Exploitation of Black Olive (Olea europaea L. cv. Piantone di Mogliano) Pomace for the Production of High-Value Bread
Abstract
:1. Introduction
2. Materials and Methods
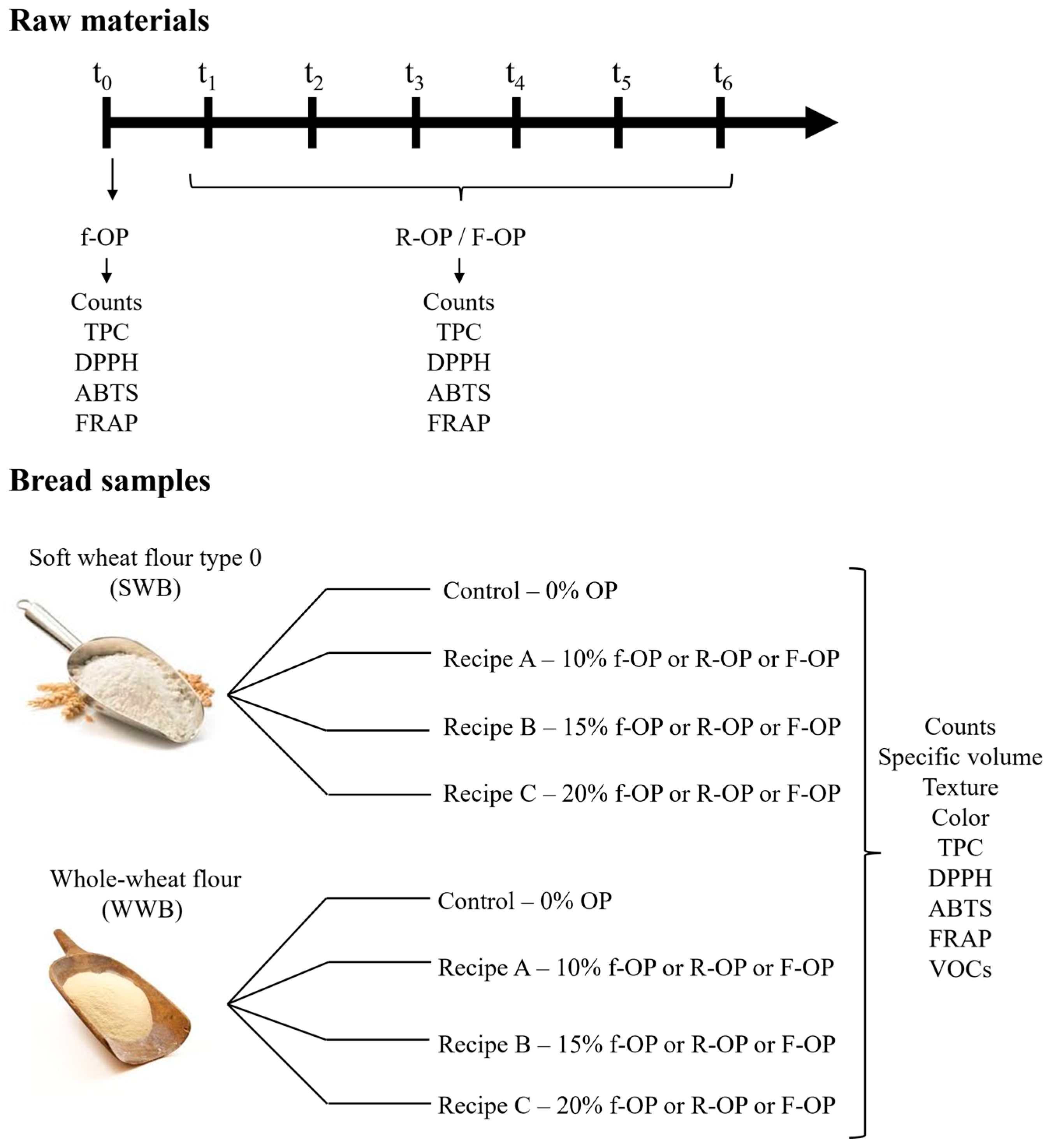
2.1. Raw Materials
2.2. Analyses of Olive Pomace during the 6-Month Storage Period
2.2.1. Microbiological Analyses
2.2.2. Evaluation of Phenolic Fraction
2.2.3. Determination of the Antioxidant Capacity (AOC)
DPPH Radical-Scavenging Activity Assay
ABTS Radical-Scavenging Activity Assay
Determination of Ferric-Ion-Reducing Power (FRAP)
2.3. Bread-Making Trials
2.3.1. Analyses of Dough and Bread Samples
Microbiological Analyses
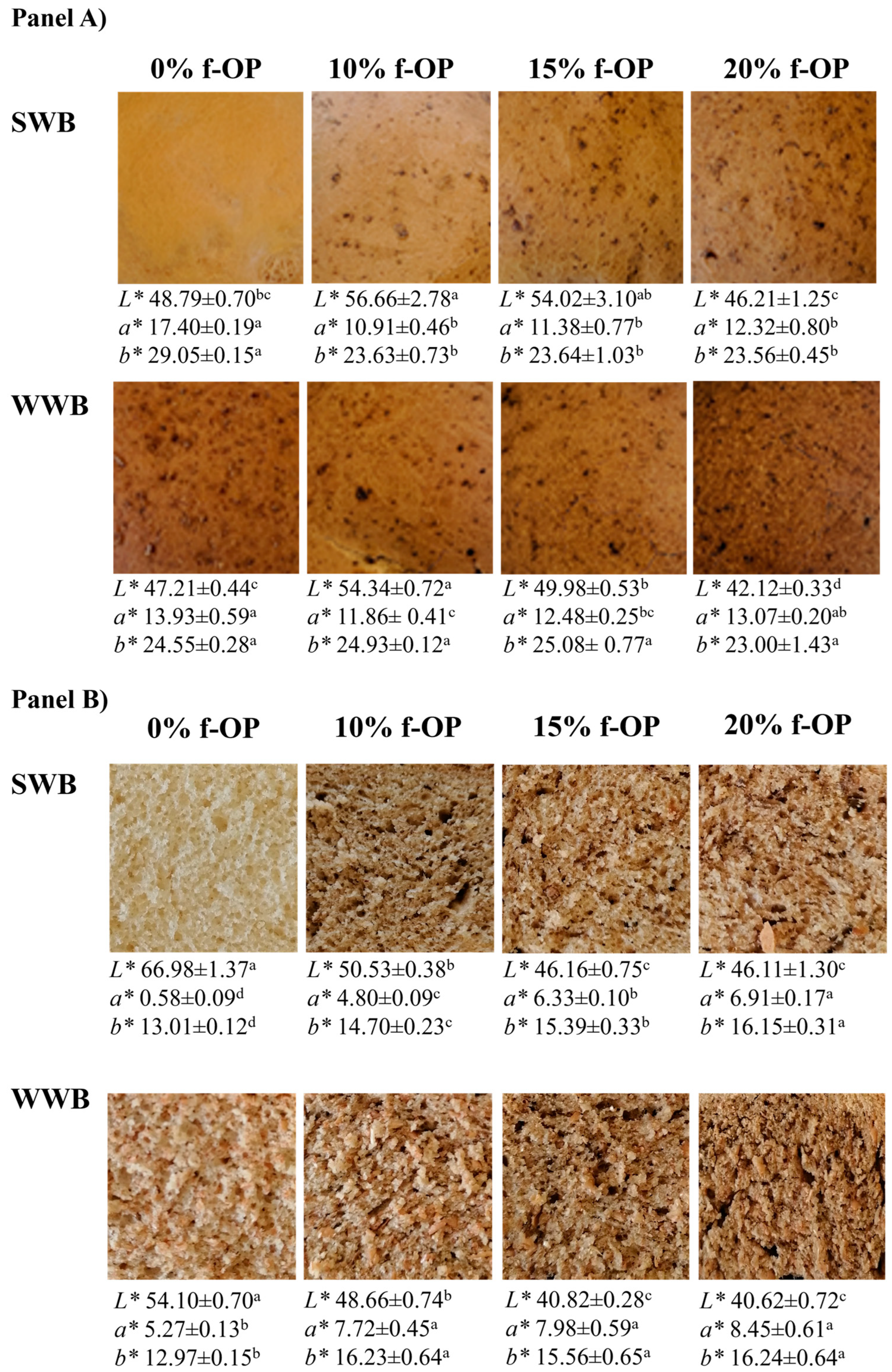
Determination of Bread Specific Volume, Hardness, and Color
Evaluation of Bread Phenolic Fraction
Evaluation of Bread Dietary Fiber
SPME–GC/MS Analysis of Bread Volatile Components
2.4. Statistical Analysis
3. Results
3.1. Microbiological Analyses of Olive Pomace
3.2. Total Phenolic Content (TPC) and Antioxidant Capacity of Olive Pomace
3.3. Microbiological Analyses of Dough and Bread
3.4. Specific Volume, Hardness, and Color of the Experimental Breads
3.5. Total Phenolic Content, Antioxidant Capacity, and Dietary Fiber of the Breads
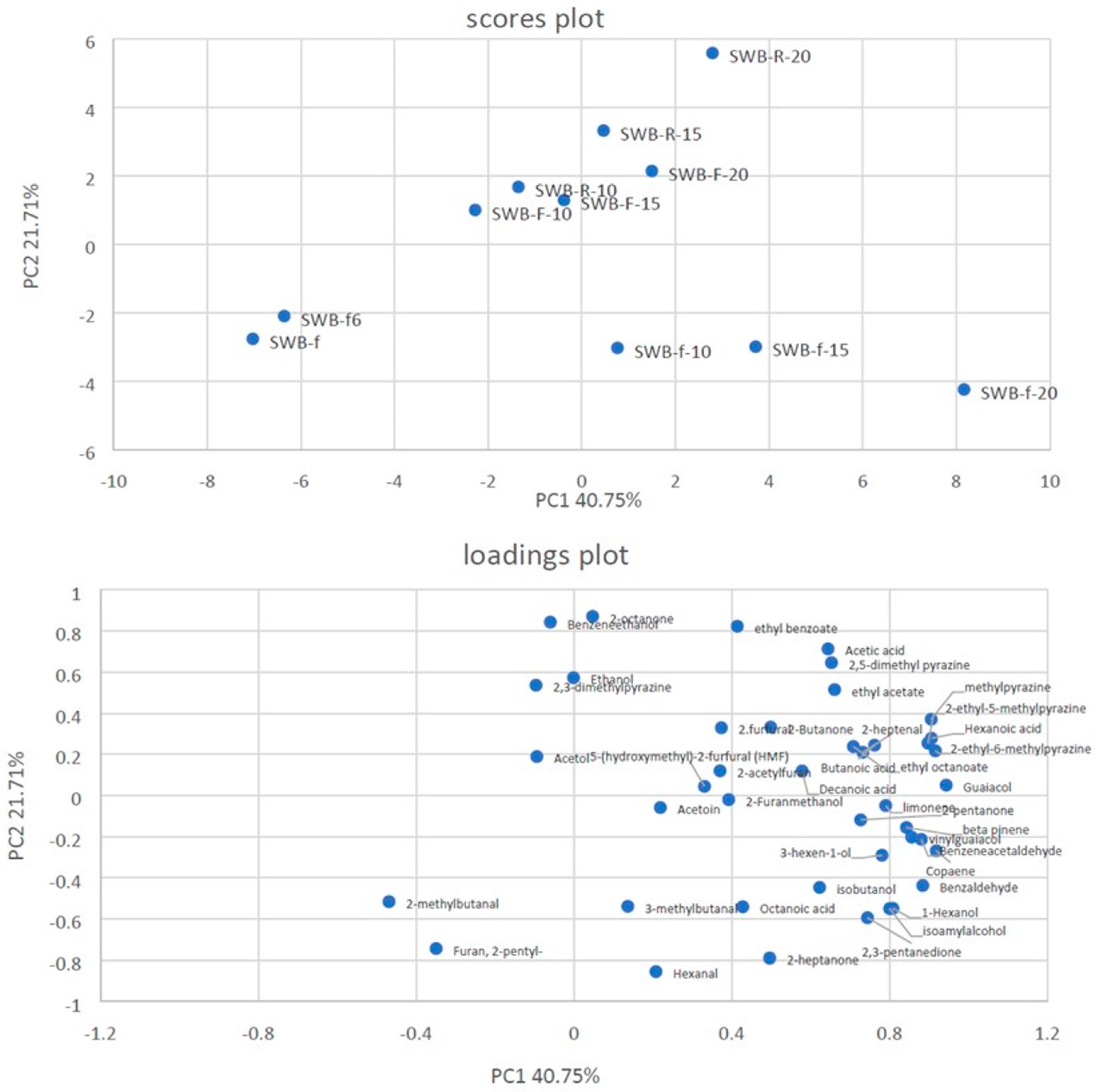
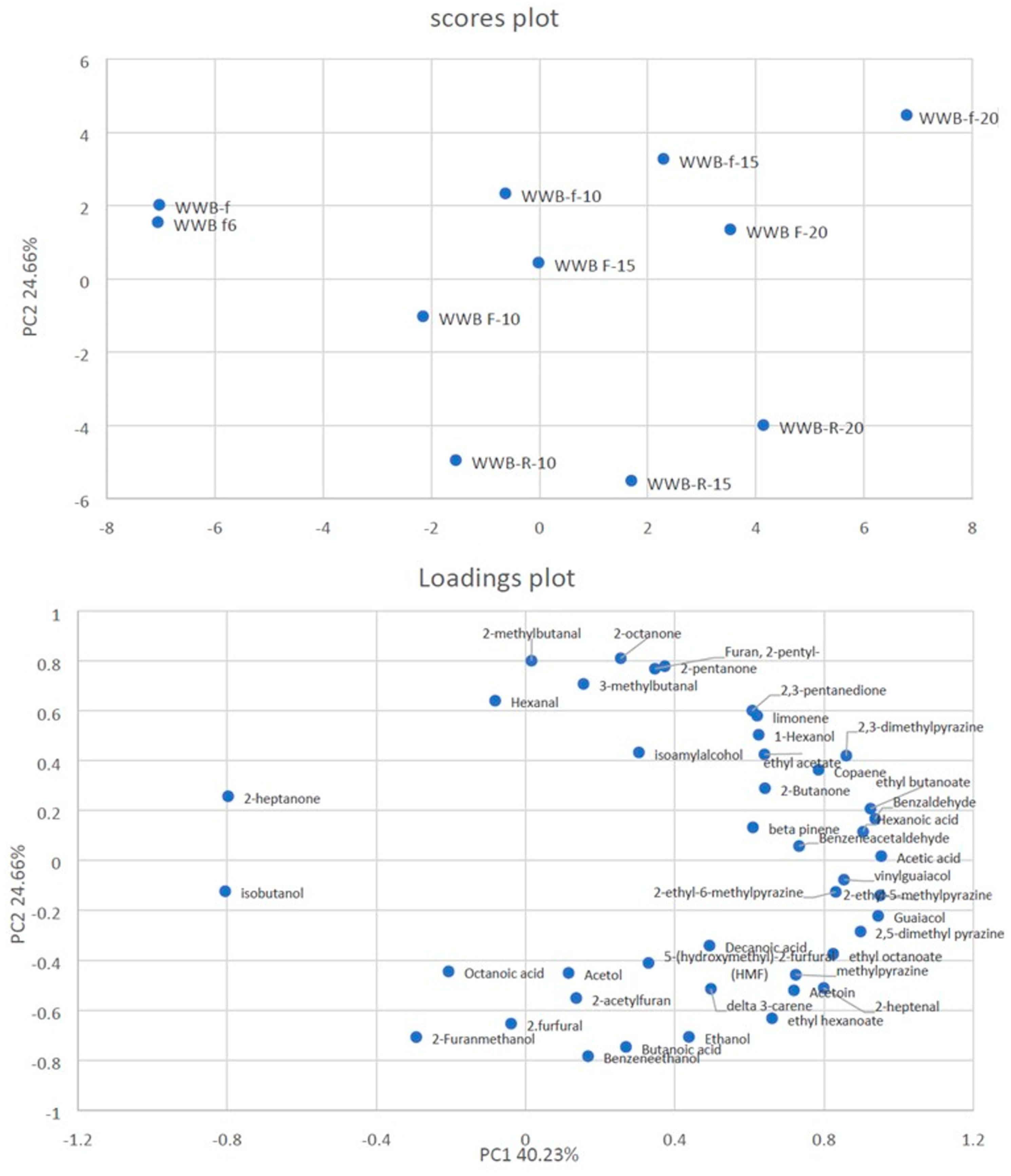
3.6. SPME–GC/MS Analysis of the Volatile Components of the Breads
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Tura, D.; Gigliotti, C.; Pedò, S.; Failla, O.; Bassi, D.; Serraiocco, A. Influence of cultivar and site of cultivation on levels of lipophilic and hydrophilic antioxidants in virgin olive oils (Olea europea L.) and correlations with oxidative stability. Sci. Hortic. 2007, 112, 108–119. [Google Scholar] [CrossRef]
- Batuecas, E.; Tommasi, T.; Battista, F.; Negro, V.; Sonetti, G.; Viotti, P.; Fino, D.; Mancini, G. Life Cycle Assessment of waste disposal from olive oil production: Anaerobic digestion and conventional disposal on soil. J. Environ. Manag. 2019, 237, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Carmona, I.; Aguirre, I.; Griffith, D.M.; García-Borrego, A. Towards a circular economy in virgin olive oil production: Valorization of the olive mill waste (OMW) “alpeorujo” through polyphenol recovery with natural deep eutectic solvents (NADESs) and vermicomposting. Sci. Total Environ. 2023, 872, 162198. [Google Scholar] [CrossRef] [PubMed]
- Goula, A.M.; Lazarides, H.N. Integrated processes can turn industrial food waste into valuable food by-products and/or ingredients: The cases of olive mill and pomegranate wastes. J. Food Eng. 2015, 167, 45–50. [Google Scholar] [CrossRef]
- Harrami, M.; El Fami, N.; Moussadik, A.; Khachani, N.; Taibi, M.; Diouri, A. Elaboration and characterization of composite clays based on “Coal waste-Olive pomace” mixtures. Mat. Today-Proc. 2022, 58, 1573–1577. [Google Scholar] [CrossRef]
- Lila, K.; Belaadi, S.; Solimando, R.; Ralida Zirour, F. Valorisation of organic waste: Use of olive kernels and pomace for cement manufacture. J. Clean. Prod. 2020, 277, 123703. [Google Scholar] [CrossRef]
- Ruschioni, S.; Loreto, N.; Foligni, R.; Mannozzi, C.; Raffaelli, N.; Zamporlini, F.; Pasquini, M.; Roncolini, A.; Cardinali, F.; Osimani, A.; et al. Addition of olive pomace to feeding substrate affects growth performance and nutritional value of mealworm (Tenebrio molitor L.) larvae. Foods 2020, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trend Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Ying, D.; Hlaing, M.M.; Lerisson, J.; Pitts, K.; Cheng, L.; Sanguansri, L.; Augustin, M.A. Physical properties and FTIR analysis of rice-oat flour and maize-oat flour based extruded food products containing olive pomace. Food Res. Int. 2017, 100, 665–673. [Google Scholar] [CrossRef]
- Balli, D.; Cecchi, L.; Innocenti, M.; Bellumori, M.; Mulinacci, N. Food by-products valorisation: Grape pomace and olive pomace (pâté) as sources of phenolic compounds and fiber for enrichment of tagliatelle pasta. Food Chem. 2021, 355, 129642. [Google Scholar] [CrossRef]
- Simonato, B.; Trevisan, S.; Tolve, R.; Favati, F.; Pasini, G. Pasta fortification with olive pomace: Effects on the technological characteristics and nutritional properties. LWT 2019, 114, 108368. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Bonifácio-Lopes, T.; Morais, P.; Miranda, A.; Nunes, J.; Vicente, A.A.; Pintado, M. Incorporation of olive pomace ingredients into yoghurts as a source of fibre and hydroxytyrosol: Antioxidant activity and stability throughout gastrointestinal digestion. J. Food Eng. 2021, 297, 110476. [Google Scholar] [CrossRef]
- Cecchi, L.; Schuster, N.; Flynn, D.; Bechtel, R.; Bellumori, M.; Innocenti, M.; Mulinacci, N.; Guinard, J.X. Sensory profiling and consumer acceptance of pasta, bread, and granola bar fortified with dried olive pomace (pâté): A byproduct from virgin olive oil production. J. Food Sci. 2019, 84, 2995–3008. [Google Scholar] [CrossRef]
