Effects of Balsa Fish Skin Gelatin, Lentinula edodes Mushrooms, Soy Protein Isolate, and Starch on the Sensory Quality and Characterization of Physicochemical and Antioxidant Properties of New Sausage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Formulations and Processing
2.4. Sensory Evaluation
2.5. Proximate Composition, Energy Value, and Color
2.6. Amino Acid Profile
2.7. Fatty Acid Profile
2.8. Volatile Compound Analysis
2.9. Total Phenolic Content
2.10. DPPH Radical Scavenging Activity
2.11. ABTS Radical Scavenging Activity
2.12. Statistical Analysis
3. Results and Discussion
3.1. Response Surface Analysis
3.1.1. Model Building and Statistical Significance Test
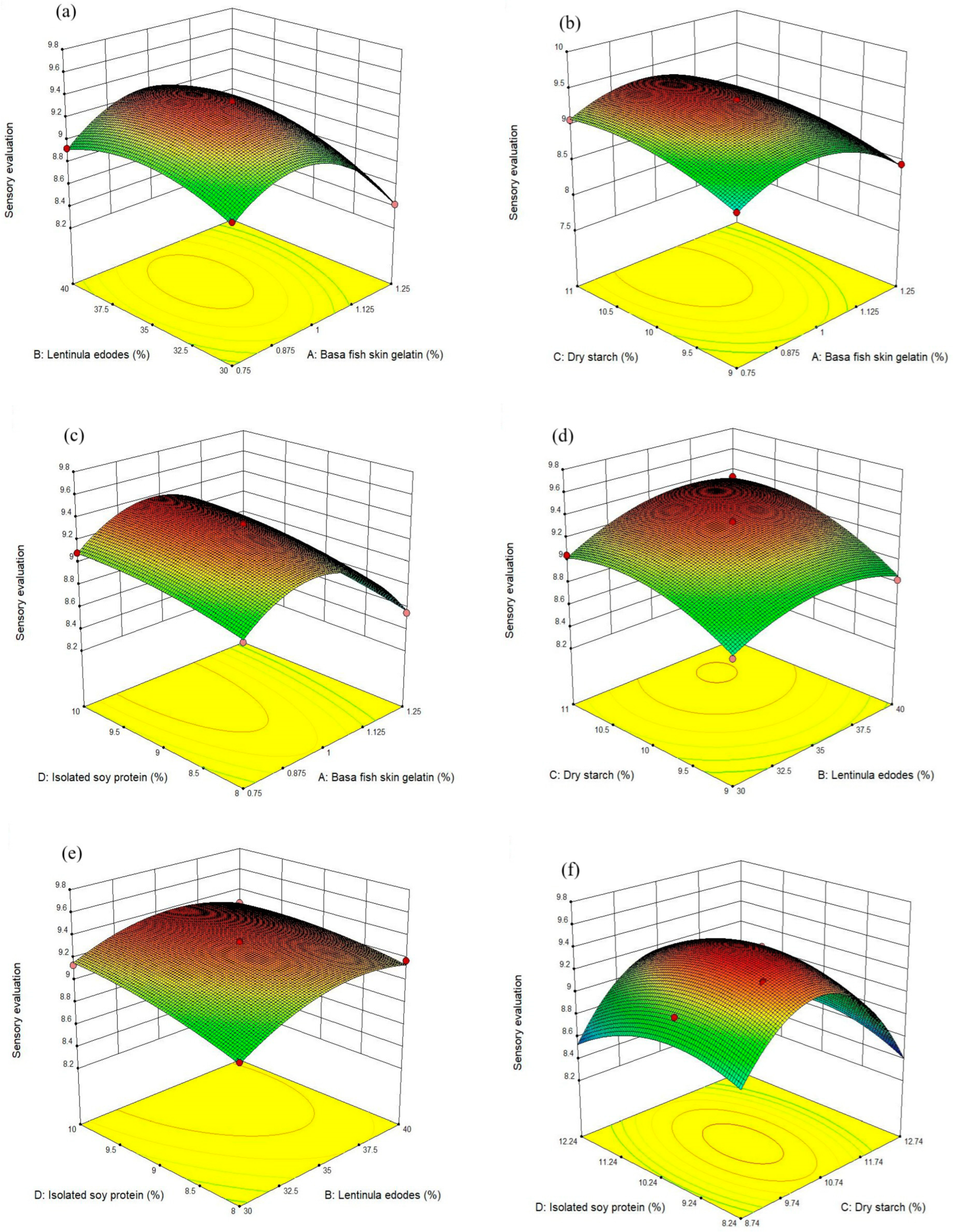
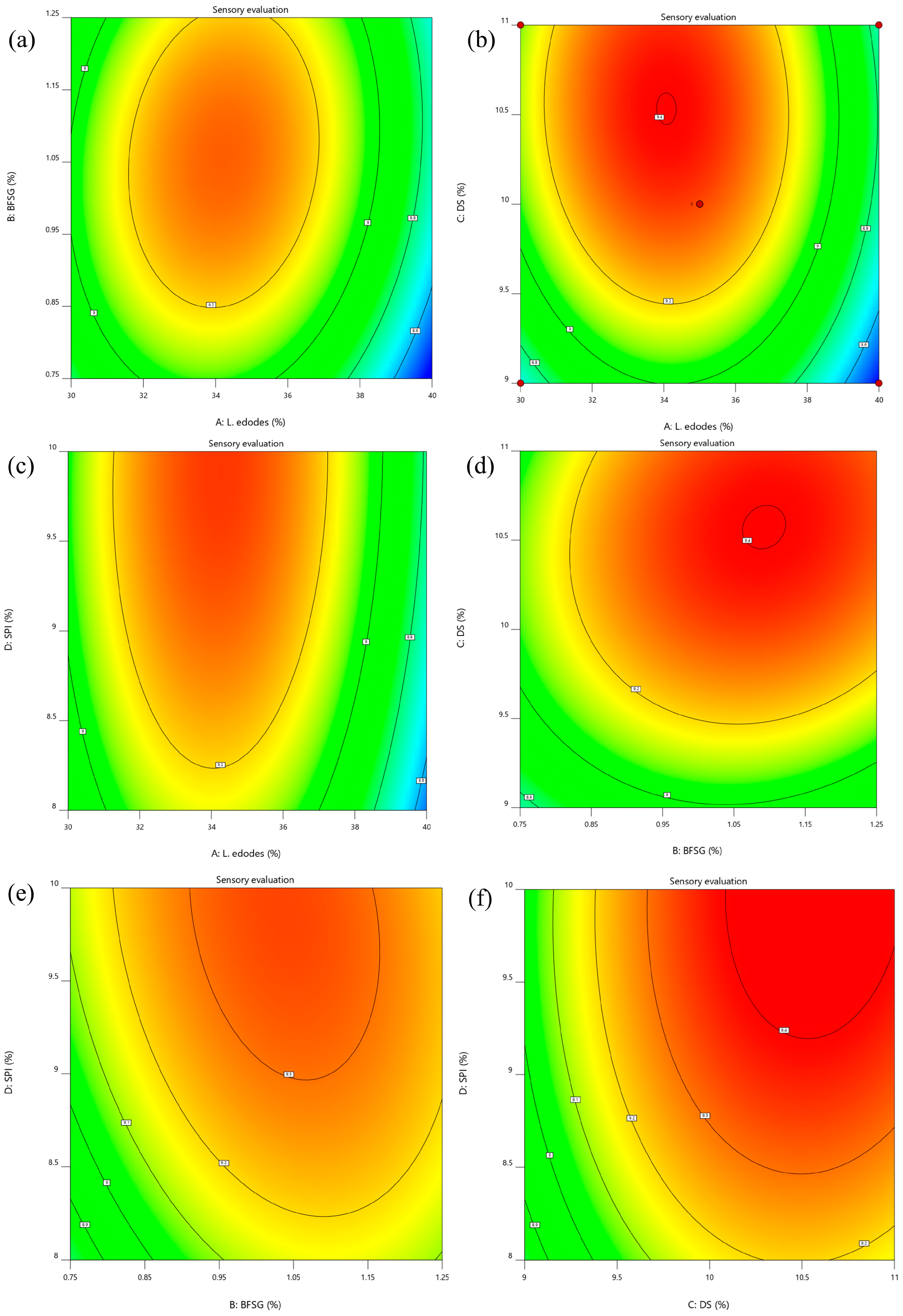
3.1.2. Three-Dimensional (3D) Response Surface Plots
3.1.3. Sensory Evaluation and Validation of the Predicted Optimal Conditions
3.2. Proximate Composition, Energy Value, and Color
3.3. Amino Acid Profile
3.4. Fatty Acid Profile
3.5. Volatile Compound Analysis
3.6. Total Phenolic Content (TPC)
3.7. DPPH Radical Scavenging Activity
3.8. ABTS Radical Scavenging Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tran, T.T.T.; Ton, N.M.N.; Nguyen, T.T.; Sajeev, D.; Schilling, M.W.; Dinh, T.T. Application of natural antioxidant extract from guava leaves (Psidium guajava L.) in fresh pork sausage. Meat Sci. 2020, 165, 108106. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Liu, X.; Jiang, G.; Li, C.; Li, X.; Li, Y. Roles of Lentinula edodes as the pork lean meat replacer in production of the sausage. Meat Sci. 2019, 156, 44–51. