5 | 1 | 0.5 | 0 | |
 | |||||||
| Visual judgment | + | + | + | + | + | + | − |
| mABS | 165.2 | 136.8 | 98.7 | 52.5 | 31.3 | 12.1 | 0 |
| Model Foods | Added Ovalbumin Concentration (µg/g) | Immunochromatography Visual Judgment | Immunochromatography mABS | Quantitative ELISA Hen Egg Protein Concentration (µg/g) |
|---|---|---|---|---|
| Udon noodles | 0 | − | 0 | 0 |
| 1 | + | 33.4 | 2.3 (equivalent to 1.15 µg/g ovalbumin) | |
| 5 | + | 143.8 | 9.0 (equivalent to 4.5 µg/g ovalbumin) | |
| Pork ham | 0 | − | 0 | 0 |
| 1 | + | 28.4 | 1.9 (equivalent to 0.95 µg/g ovalbumin) | |
| 5 | + | 129.9 | 8.5 (equivalent to 4.25 µg/g ovalbumin) | |
| Processed chicken breast | 0 | − | 0 | 0 |
| 1 | + | 24.6 | 1.8 (equivalent to 0.9 µg/g ovalbumin) | |
| 5 | + | 118.2 | 9.2 (equivalent to 4.6 µg/g ovalbumin) | |
| Lactic-fermented beverage | 0 | − | 0 | 0 |
| 1 | + | 29.1 | 2.2 (equivalent to 1.1 µg/g ovalbumin) | |
| 5 | + | 132.7 | 8.7 (equivalent to 4.35 µg/g ovalbumin) | |
| Pumpkin soup | 0 | − | 0 | 0 |
| 1 | + | 31.9 | 2.6 (equivalent to 1.3 µg/g ovalbumin) | |
| 5 | + | 140.5 | 9.4 (equivalent to 4.7 µg/g ovalbumin) |
| Commercially Available Food Products | Allergen Labeling | Immunochromatography Visual Judgment | Quantitative ELISA Hen Egg Protein Concentration (µg/g) |
|---|---|---|---|
| Bread | Egg | + | >20 |
| Instant noodles | Egg | + | >20 |
| Pasta sauce | Egg | + | >20 |
| Pudding | Egg | + | >20 |
| Potato salad | Egg | + | >20 |
| Chinese dumplings | Egg | + | >20 |
| Fried rice | Egg | + | >20 |
| Egg soup | Egg | + | >20 |
| Sandwich | Egg | + | >20 |
| Whipped cream mochi | Egg (partial ingredient) | + | 7.4 |
| Bread | Egg-free | − | <1.0 |
| Instant noodles | Egg-free | − | <1.0 |
| Pasta sauce | Egg-free | − | <1.0 |
| Fruit jelly | Egg-free | − | <1.0 |
| Vegetable salad | Egg-free | − | <1.0 |
| Rice ball | Egg-free | − | <1.0 |
| Potato chips | Egg-free | − | <1.0 |
| Gratin | Egg-free | − | <1.0 |
| Fried snack | Egg-free | − | <1.0 |
| Strawberry jam | Egg-free | − | <1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terada, Y.; Akimoto, M.; Sakoda, H.; Yamamoto, S.; Kubota, M.; Motoyama, T.; Imanaka, Y.; Nakano, S.; Ito, S.; Kato, S.; et al. Comprehensive Epitope Analysis of Monoclonal Antibodies Binding to Hen Egg Ovalbumin Using a Peptide Array. Foods 2024, 13, 407. https://doi.org/10.3390/foods13030407
Terada Y, Akimoto M, Sakoda H, Yamamoto S, Kubota M, Motoyama T, Imanaka Y, Nakano S, Ito S, Kato S, et al. Comprehensive Epitope Analysis of Monoclonal Antibodies Binding to Hen Egg Ovalbumin Using a Peptide Array. Foods. 2024; 13(3):407. https://doi.org/10.3390/foods13030407
Chicago/Turabian StyleTerada, Yuko, Masanobu Akimoto, Hirofumi Sakoda, Shunsuke Yamamoto, Mayuka Kubota, Tomoharu Motoyama, Yo Imanaka, Shogo Nakano, Sohei Ito, Shigeki Kato, and et al. 2024. "Comprehensive Epitope Analysis of Monoclonal Antibodies Binding to Hen Egg Ovalbumin Using a Peptide Array" Foods 13, no. 3: 407. https://doi.org/10.3390/foods13030407
APA StyleTerada, Y., Akimoto, M., Sakoda, H., Yamamoto, S., Kubota, M., Motoyama, T., Imanaka, Y., Nakano, S., Ito, S., Kato, S., & Ito, K. (2024). Comprehensive Epitope Analysis of Monoclonal Antibodies Binding to Hen Egg Ovalbumin Using a Peptide Array. Foods, 13(3), 407. https://doi.org/10.3390/foods13030407