- Cedola, A.; Cardinali, A.; D’Antuono, I.; Conte, A.; Del Nobile, M.A. Cereal foods fortified with by-products from the olive oil industry. Food Biosci. 2020, 33, 100490. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Chiarello, E.; Caboni, M.F.; Capozzi, F.; Gianotti, A.; Bordoni, A. Olive oil by-product as functional ingredient in bakery products. Influence of processing and evaluation of biological effects. Food Res. Int. 2020, 131, 108940. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Bleve, G.; Selvaggini, R.; Veneziani, G.; Servili, M.; Mita, G. Bioactive compounds and stability of a typical Italian bakery products “Taralli” enriched with fermented olive paste. Molecules 2019, 24, 3258. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Ramos, K.C.; Sanz-Ponce, N.; Haros, C.M. Evaluation of technological and nutritional quality of bread enriched with amaranth flour. LWT 2019, 114, 108418. [Google Scholar] [CrossRef]
- Taccari, M.; Aquilanti, L.; Polverigiani, S.; Osimani, A.; Garofalo, C.; Milanović, V.; Clementi, F. Microbial diversity of type I sourdoughs prepared and back-slopped with wholemeal and refined soft (Triticum aestivum) wheat flours. J. Food Sci. 2016, 81, M1996–M2005. [Google Scholar] [CrossRef] [PubMed]
- Saka, M.; Özkaya, B.; Saka, İ. The effect of bread-making methods on functional and quality characteristics of oat bran blended bread. Int. J. Gastr. Food Sci. 2021, 26, 100439. [Google Scholar] [CrossRef]
- Venturi, M.; Cappelli, A.; Pini, N.; Galli, V.; Lupori, L.; Granchi, L.; Cini, E. Effects of kneading machine type and total element revolutions on dough rheoLogy and bread characteristics: A focus on straight dough and indirect (biga) methods. LWT 2022, 153, 112500. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Cirlini, M.; Morini, E.; Rinaldi, M.; Ganino, T.; Chiavaro, E. Effect of chestnut flour supplementation on physico-chemical properties and volatiles in bread making. LWT 2013, 53, 233–239. [Google Scholar] [CrossRef]
- Martin-Diana, A.B.; Izquierdo, N.; Albertos, I.; Sanchez, M.S.; Herrero, A.; Sanz, M.A.; Rico, D. Valorization of Carob’s Germ and Seed Peel as Natural Antioxidant Ingredients in Gluten-Free Crackers. J. Food Process. Preserv. 2017, 41, e12770. [Google Scholar] [CrossRef]
- Jemai, H.; Bouaziz, M.; Sayadi, S. Phenolic composition, sugar contents and antioxidant activity of Tunisian sweet olive cultivar with regard to fruit ripening. J. Agric. Food Chem. 2009, 57, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Belleggia, L.; Botta, C.; Ferrocino, I.; Milanović, V.; Cardinali, F.; Haouet, M.N.; Garofalo, C.; Mozzon, M.; Foligni, R.; et al. Journey to the morpho-textural traits, microbiota, and volatilome of Ciauscolo PGI salami. Food Biosci. 2023, 53, 102582. [Google Scholar] [CrossRef]
- Dreher, J.; Konig, M.; Herrmann, K.; Terjung, N.; Gibis, M.; Weiss, J. Varying the amount of solid fat in animal fat mimetics for plant-based salami analogues influences texture, appearance and sensory characteristics. LWT 2021, 143, 111140. [Google Scholar] [CrossRef]
- Cardinali, F.; Garofalo, C.; Reale, A.; Boscaino, F.; Osimani, A.; Milanovic, V.; Taccari, M.; Aquilanti, L. Liquid sourdough from stone-ground soft wheat (Triticum aestivum) flour: Development and exploitation in the breadmaking process. Food Res. Int. 2022, 161, 111796. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Foti, P.; Russo, N.; Randazzo, C.L.; Choupina, A.B.; Pino, A.; Caggia, C.; Romeo, F.V. Profiling of phenol content and microbial community dynamics during pâté olive cake fermentation. Food Biosci. 2023, 52, 102358. [Google Scholar] [CrossRef]
- Zhou, D.; Zhong, J.; Huang, Y.; Cheng, Y. Effect of free and bound polyphenols from Rosa roxburghii Tratt distiller’s grains on moderating fecal microbiota. Food Chem. X 2023, 19, 100747. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, A.; De Bruno, A.; Zappia, A.; Poiana, M. Antioxidant activity of dried green olives (Carolea cv.). LWT 2014, 58, 49–54. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Marinopoulou, A.; Papageorgiou, M.; Irakli, M.; Gerasopoulos, D. Effect of Olive Pulp Enrichment on Physicochemical and Antioxidant Properties of Wheat Bread. Int. J. Food Stud. 2020, 9, 178–192. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Cheng, K.W.; Jiang, Y.; Chen, F.; Wang, M. The effects of grape seed extract fortification on the antioxidant activity and quality attributes of bread. Food Chem. 2010, 119, 49–53. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as Functional Ingredients in Savory Food Products: Application to Wheat Crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef]