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Ren, L.; Guo, H.; Li, Y. Production of Pork Sausages Using Pleaurotus eryngii with Different Treatments as Replacements for Pork Back Fat. J. Food Sci. 2019, 84, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Font-i-Furnols, M. Meat Consumption, Sustainability and Alternatives: An Overview of Motives and Barriers. Foods 2023, 12, 2144. [Google Scholar] [CrossRef] [PubMed]
- Aisala, H.; Laaksonen, O.; Manninen, H.; Raittola, A.; Hopia, A.; Sandell, M. Sensory properties of Nordic edible mushrooms. Food Res. Int. 2018, 109, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.; Drummond, L.; Mullen, A.M. Protein recovered from meat co-products and processing streams as pork meat replacers in Irish breakfast sausages formulations. LWT—Food Sci. Technol. 2018, 96, 679–685. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Terent’ev, A.O.; Levitsky, D.O. Amino and fatty acids of wild edible mushrooms of the genus Boletus. Rec. Nat. Prod. 2010, 4, 218–223. [Google Scholar]
- Zhang, Y.; Hartung, N.M.; Fraatz, M.A.; Zorn, H. Quantification of key odor-active compounds of a novel nonalcoholic beverage produced by fermentation of wort by shiitake (Lentinula edodes) and aroma genesis studies. Food Res. Int. 2015, 70, 23–30. [Google Scholar] [CrossRef]
- Ren, Z.; Li, J.; Song, X.; Zhang, J.; Wang, W.; Wang, X.; Gao, Z.; Jing, H.; Li, S.; Jia, L. The regulation of inflammation and oxidative status against lung injury of residue polysaccharides by Lentinula edodes. Int. J. Biol. Macromol. 2018, 106, 185–192. [Google Scholar] [CrossRef]