- Pereira, A.P.M.; Stradiotto, G.C.; Freire, L.; Alvarenga, V.O.; Crucello, A.; Morassi, L.L.P.; Silva, F.P.; Sant’Ana, A.S. Occurrence and enumeration of rope-producing spore forming bacteria in flour and their spoilage potential in different bread formulations. LWT 2020, 133, 110108. [Google Scholar] [CrossRef]
- O’Shea, N.; Kilcawley, K.N.; Gallagher, E. Aromatic composition and physicochemical characteristics of crackers containing barley fractions. Cereal Chem. 2017, 94, 611–618. [Google Scholar] [CrossRef]
- de Gennaro, G.; Difonzo, G.; Summo, C.; Pasqualone, A.; Caponio, F. Olive cake powder as functional ingredient to improve the quality of gluten-free breadsticks. Foods 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M.; Summo, C.; Gambacorta, G.; Caponio, F.; Blando, A. Production and characterization of functional biscuits obtained from purple wheat. Food Chem. 2015, 180, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
| Raw Material | Control | Recipe A | Recipe B | Recipe C | ||||
|---|---|---|---|---|---|---|---|---|
| SWB | WWB | SWB | WWB | SWB | WWB | SWB | WWB | |
| Olive pomace | 0% | 10.0% | 15.0% | 20.0% | ||||
| Whole wheat flour | 38.2% | 31.7% | 28.4% | 25.2% | ||||
| Type 0 soft wheat flour | 60.1% | 20.6% | 50.1% | 17.1% | 45.1% | 15.4% | 40.1% | 13.6% |
| Biga | 6.0% | 5.9% | 6.0% | 5.9% | 6.0% | 5.9% | 6.0% | 5.9% |
| Water | 33.5% | 35.1% | 33.5% | 35.1% | 33.5% | 35.1% | 33.5% | 35.1% |
| Baker’s yeast | 0.4% | 0.2% | 0.4% | 0.2% | 0.4% | 0.2% | 0.4% | 0.2% |
| Panel a | |||||
|---|---|---|---|---|---|
| Total Mesophilic Aerobes | Presumptive Lactobacilli | Enterobacteriaceae | Eumycetes | ||
| f-OP | t0 | 5.00 ± 0.12 a | 2.96 ± 0.01 a | <1.00 | 4.81 ± 0.15 d |
| R-OP | t1 | 5.02 ± 0.04 a | 1.87 ± 0.04 b | <1.00 | 5.69 ± 0.02 c |
| t2 | 4.07 ± 0.16 b | 1.65 ± 0.07 c | <1.00 | 6.04 ± 0.07 b | |
| t3 | 1.95 ± 0.07 c | 1.54 ± 0.09 c | <1.00 | 6.24 ± 0.02 b | |
| t4 | 1.83 ± 0.18 c | 1.86 ± 0.03 b | <1.00 | 6.64 ± 0.00 a | |
| t5 | 1.69 ± 0.12 c | <1.00 | <1.00 | 6.54 ± 0.01 a | |
| t6 | <1.00 | <1.00 | <1.00 | 6.54 ± 0.00 a | |
| Panel b | |||||
| Total Mesophilic Aerobes | Presumptive Lactobacilli | Enterobacteriaceae | Eumycetes | ||
| f-OP | t0 | 5.00 ± 0.12 a | 2.96 ± 0.01 a | <1.00 | 4.81 ± 0.15 a |
| F-OP | t1 | 3.59 ± 0.03 b | 2.13 ± 0.07 b | <1.00 | 3.33 ± 0.02 b |
| t2 | 3.51 ± 0.00 b | 2.06 ± 0.03 b | <1.00 | 3.33 ± 0.01 b | |
| t3 | 3.09 ± 0.01 c | 1.99 ± 0.02 b | <1.00 | 3.27 ± 0.01 b | |
| t4 | 2.74 ± 0.03 d | 1.98 ± 0.04 b | <1.00 | 3.05 ± 0.05 bc | |
| t5 | 2.87 ± 0.04 d | 1.81 ± 0.05 c | <1.00 | 2.75 ± 0.21 c | |
| t6 | 2.89 ± 0.06 cd | 2.08 ± 0.05 b | <1.00 | 3.08 ± 0.01 bc |
| Panel a | |||||
|---|---|---|---|---|---|
| TPC mg GAE g−1 | DPPH mg TEAC g−1 | ABTS mg TEAC g−1 | FRAP mg TEAC g−1 | ||
| f-OP | t0 | 7.18 ± 0.12 a | 16.21 ± 0.41 a | 15.36 ± 0.51 a | 15.95 ± 0.19 a |
| R-OP | t1 | 7.35 ± 0.16 a | 16.15 ± 0.11 a | 14.69 ± 0.18 a | 16.48 ± 0.24 a |
| t2 | 6.80 ± 0.19 a | 15.47 ± 0.08 a | 14.78 ± 0.02 a | 14.67 ± 0.66 ab | |
| t3 | 7.01 ± 0.12 a | 15.41 ± 0.32 a | 14.57 ± 0.25 a | 14.65 ± 0.35 ab | |
| t4 | 7.40 ± 0.09 a | 16.47 ± 0.67 a | 16.49 ± 0.32 a | 15.71 ± 0.60 a | |
| t5 | 6.67 ± 0.15 b | 14.87 ± 0.28 a | 14.42 ± 0.32 a | 14.34 ± 0.28 b | |
| t6 | 7.39 ± 0.28 a | 16.23 ± 0.52 a | 15.86 ± 0.28 a | 15.36 ± 0.02 a | |
| Panel b | |||||
| TPC mg GAE g−1 | DPPH mg TEAC g−1 | ABTS mg TEAC g−1 | FRAP mg TEAC g−1 | ||
| f-OP | t0 | 7.18 ± 0.12 a | 16.21 ± 0.41 a | 15.36 ± 0.51 a | 15.95 ± 0.19 a |
| F-OP | t1 | 8.40 ± 0.59 a | 17.25 ± 0.44 a | 16.42 ± 0.27 ab | 18.16 ± 0.46 ab |
| t2 | 7.92 ± 0.40 a | 17.03 ± 0.45 a | 16.94 ± 0.04 ab | 18.56 ± 0.62 b | |
| t3 | 7.38 ± 0.82 a | 16.27 ± 0.04 a | 17.13 ± 0.50 ab | 17.26 ± 0.31 a | |
| t4 | 8.24 ± 0.31 a | 16.77 ± 0.22 a | 17.31 ± 0.58 ab | 18.88 ± 0.31 b | |
| t5 | 7.98 ± 1.11 a,* | 16.80 ± 0.06 a,* | 18.33 ± 0.82 b,* | 18.99 ± 0.70 b,* | |
| t6 | 9.05 ± 0.29 a,* | 17.41 ± 0.55 a,* | 15.80 ± 1.03 a | 20.20 ± 0.47 b,* |
| % OP | Presumptive Lactobacilli | Eumycetes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SWD | WWD | SWD | WWD | ||||||
| BL | AL | BL | AL | BL | AL | BL | AL | ||
| t0-f | 0% | 1.83 ± 0.18 b,A | 2.02 ± 0.09 a,A | 1.72 ± 0.17 b,A | 1.39 ± 0.12 b,A | 6.40 ± 0.00 b,A | 6.30 ± 0.06 ab,A | 6.50 ± 0.01 a,A | 6.30 ± 0.00 b,B |
| 10% | 2.60 ± 0.18 a,A | 1.98 ± 0.71 a,A | 2.40 ± 0.02 ab,A | 2.55 ± 0.04 a,A | 6.59 ± 0.02 a,A | 6.37 ± 0.04 a,B | 6.45 ± 0.03 a,A | 6.24 ± 0.05 b,B | |
| 15% | 2.76 ± 0.11 a,A | 2.94 ± 0.12 a,A | 2.69 ± 0.51 ab,A | 2.88 ± 0.18 a,A | 6.57 ± 0.00 ab,A | 6.20 ± 0.01 b,B | 6.54 ± 0.10 a,A | 6.78 ± 0.03 a,A | |
| 20% | 2.69 ± 0.04 a,A | 3.04 ± 0.33 a,A | 3.04 ± 0.10 a,A | 3.02 ± 0.13 a,A | 6.55 ± 0.09 ab,A | 6.21 ± 0.04 ab,B | 6.59 ± 0.01 a,A | 5.96 ± 0.01 c,B | |
| t6-R | 0% | 1.48 ± 0.40 b,A | 1.50 ± 0.24 c,A | 1.86 ± 0.45 b,A | 2.32 ± 0.22 a,A | 6.92 ± 0.10 b,A | 7.05 ± 0.08 a,A | 6.26 ± 0.10 b,A | 6.72 ± 0.03 c,A |
| 10% | 3.21 ± 0.24 a,A | 3.06 ± 0.33 b,A | 2.96 ± 0.36 ab,A | 2.73 ± 0.84 a,A | 7.28 ± 0.13 a,A | 7.08 ± 0.10 a,A | 7.14 ± 0.04 a,A | 7.00 ± 0.01 a,B | |
| 15% | 3.31 ± 0.25 a,A | 3.32 ± 0.32 ab,A | 3.48 ± 0.92 a,A | 3.33 ± 1.11 a,A | 7.27 ± 0.17 a,A | 7.10 ± 0.12 a,A | 7.03 ± 0.02 a,A | 7.05 ± 0.01 a,A | |
| 20% | 3.48 ± 0.13 a,A | 3.66 ± 0.20 a,A | 3.69 ± 0.66 a,A | 3.34 ± 0.77 a,A | 7.35 ± 0.11 a,A | 7.17 ± 0.11 a,A | 7.09 ± 0.01 a,A | 6.81 ± 0.06 b,B | |
| t6-F | 0% | 1.48 ± 0.40 b,A | 1.50 ± 0.24 a,A | 1.86 ± 0.45 a,A | 2.32 ± 0.22 a,A | 6.92 ± 0.10 b,A | 7.05 ± 0.08 ab,A | 6.26 ± 0.10 d,B | 6.72 ± 0.03 c,A |
| 10% | 1.86 ± 0.28 ab,A | 1.56 ± 0.40 a,A | 1.76 ± 0.15 a,A | 1.84 ± 0.17 a,A | 7.29 ± 0.14 a,A | 7.29 ± 0.12 a,A | 6.38 ± 0.02 c,A | 6.23 ± 0.06 d,B | |
| 15% | 2.31 ± 0.39 a,A | 1.51 ± 0.37 a,B | 2.08 ± 0.27 a,A | 1.75 ± 0.50 a,A | 7.41 ± 0.14 a,A | 7.15 ± 0.12 ab,B | 7.10 ± 0.01 a,A | 6.91 ± 0.04 b,B | |
| 20% | 1.15 ± 0.30 b,A | 1.80 ± 0.55 a,A | 2.60 ± 0.96 a,A | 1.83 ± 1.59 a,A | 7.37 ± 0.14 a,A | 7.23 ± 0.07 ab,A | 6.83 ± 0.01 b,B | 7.07 ± 0.01 a,A | |
| %OP | Specific Volume (cm3 g−1) | Hardness (N) | |||
|---|---|---|---|---|---|
| SWB | WWB | SWB | WWB | ||
| t0-f | 0% | 22.14 ± 0.01 a,A | 20.81 ± 0.05 a,B | 22.75 ± 3.17 a,A | 25.86 ± 1.73 a,A |
| 10% | 21.23 ± 1.06 a,A | 20.76 ± 0.03 a,A | 10.83 ± 1.20 c,B | 21.57 ± 2.31 c,A | |
| 15% | 20.91 ± 1.30 a,A | 21.93 ± 0.72 a,A | 11.74 ± 1.22 c,B | 26.29 ± 1.73 b,A | |
| 20% | 22.56 ± 0.20 a,A | 21.95 ± 0.07 a,A | 18.75 ± 0.47 b,B | 23.00 ± 2.01 b,A | |
| t6-R | 0% | 22.65 ± 0.82 a,A | 19.21 ± 1.20 a,B | 22.40 ± 2.22 a,B | 26.90 ± 0.95 a,A |
| 10% | 20.40 ± 1.04 a,A | 20.11 ± 0.77 a,A | 8.09 ± 0.94 b,B | 16.66 ± 1.95 b,A | |
| 15% | 20.31 ± 1.35 a,A | 21.48 ± 1.01 a,A | 9.73 ± 1.33 b,B | 24.30 ± 1.87 a,A | |
| 20% | 21.68 ± 0.76 a,A | 21.15 ± 2.59 a,A | 13.34 ± 1.52 b,B | 24.01 ± 1.51 ab,A | |
| t6-F | 0% | 22.65 ± 0.82 a,A | 19.21 ± 1.20 a,B | 22.40 ± 2.22 b,B | 26.90 ± 0.94 b,A |
| 10% | 21.07 ± 0.62 a,A | 19.41 ± 2.34 a,A | 19.26 ± 2.74 b,B | 25.59 ± 4.91 b,A | |
| 15% | 21.07 ± 1.09 a,A | 21.67 ± 1.48 a,A | 28.21 ± 1.18 a,B | 31.26 ± 2.47 a,A | |
| 20% | 22.56 ± 0.95 a,A | 20.80 ± 0.43 a,B | 29.12 ± 3.73 a,B | 33.72 ± 1.27 a,A | |
| %OP | TPC (mg GAE g−1) | DPPH (mg TEAC g−1) | ABTS (mg TEAC g−1) | FRAP (mg TEAC g−1) | Dietary Fiber (g 100 g−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SWB | WWB | SWB | WWB | SWB | WWB | SWB | WWB | SWB | WWB | ||
| t0-f | 0% | 0.27 ± 0.01 a, A | 0.29± 0.061 a,A | 0.19 ± 0.03 a,A | 0.28 ± 0.10 a,A | 0.32 ± 0.01 a,A | 0.56 ± 0.13 a,A | 0.14 ± 0.01 a,A | 0.25 ± 0.47 a,A | 3.27 ± 0.08 c,B | 6.09 ± 0.83 a,A |
| 10% | 0.70 ± 0.08 b,A | 0.68 ± 0.05 b,A | 1.26 ± 0.17 bc,A | 1.29 ± 0.00 b,A | 1.23 ± 0.22 b,A | 1.41 ± 0.04 b,A | 1.15 ± 0.10 b,A | 1.23 ± 0.04 b,A | 4.43 ± 0.52 b,A | 6.83 ± 0.59 a,A | |
| 15% | 0.84 ± 0.09 bc,A | 0.90 ± 0.10 bc,A | 1.67 ± 0.09 bc,A | 1.78 ± 0.05 bc,A | 1.55 ± 0.01 bc,A | 1.73 ± 0.10 b,A | 1.63 ± 0.07 b,A | 1.65 ± 0.05 b,A | 5.49 ± 0.09 ab,A | 7.00 ± 0.69 a,A | |
| 20% | 0.91 ± 0.05 c,A | 0.75 ± 0.11 bc,A | 1.90 ± 0.18 c,A | 1.12 ± 0.00 b,A | 1.76 ± 0.05 c,A | 1.24 ± 0.04 b,B | 1.76 ± 0.16 b,A | 1.11 ± 0.01 b,B | 6.57 ± 0.09 a,A | 8.71 ± 0.83 a,A | |
| t6-R | 0% | 0.19 ± 0.01 a,A | 0.37 ± 0.04 a,B | 0.25 ± 0.04 a,A | 0.33 ± 0.03 a,A | 0.77 ± 0.11 a,A | 0.97 ± 0.05 a,A | 0.21 ± 0.00 a,A | 0.31 ± 0.01 a,A | 3.36 ± 0.20 b,B | 5.20 ± 0.44 a,A |
| 10% | 1.08 ± 0.28 b,A | 0.99 ± 0.05 b,A | 0.89 ± 0.21 b,A | 0.82 ± 0.19 b,A | 1.93 ± 0.49 b,A | 1.82 ± 0.04 b,A | 1.38 ± 0.49 b,A | 1.21 ±0.30 b,A | 4.83 ± 0.60 ab,A | 6.58 ± 1.27 a,A | |
| 15% | 1.46 ± 0.33 b,A | 1.20 ± 0.13 b,A | 1.21 ± 0.21 b,A | 0.97 ± 0.01 b,A | 2.57 ± 0.50 c,A | 2.15 ± 0.04 bc,A | 2.02 ± 0.59 bc,A | 1.45 ±0.03 b,B | 4.87 ± 1.03 ab,A | 6.65 ± 0.88 a,A | |
| 20% | 1.70 ± 0.20 b,A | 1.61 ± 0.52 b,A | 1.36 ± 0.27 b,A | 1.34 ± 0.33 b,A | 2.81 ± 0.45 c,A | 2.64 ± 0.58 c,A | 2.35 ± 0.51 c,A | 2.14 ±0.81 c,A | 6.44 ± 0.59 a,A | 7.34 ± 0.44 a,A | |
| t6-F | 0% | 0.19 ± 0.01 a,A | 0.37 ± 0.04 a,B | 0.25 ± 0.04 a,A | 0.33 ± 0.03 a,A | 0.77 ± 0.11 a,A | 0.97 ± 0.05 a,A | 0.21 ± 0.00 a,A | 0.31 ± 0.01 a,A | 3.36 ± 0.20 b,B | 5.20 ± 0.44 a,A |
| 10% | 0.91 ± 0.18 b,A | 0.95 ± 0.05 b,A | 0.62 ± 0.08 b,A | 0.66 ± 0.16 b,A | 1.56 ± 0.18 b,A | 1.64 ± 0.71 b,A | 1.01 ± 0.22 b,A | 0.99 ± 0.21 b,A | 4.32 ± 0.72 ab,A | 5.32 ± 0.71 a,A | |
| 15% | 1.01 ± 0.33 b,A | 0.72 ± 0.14 b,A | 1.01 ± 0.38 b,A | 0.82 ± 0.03 b,A | 2.11 ± 0.72 c,A | 1.58 ± 0.13 b,A | 1.53 ± 0.73 b,A | 1.13 ± 0.10 b,A | 5.65 ± 0.83 a,A | 5.97 ± 1.35 a,A | |
| 20% | 1.39 ± 0.60 b,A | 1.22 ± 0.13 b,A | 1.07 ± 0.39 b,A | 1.27 ± 0.26 b,A | 2.36 ± 0.40 c,A | 2.30 ± 0.01 c,A | 1.85 ± 0.64 c,A | 1.69 ± 0.24 c,A | 6.21 ± 0.67 a,A | 6.75 ± 0.50 a,A | |
| RI | Compounds | t0-f 0% | t0-f 10% | t0-f 15% | t0-f 20% | t6-(R or F) 0% | t6-R 10% | t6-R 15% | t6-R 20% | t6-F 10% | t6-F 15% | t6-F 20% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Terpenoids | ||||||||||||
| 1084 | beta pinene | 1.90 ± 0.03 a | 3.00 ± 0.05 c | 4.10 ± 0.05 d | 5.46 ± 0.51 e | 1.95 ± 0.04 a | 1.43 ± 0.26 b | 3.19 ± 0.53 c | 3.17 ± 0.12 c | 2.98 ± 0.20 c | 3.48 ± 0.17 c | 4.44 ± 0.23 d |
| 1191 | limonene | 0.20 ± 0.01 b | 0.30 ± 0.02 c | 0.35 ± 0.03 c | 0.50 ± 0.02 d | 0.26 ± 0.01 a | 0.24 ± 0.05 a | 0.28 ± 0.01 a | 0.34 ± 0.05 c | 0.30 ± 0.08 c | 0.32 ± 0.05 c | 0.49 ± 0.08 d |
| 1480 | Copaene | Nd | 2.14 ± 0.00 d | 2.50 ± 0.02 e | 2.84 ± 0.01 f | Nd | 0.87 ± 0.02 a | 1.04 ± 0.06 b | 1.33 ± 0.19 c | 1.20 ± 0.04 c | 1.26 ± 0.29 c | 1.40 ± 0.05 c |
| Ketones | ||||||||||||
| 812 | 2-Butanone | Nd | 0.20 ± 0.00 d | 0.21 ± 0.01 d | 0.37 ± 0.01 b | Nd | 0.60 ± 0.12 a | 0.49 ± 0.03 a | 0.37 ± 0.02 b | Nd | Nd | 0.16 ± 0.01 c |
| 938 | 2-pentanone | Nd | 5.61 ± 0.14 a | 6.56 ± 0.52 a | 6.51 ± 0.42 a | Nd | Nd | 6.01 ± 1.06 a | 5.73 ± 0.34 a | Nd | Nd | Nd |
| 1065 | 2,3-pentanedione | 0.22 ± 0.01 b | 2.18 ± 0.09 f | 5.88 ± 0.51 g | 7.48 ± 0.51 h | 0.26 ± 0.01 a | 0.92 ± 0.17 c | 0.79 ± 0.21 c | 0.73 ± 0.12 c | 0.54 ± 0.02 d | 0.22 ± 0.01 a | 0.15 ± 0.03 e |
| 1198 | 2-heptanone | 0.48 ± 0.15 a | 0.48 ± 0.04 a | 1.10 ± 0.09 b | 1.85 ± 0.04 c | 0.52 ± 0.06 a | Nd | Nd | Nd | Nd | Nd | Nd |
| 1283 | 2-octanone | 0.16 ± 0.01 b | Nd | Nd | Nd | 0.11 ± 0.01 a | 0.35 ± 0.04 c | 0.47 ± 0.08 d | 0.78 ± 0.03 e | Nd | 0.22 ± 0.02 f | 0.33 ± 0.01 c |
| 1281 | Acetoin | 0.77 ± 0.03 b | 2.18 ± 0.01 d | 2.57 ± 0.04 d | 2.75 ± 0.01 d | 3.16 ± 0.59 a | 4.01 ± 0.84 c | 2.21 ± 0.49 d | 1.85 ± 0.47 ed | 1.34 ± 0.08 e | 2.04 ± 0.22 d | 2.10 ± 0.35 d |
| 1303 | Acetol | 0.50 ± 0.01 b | 1.40 ± 0.00 c | 0.99 ± 0.00 c | 0.12 ± 0.03 e | 0.66 ± 0.03 a | 1.38 ± 0.19 c | 1.08 ± 0.24 c | 0.90 ± 0.09 c | 0.57 ± 0.04 d | 0.42 ± 0.09 b | 0.40 ± 0.08 b |
| Aldehydes | ||||||||||||
| 820 | 2-methylbutanal | 11.96 ± 0.63 b | 16.28 ± 0.49 d | 10.22 ± 0.22 a | 5.52 ± 0.12 c | 10.18 ± 0.06 a | 10.38 ± 0.78 a | 5.71 ± 0.42 c | 5.11 ± 1.10 c | 10.89 ± 0.12 b | 9.68 ± 0.14 a | 5.28 ± 0.13 c |
| 825 | 3-methylbutanal | 14.07 ± 0.59 b | 30.84 ± 0.46 g | 21.18 ± 0.32 h | 13.62 ± 0.17 b | 10.63 ± 0.08 a | 16.75 ± 1.01 c | 10.54 ± 0.43 a | 10.25 ± 1.22 d | 10.99 ± 0.18 ad | 9.21 ± 0.15 e | 7.25 ± 0.24 f |
| 1078 | Hexanal | 7.78 ± 0.50 b | 6.46 ± 0.19 f | 8.99 ± 0.47 g | 9.56 ± 0.20 h | 5.62 ± 0.43 a | 3.81 ± 0.46 c | 2.55 ± 0.19 d | 2.46 ± 0.13 d | 1.17 ± 0.22 e | 1.58 ± 0.05 e | 1.68 ± 0.32 e |
| 1325 | 2-heptenal | 0.10 ± 0.00 a | 1.24 ± 0.08 e | 1.07 ± 0.05 b | 0.92 ± 0.02 b | Nd | 1.07 ± 0.13 b | 0.90 ± 0.05 c | 1.19 ± 0.20 b | 0.54 ± 0.14 d | 0.45 ± 0.09 d | 0.63 ± 0.07 d |
| 1522 | Benzaldehyde | 1.16 ± 0.06 b | 9.76 ± 0.19 e | 12.98 ± 0.75 f | 15.90 ± 0.10 g | 0.97 ± 0.04 a | 3.91 ± 0.88 c | 4.46 ± 0.33 c | 4.73 ± 0.92 c | 4.28 ± 0.72 c | 4.97 ± 0.23 c | 7.72 ± 1.24 d |
| 1640 | Benzeneacetaldehyde | Nd | 0.87 ± 0.09 c | 1.18 ± 0.03 d | 1.23 ± 0.03 d | Nd | 0.23 ± 0.05 a | 0.52 ± 0.11 b | 0.60 ± 0.10 b | Nd | 0.84 ± 0.10 c | 0.78 ± 0.12 c |
| Esters and acetates | ||||||||||||
| 1433 | Ethyl octanoate | 0.30 ± 0.01 a | 0.30 ± 0.00 c | 0.32 ± 0.00 d | 0.86 ± 0.04 e | Nd | 0.25 ± 0.05 a | 0.30 ± 0.02 a | 0.74 ± 0.17 b | 0.50 ± 0.11 b | 0.61 ± 0.05 b | 0.64 ± 0.10 b |
| 1660 | Ethyl benzoate | Nd | 0.30 ± 0.01 a | 0.31 ± 0.02 a | 0.28 ± 0.01 a | Nd | 0.33 ± 0.09 a | 0.87 ± 0.10 b | 0.88 ± 0.03 b | 0.57 ± 0.03 c | 0.55 ± 0.04 c | 0.47 ± 0.07 c |
| 801 | Ethyl acetate | 1.96 ± 0.01 a | 2.60 ± 0.00 e | 3.75 ± 0.04 b | 4.01 ± 0.05 d | 2.18 ± 0.35 a | 1.86 ± 0.13 a | 3.64 ± 0.67 b | 5.75 ± 0.48 c | 3.40 ± 0.31 b | 3.88 ± 0.19 b | 4.18 ± 0.24 d |
| Alcohols | ||||||||||||
| 888 | Ethanol | 134.31 ± 8.04 b | 86.71 ± 7.33 f | 128.4 ± 3.27 g | 120.2 ± 0.67 h | 105.61 ± 5.58 a | 101.1 ± 20.77 a | 183.8 ± 37.12 c | 174.2 ± 9.15 c | 254.3 ± 12.73 d | 225.7 ± 36.31 d | 212.3 ± 25.10 e |
| 1211 | Isoamylalcohol | 17.53 ± 0.12 a | 26.90 ± 3.63 a | 30.08 ± 0.28 b | 42.26 ± 0.17 b | 16.57 ± 3.19 a | 17.95 ± 4.31 a | 20.86 ± 0.86 a | 20.15 ± 3.22 a | 21.12 ± 4.88 a | 19.09 ± 4.92 a | 19.33 ± 2.98 a |
| 1108 | Isobutanol | 2.29 ± 0.07 b | 2.50 ± 0.01 b | 3.40 ± 0.02 a | 9.46 ± 0.44 c | 3.50 ± 0.47 a | 2.85 ± 0.51 b | 2.47 ± 0.25 b | 2.38 ± 0.13 b | 3.18 ± 0.31 a | 3.58 ± 0.31 a | 3.94 ± 1.18 a |
| 1355 | 1-Hexanol | 1.72 ± 0.12 a | 7.37 ± 0.04 d | 8.10 ± 0.26 e | 15.03 ± 0.90 f | 1.57 ± 0.43 a | 2.83 ± 0.53 b | 2.99 ± 0.80 b | 2.50 ± 0.20 b | 3.94 ± 0.85 c | 4.38 ± 0.28 c | 5.21 ± 0.78 c |
| 1383 | 3-hexen-1-ol | Nd | Nd | 1.10 ± 0.10 c | 1.78 ± 0.02 d | Nd | Nd | Nd | 0.39 ± 0.02 a | Nd | 0.82 ± 0.02 b | 0.90 ± 0.10 b |
| 1905 | Benzeneethanol | 1.74 ± 0.01 b | 1.21 ± 0.01 d | 1.31 ± 0.03 e | 1.51 ± 0.00 f | 2.14 ± 0.61 a | 2.31 ± 0.27 a | 2.49 ± 0.18 a | 3.40 ± 0.2 c | 1.69 ± 0.26 b | 1.83 ± 0.26 b | 2.09 ± 0.04 a |
| Pyrazines | ||||||||||||
| 1268 | Methylpyrazine | 5.00 ± 0.11 b | 5.50 ± 0.01 b | 7.47 ± 0.42 c | 9.86 ± 0.01 f | 4.50 ± 0.18 a | 5.85 ± 1.03 b | 8.72 ± 1.05 c | 10.27 ± 1.35 d | 3.26 ± 0.19 e | 4.81 ± 0.55 a | 7.61 ± 1.18 c |
| 1325 | 2,5-dimethyl pyrazine | 0.71 ± 0.02 a | 0.75 ± 0.01 a | 0.71 ± 0.04 a | 1.50 ± 0.01 e | 0.65 ± 0.11 a | 0.93 ± 0.12 b | 1.21 ± 0.09 c | 2.96 ± 0.57 d | 0.86 ± 0.16 b | 0.81 ± 0.04 b | 1.55 ± 0.07 e |
| 1349 | 2,3-dimethylpyrazine | 1.11 ± 0.14 a | Nd | Nd | Nd | 1.00 ± 0.12 a | 1.45 ± 0.15 b | 0.45 ± 0.02 c | 0.29 ± 0.03 d | 0.71 ± 0.04 e | 0.19 ± 0.01 f | 0.51 ± 0.02 g |
| 1386 | 2-ethyl-6-methylpyrazine | 0.78 ± 0.12 b | 1.98 ± 0.06 g | 2.15 ± 0.03 f | 2.35 ± 0.01 h | 0.97 ± 0.07 a | 0.98 ± 0.13 a | 1.38 ± 0.17 c | 2.60 ± 0.13 d | 1.11 ± 0.01 e | 1.52 ± 0.02 c | 2.12 ± 0.03 f |
| 1391 | 2-ethyl-5-methylpyrazine | 1.55 ± 0.09 a | 1.77 ± 0.04 e | 2.01 ± 0.02 f | 2.65 ± 0.02 g | 1.65 ± 0.08 a | 1.17 ± 0.12 b | 1.58 ± 0.10 a | 3.18 ± 0.25 c | 0.91 ± 0.16 b | 1.20 ± 0.04 b | 1.35 ± 0.11 d |
| Acids | ||||||||||||
| 1447 | Acetic acid | 11.83 ± 0.47 b | 16.41 ± 0.25 a | 19.83 ± 0.43 c | 20.02 ± 0.53 c | 13.77 ± 0.64 a | 21.44 ± 1.76 c | 23.16 ± 2.46 c | 27.76 ± 0.45 | 15.40 ± 2.06 a | 20.39 ± 2.73 c | 22.57 ± 2.89 c |
| 1628 | Butanoic acid | 0.54 ± 0.00 b | 0.85 ± 0.02 c | 1.03 ± 0.03 d | 1.15 ± 0.02 e | 0.62 ± 0.04 a | 0.76 ± 0.06 c | 0.94 ± 0.13 d | 0.98 ± 0.11 d | 0.91 ± 0.22 d | 1.23 ± 0.15 e | 1.23 ± 0.18 e |
| 1840 | Hexanoic acid | Nd | 0.45 ± 0.01 c | 0.54 ± 0.01 d | 0.59 ± 0.00 d | 0.21 ± 0.01 a | 0.35 ± 0.04 b | 0.48 ± 0.08 c | 0.58 ± 0.08 d | 0.41 ± 0.03 c | 0.40 ± 0.01 c | 0.50 ± 0.09 c |
| 2058 | Octanoic acid | 0.25 ± 0.01 b | 0.22 ± 0.00 a | 0.26 ± 0.06 b | 0.52 ± 0.10 f | 0.21 ± 0.04 a | 0.37 ± 0.06 c | 0.20 ± 0.00 a | 0.14 ± 0.01 d | 0.09 ± 0.00 e | 0.14 ± 0.01 d | 0.16 ± 0.01 d |
| 2281 | Decanoic acid | 0.30 ± 0.01 b | 0.34 ± 0.03 a | 0.46 ± 0.04 f | 0.70 ± 0.04 g | 0.35 ± 0.02 a | 0.10 ± 0.01 c | 0.32 ± 0.02 ab | 0.94 ± 0.17 d | 0.23 ± 0.03 e | 0.25 ± 0.04 e | 0.35 ± 0.04 a |
| Furans and pyrans | ||||||||||||
| 1223 | 2-pentylfuran | 4.02 ± 0.03 b | 3.78 ± 0.10 f | 1.97 ± 0.04 g | 1.91 ± 0.01 h | 3.50 ± 0.02 a | Nd | 0.49 ± 0.05 c | 0.81 ± 0.03 d | 0.49 ± 0.06 c | 0.45 ± 0.05 c | 0.40 ± 0.01 e |
| 1462 | 2-furfural | 2.10 ± 0.02 b | 5.08 ± 0.06 g | 5.24 ± 0.34 g | 5.50 ± 0.31 d | 2.64 ± 0.31 a | 9.65 ± 1.87 c | 7.70 ± 1.49 d | 6.35 ± 0.65 d | 3.37 ± 0.49 e | 4.05 ± 0.21 f | 2.04 ± 0.91 b |
| 1658 | 2-Furanmethanol | 1.61 ± 0.06 a | 8.43 ± 0.47 c | 7.59 ± 0.24 g | 7.38 ± 0.21 g | 2.10 ± 0.62 a | 12.11 ± 2.41 b | 8.61 ± 0.57 c | 3.88 ± 0.25 d | 5.89 ± 0.92 e | 3.36 ± 0.17 f | 2.27 ± 0.58 a |
| 1503 | 2-acetylfuran | 0.28 ± 0.00 a | 1.07 ± 0.01 c | 0.79 ± 0.05 f | 0.73 ± 0.03 f | 0.27 ± 0.06 a | 1.27 ± 0.17 b | 1.14 ± 0.21 c | 0.49 ± 0.06 d | 0.64 ± 0.06 e | 0.59 ± 0.08 de | 0.55 ± 0.11 d |
| 2501 | 5-(hydroxymethyl)-2-furfural (HMF) | 0.31 ± 0.00 b | 0.46 ± 0.02 a | 0.51 ± 0.03 a | 0.62 ± 0.03 d | 0.45 ± 0.12 a | 0.84 ± 0.15 c | 0.52 ± 0.07 a | 0.41 ± 0.06 a | 0.45 ± 0.03 a | 0.52 ± 0.01 a | 0.44 ± 0.06 a |
| Phenols | ||||||||||||
| 2203 | Vinylguaiacol | Nd | 0.30 ± 0.05 c | 0.35 ± 0.04 c | 0.42 ± 0.02 d | Nd | 0.19 ± 0.01 a | 0.18 ± 0.04 ab | 0.13 ± 0.03 b | 0.24 ± 0.04 c | 0.24 ± 0.05 c | 0.31 ± 0.05 c |
| 1862 | Guaiacol | Nd | 0.48 ± 0.02 c | 0.51 ± 0.01 c | 0.60 ± 0.02 d | Nd | 0.32 ± 0.07 a | 0.36 ± 0.01 a | 0.42 ± 0.04 b | 0.32 ± 0.07 a | 0.40 ± 0.03 b | 0.41 ± 0.04 b |
| RI | Compounds | t0-f 0% | t0-f 10% | t0-f 15% | t0-f 20% | t6-(R or F) 0% | t6-R 10% | t6-R 15% | t6-R 20% | t6-F 10% | t6-F 15% | t6-F 20% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Terpenoids | ||||||||||||
| 1084 | Beta pinene | 3.23 ± 0.65 a | 4.60 ± 0.50 a | 4.80 ± 0.60 a | 6.10 ± 0.50 b | 3.50 ± 1.0 a | 4.10 ± 0.36 a | 4.50 ± 0.73 a | 4.60 ± 0.47 a | 4.48 ± 0.58 a | 4.85 ± 0.98 c | 2.98 ± 0.15 a |
| 1191 | Limonene | 0.55 ± 0.04 a | 0.57 ± 0.05 a | 0.95 ± 0.01 d | 2.44 ± 0.15 e | 0.60 ± 0.05 a | 0.47 ± 0.01 b | 0.47 ± 0.02 b | 0.60 ± 0.02 a | 0.60 ± 0.03 a | 0.74 ± 0.10 a | 1.12 ± 0.01 c |
| 1480 | Copaene | Nd | 1.57 ± 0.03 c | 2.94 ± 0.06 d | 3.18 ± 0.02 e | Nd | 0.95 ± 0.05 a | 1.14 ± 0.28 a,b | 1.23 ± 0.21 b | 0.85 ± 0.01 c,b | 0.87 ± 0.08 c,b | 1.04 ± 0.04 b |
| Ketones | ||||||||||||
| 812 | 2-Butanone | 0.33 ± 0.00 a | 0.31 ± 0.00 a | 0.36 ± 0.03 a | 0.85 ± 0.03 e | 0.34 ± 0.02 a | 0.21 ± 0.04 b | 0.39 ± 0.02 c | 0.57 ± 0.09 d | 0.19 ± 0.04 b | 0.25 ± 0.03 b | 0.36 ± 0.03 a |
| 938 | 2-pentanone | 3.84 ± 0.36 b | 4.78 ± 0.15 e | 4.91 ± 0.05 e | 7.20 ± 0.40 f | 3.10 ± 0.05 a | 2.04 ± 0.20 c | 2.47 ± 0.20 d | 2.54 ± 0.05 d | 2.32 ± 0.23 c,d | 2.80 ± 0.30 d | 2.96 ± 0.59 d |
| 1065 | 2,3-pentanedione | 0.40 ± 0.08 a | 1.10 ± 0.03 d | 2.08 ± 0.03 e | 2.20 ± 0.05 f | 0.38 ± 0.02 a | 0.31 ± 0.03 a | 0.47 ± 0.05 a | 0.72 ± 0.04 b | 0.23 ± 0.02 c | 0.32 ± 0.02 a | 0.67 ± 0.02 b |
| 1198 | 2-heptanone | 0.70 ± 0.01 a | Nd | Nd | Nd | 0.75 ± 0.07 a | Nd | Nd | Nd | Nd | Nd | Nd |
| 1283 | 2-octanone | 1.10 ± 0.05 a | 0.95 ± 0.05 c | 1.10 ± 0.06 a | 1.45 ± 0.02 e | 1.18 ± 0.27 a | 0.62 ± 0.12 b | 0.85 ± 0.05 c | 0.90 ± 0.05 c | 0.87 ± 0.03 c | 1.10 ± 0.05 a | 1.29 ± 0.07 d |
| 1281 | Acetoin | 1.17 ± 0.08 b | 2.29 ± 0.04 c | 2.34 ± 0.03 c | 2.85 ± 0.04 c | 2.34 ± 0.26 a | 2.81 ± 0.13 c | 3.18 ± 0.14 d | 3.44 ± 0.54 d,a | 2.60 ± 0.53 c | 2.68 ± 0.34 c | 3.06 ± 0.07 d |
| 1303 | Acetol | 0.85 ± 0.03 a | 0.20 ± 0.03 e | 0.34 ± 0.01 f | 0.74 ± 0.00 g | 0.90 ± 0.04 a | 0.99 ± 0.04 b | 1.09 ± 0.07 b | 1.04 ± 0.13 b | 0.47 ± 0.03 c | 0.57 ± 0.03 d | 1.37 ± 0.02 d |
| Aldehydes | ||||||||||||
| 820 | 2-methylbutanal | 9.56 ± 0.21 a | 10.69 ± 0.43 g | 11.49 ± 0.35 g | 8.84 ± 0.35 a | 8.79 ± 0.67 a | 3.25 ± 0.46 c | 3.67 ± 0.11 d | 7.13 ± 0.50 a | 2.87 ± 0.34 c | 7.30 ± 0.05 e | 7.75 ± 0.05 f |
| 825 | 3-methylbutanal | 13.31 ± 2.41 a | 21.62 ± 0.01 c | 20.66 ± 0.19 c | 18.67 ± 0.76 d | 12.97 ± 2.17 a | 15.36 ± 0.48 a | 9.40 ± 0.24 b | 8.91 ± 0.67 b | 14.98 ± 2.11 a,c | 15.06 ± 2.11 a,c | 15.81 ± 0.19 a |
| 1078 | Hexanal | 6.91 ± 0.84 a | 12.90 ± 0.39 d | 9.83 ± 0.68 e | 8.40 ± 0.30 f | 6.41 ± 1.09 a | 4.72 ± 0.41 b | 3.81 ± 0.58 b | 2.84 ± 0.12 c | 4.88 ± 0.99 b | 3.85 ± 0.31 b | 3.08 ± 0.18 b |
| 1325 | 2-heptenal | 0.19 ± 0.01 a | 0.57 ± 0.03 c | 0.73 ± 0.05 e | 0.95 ± 0.02 f | 0.20 ± 0.01 a | 1.14 ± 0.04 b | 1.11 ± 0.05 b | 1.12 ± 0.21 b | 0.56 ± 0.09 c | 0.85 ± 0.02 d | 1.12 ± 0.09 b |
| 1522 | Benzaldehyde | 1.92 ± 0.02 a | 5.03 ± 0.06 b | 7.15 ± 0.42 d | 7.65 ± 0.41 d | 1.72 ± 0.19 a | 4.07 ± 0.93 b | 4.90 ± 0.86 b | 5.87 ± 0.44 b | 3.75 ± 0.23 c | 4.34 ± 0.30 b | 5.20 ± 0.75 b |
| 1640 | Benzeneacetaldehyde | Nd | 2.10 ± 0.05 b | 2.40 ± 0.06 b | 3.12 ± 0.07 d | Nd | 1.76 ± 0.19 a | 2.32 ± 0.19 b | 2.45 ± 0.09 b | 1.78 ± 0.04 a | 1.85 ± 0.01 a | 6.65 ± 0.05 c |
| Esters and acetates | ||||||||||||
| 1020 | Ethyl butanoate | Nd | 0.30 ± 0.01 d | 0.33 ± 0.02 d | 0.45 ± 0.03 c | Nd | 0.15 ± 0.01 a | 0.26 ± 0.02 b | 0.26 ± 0.01 b | 0.23 ± 0.02 b | 0.26 ± 0.01 b | 0.42 ± 0.04 c |
| 1229 | Ethyl hexanoate | Nd | 0.71 ± 0.03 c | 1.20 ± 0.03 d | 1.39 ± 0.05 b | Nd | 2.24 ± 0.13 a | 2.20 ± 0.14 a | 1.53 ± 0.24 b | 0.80 ± 0.14 c | 1.11 ± 0.20 d | 1.36 ± 0.01 b |
| 1433 | Ethyl octanoate | 0.14 ± 0.01 a | 0.72 ± 0.03 e | 0.78 ± 0.00 f | 0.82 ± 0.00 b | Nd | 0.87 ± 0.10 b | 0.82 ± 0.10 b | 0.92 ± 0.06 b | 0.67 ± 0.01 c | 0.60 ± 0.03 d | 0.68 ± 0.11 c |
| 801 | Ethyl acetate | 1.94 ± 0.04 b | 2.71 ± 0.03 f | 3.20 ± 0.05 h | 3.77 ± 0.09 i | 1.14 ± 0.01 a | 1.43 ± 0.09 c | 2.30 ± 0.15 d | 2.02 ± 0.31 d | 1.74 ± 0.06 a | 2.55 ± 0.15 d,f | 5.71 ± 1.23 g |
| Alcohols | ||||||||||||
| 888 | Ethanol | 93.06 ± 0.10 b | 97.78 ± 4.10 a,b | 103.00 ± 0.69 f | 110.00 ± 1.66 c | 95.53 ± 0.23 a | 119.94 ± 6.55 c | 186.97 ± 2.70 d | 215.73 ± 4.86 a | 92.78 ± 4.10 a,b | 104.77 ± 0.69 f | 105.93 ± 1.66 f |
| 1211 | Isoamylalcohol | 26.73 ± 0.42 a | 24.17 ± 0.07 f | 27.96 ± 0.58 g | 34.36 ± 0.14 h | 28.97 ± 2.15 a | 17.48 ± 0.50 b | 20.23 ± 1.20 c | 32.55 ± 0.51 d | 12.60 ± 0.60 e | 16.90 ± 0.15 b | 24.46 ± 0.50 f |
| 1108 | Isobutanol | 3.25 ± 0.15 a | 3.60 ± 0.50 a | 2.64 ± 0.75 b | 1.76 ± 0.04 c | 3.30 ± 0.48 a | 3.31 ± 0.99 a | 2.51 ± 0.16 b | 2.33 ± 0.29 b | 3.33 ± 0.60 a | 2.51 ± 0.06 b | 1.81 ± 0.03 c |
| 1355 | 1-Hexanol | 3.97 ± 0.14 a | 5.10 ± 0.55 a | 8.64 ± 0.56 e | 12.13 ± 0.69 f | 4.32 ± 0.30 a | 6.70 ± 0.36 b | 4.64 ± 0.42 a | 3.61 ± 0.41 c | 4.74 ± 0.15 d | 8.60 ± 0.21 e | 9.43 ± 0.40 f |
| 1905 | Benzeneethanol | 0.92 ± 0.02 a | 1.23 ± 0.01 c | 1.14 ± 0.03 c | 1.02 ± 0.06 a | 0.92 ± 0.05 a | 3.35 ± 0.51 b | 3.12 ± 0.65 b | 2.34 ± 0.58 b | 1.30 ± 0.09 c | 3.27 ± 0.19 b | 1.30 ± 0.21 c |
| Pyrazines | ||||||||||||
| 1268 | Methylpyrazine | 2.60 ± 0.74 a | 5.39 ± 0.03 b | 5.48 ± 0.07 b | 6.10 ± 0.07 b | 2.80 ± 0.50 a | 5.86 ± 0.52 b | 7.79 ± 1.55 c | 12.89 ± 1.51 d | 5.26 ± 0.35 b | 5.87 ± 0.35 b | 9.63 ± 0.60 c |
| 1325 | 2,5-dimethyl pyrazine | 0.75 ± 0.11 a | 1.42 ± 0.04 d | 1.50 ± 0.01 d | 1.67 ± 0.00 e | 0.80 ± 0.11 a | 1.19 ± 0.10 b | 1.83 ± 0.47 c | 1.91 ± 0.12 c | 1.10 ± 0.03 b | 1.25 ± 0.02 b | 1.47 ± 0.29 d |
| 1349 | 2.3-dimethylpyrazine | Nd | 0.80 ± 0.04 c | 1.20 ± 0.06 f | 1.98 ± 0.05 g | Nd | 0.35 ± 0.01 a | 0.42 ± 0.03 b | 0.86 ± 0.05 c | 0.74 ± 0.01 d | 0.85 ± 0.05 c | 1.74 ± 0.01 e |
| 1386 | 2-ethyl-6-methylpyrazine | 1.12 ± 0.10 b | 0.90 ± 0.02 g | 1.20 ± 0.04 b | 1.84 ± 0.00 d | 0.10 ± 0.01 a | 1.15 ± 0.04 b | 1.38 ± 0.04 c | 1.83 ± 0.04 d | 0.97 ± 0.02 e | 1.40 ± 0.04 c | 1.60 ± 0.02 f |
| 1391 | 2-ethyl-5-methylpyrazine | 0.65 ± 0.02 b | 1.30 ± 0.05 f | 1.34 ± 0.10 g | 1.85 ± 0.20 e | 0.79 ± 0.02 a | 1.14 ± 0.07 c | 1.64 ± 0.12 d | 1.87 ± 0.13 e | 1.25 ± 0.01 f | 1.45 ± 0.03 g | 1.87 ± 0.02 e |
| Acids | ||||||||||||
| 1447 | Acetic acid | 12.19 ± 0.30 b | 20.50 ± 0.40 c | 22.35 ± 0.55 c | 27.20 ± 0.43 e | 13.76 ± 0.79 a | 20.73 ± 0.72 c | 21.13 ± 0.75 c | 22.71 ± 2.00 c,d | 20.17 ± 3.66 c | 22.42 ± 1.01 c | 25.02 ± 1.53 d |
| 1628 | Butanoic acid | 0.64 ± 0.01 a | 0.43 ± 0.01 c | 0.55 ± 0.12 a | 0.67 ± 0.08 a | 0.59 ± 0.07 a | 1.03 ± 0.11 b | 0.98 ± 0.05 b | 1.03 ± 0.10 b | 0.44 ± 0.09 a,c | 0.45 ± 0.02 c | 0.70 ± 0.07 a |
| 1840 | Hexanoic acid | 0.20 ± 0.01 b | 1.12 ± 0.03 e | 1.23 ± 0.01 e | 1.40 ± 0.05 f | 0.27 ± 0.02 a | 0.70 ± 0.11 c | 0.90 ± 0.04 d | 1.19 ± 0.04 e | 1.07 ± 0.02 f | 1.08 ± 0.02 f | 1.17 ± 0.09 e |
| 2058 | Octanoic acid | 0.18 ± 0.00 a | 0.12 ± 0.00 c | 0.16 ± 0.06 c | 0.18 ± 0.06 c,a | 0.22 ± 0.04 a | 0.35 ± 0.02 b | 0.23 ± 0.02 a | 0.13 ± 0.01 c | 0.11 ± 0.02 c | 0.12 ± 0.00 c | 0.15 ± 0.01 c |
| 2281 | Decanoic acid | 0.30 ± 0.04 a | 0.34 ± 0.06 a | 0.40 ± 0.05 a | 0.48 ± 0.01 e | 0.35 ± 0.04 a | 0.25 ± 0.06 b | 0.56 ± 0.02 c | 0.81 ± 0.01 d | 0.30 ± 0.01 a | 0.25 ± 0.01 b | 0.28 ± 0.03 b |
| Furans and pyrans | ||||||||||||
| 1223 | 2-pentylfuran | 3.43 ± 0.26 a | 3.51 ± 0.05 g | 3.83 ± 0.05 d | 4.85 ± 0.68 e | 3.37 ± 0.35 a | 1.25 ± 0.04 b | 1.38 ± 0.03 c | 3.80 ± 0.09 d | 1.82 ± 0.02 e | 2.65 ± 0.10 f | 3.88 ± 0.6 d |
| 1462 | 2-furfural | 4.22 ± 0.07 b | 6.10 ± 0.01 g | 5.48 ± 0.04 e | 4.25 ± 0.03 b | 3.80 ± 0.17 a | 10.37 ± 0.22 c | 8.61 ± 0.79 d | 4.25 ± 0.85 b | 5.42 ± 0.12 e | 3.95 ± 0.14 a,b | 3.05 ± 0.01 f |
| 1658 | 2-Furanmethanol | 3.48 ± 0.39 a | 6.71 ± 0.06 e | 2.06 ± 0.08 f | 1.44 ± 0.08 g | 3.81 ± 0.55 a | 9.15 ± 0.07 c | 7.13 ± 0.54 d | 4.1 ± 0.56 a | 6.64 ± 0.11 d,e | 2.11 ± 0.28 f | 2.17 ± 0.25 f |
| 1503 | 2-acetylfuran | 0.65 ± 0.05 b | 0.88 ± 0.02 i | 0.81 ± 0.00 a | 0.73 ± 0.02 a | 0.77 ± 0.06 a | 0.80 ± 0.03 a | 1.17 ± 0.22 d | 1.77 ± 0.10 e | 1.31 ± 0.02 f | 0.47 ± 0.01 g | 0.27 ± 0.01 h |
| 2501 | 5-(hydroxymethyl)-2-furfural (HMF) | 0.35 ± 0.01 a | 0.30 ± 0.01 c | 0.35 ± 0.09 a | 0.46 ± 0.02 b | 0.31 ± 0.07 a | 0.46 ± 0.04 b | 0.48 ± 0.01 b | 0.39 ± 0.02 a | 0.27 ± 0.04 a | 0.24 ± 0.01 a | 0.28 ± 0.02 a |
| Phenols | ||||||||||||
| 2203 | Vinylguaiacol | Nd | 0.71 ± 0.00 b | 0.76 ± 0.01 c | 0.81 ± 0.00 d | Nd | 0.61 ± 0.02 a | 0.63 ± 0.05 a | 0.69 ± 0.01 a, b | 0.70 ± 0.01 b | 0.75 ± 0.01 c | 0.79 ± 0.03 d |
| 1862 | Guaiacol | Nd | 0.60 ± 0.01 c | 0.75 ± 0.02 d | 0.88 ± 0.01 b | Nd | 0.46 ± 0.01 a | 0.87 ± 0.07 b | 0.99 ± 0.08 b | 0.52 ± 0.05 a | 0.63 ± 0.05 c | 0.72 ± 0.03 d |
| Axis | Eigenvalue | Difference | Proportion (%) | Cumulative (%) |
|---|---|---|---|---|
| 1 | 17.114715 | 7.994542 | 40.75% | 40.75% |
| 2 | 9.120173 | 1.990407 | 21.71% | 62.46% |
| 3 | 7.129766 | 3.920977 | 16.98% | 79.44% |
| 4 | 3.208789 | 0.33098 | 7.64% | 87.08% |
| 5 | 2.877809 | 2.013748 | 6.85% | 93.93% |
| 6 | 0.864061 | 0.210451 | 2.06% | 95.99% |
| 7 | 0.65361 | 0.225627 | 1.56% | 97.55% |
| 8 | 0.427982 | 0.077306 | 1.02% | 98.56% |
| 9 | 0.350676 | 0.098257 | 0.83% | 99.40% |
| 10 | 0.25242 | 0.25242 | 0.60% | 100.00% |
| Axis | Eigen Value | Difference | Proportion (%) | Cumulative (%) |
|---|---|---|---|---|
| 1 | 17.29995 | 6.696625 | 40.23% | 40.23% |
| 2 | 10.603324 | 5.611939 | 24.66% | 64.89% |
| 3 | 4.991385 | 0.937016 | 11.61% | 76.50% |
| 4 | 4.054369 | 1.117212 | 9.43% | 85.93% |
| 5 | 2.937157 | 1.670838 | 6.83% | 92.76% |
| 6 | 1.266318 | 0.531867 | 2.94% | 95.70% |
| 7 | 0.734452 | 0.244383 | 1.71% | 97.41% |
| 8 | 0.490069 | 0.152634 | 1.14% | 98.55% |
| 9 | 0.337434 | 0.051891 | 0.78% | 99.34% |
| 10 | 0.285543 | 0.285543 | 0.66% | 100.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardinali, F.; Belleggia, L.; Reale, A.; Cirlini, M.; Boscaino, F.; Di Renzo, T.; Del Vecchio, L.; Cavalca, N.; Milanović, V.; Garofalo, C.; et al. Exploitation of Black Olive (Olea europaea L. cv. Piantone di Mogliano) Pomace for the Production of High-Value Bread. Foods 2024, 13, 460. https://doi.org/10.3390/foods13030460
Cardinali F, Belleggia L, Reale A, Cirlini M, Boscaino F, Di Renzo T, Del Vecchio L, Cavalca N, Milanović V, Garofalo C, et al. Exploitation of Black Olive (Olea europaea L. cv. Piantone di Mogliano) Pomace for the Production of High-Value Bread. Foods. 2024; 13(3):460. https://doi.org/10.3390/foods13030460
Chicago/Turabian StyleCardinali, Federica, Luca Belleggia, Anna Reale, Martina Cirlini, Floriana Boscaino, Tiziana Di Renzo, Lorenzo Del Vecchio, Natascia Cavalca, Vesna Milanović, Cristiana Garofalo, and et al. 2024. "Exploitation of Black Olive (Olea europaea L. cv. Piantone di Mogliano) Pomace for the Production of High-Value Bread" Foods 13, no. 3: 460. https://doi.org/10.3390/foods13030460
APA StyleCardinali, F., Belleggia, L., Reale, A., Cirlini, M., Boscaino, F., Di Renzo, T., Del Vecchio, L., Cavalca, N., Milanović, V., Garofalo, C., Cesaro, C., Rampanti, G., Osimani, A., & Aquilanti, L. (2024). Exploitation of Black Olive (Olea europaea L. cv. Piantone di Mogliano) Pomace for the Production of High-Value Bread. Foods, 13(3), 460. https://doi.org/10.3390/foods13030460