- Du, B.; Zhu, F.; Xu, B. An insight into the anti-inflammatory properties of edible and medicinal mushrooms. J. Funct. Foods 2018, 47, 334–342. [Google Scholar] [CrossRef]
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Amirullah, N.A.; Abidin, N.Z.; Abdullah, N. The potential applications of mushrooms against some facets of atherosclerosis: A review. Food Res. Int. 2018, 105, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Kamani, M.H.; Meera, M.S.; Bhaskar, N.; Modi, V.K. Partial and total replacement of meat by plant-based proteins in chicken sausage: Evaluation of mechanical, physico-chemical and sensory characteristics. J. Food Sci. Technol. 2019, 56, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Majzoobi, M.; Talebanfar, S.; Eskandari, M.H.; Farahnaky, A. Improving the quality of meat-free sausages using j-carrageenan, konjac mannan and xanthan gum. Int. J. Food Sci. Technol. 2017, 52, 1269–1275. [Google Scholar] [CrossRef]
- Malav, O.P.; Talukder, S.; Gokulakrishnan, P.; Chand, S. Meat analog: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Asgar, M.A.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A.A. Nonmeat protein alternatives as meat extenders and meat analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kang, Z.; Sukmanov, V.; Ma, H. Effects of soy protein isolate on gel properties and water holding capacity of low-salt pork myofibrillar protein under high pressure processing. Meat Sci. 2021, 176, 108471. [Google Scholar] [CrossRef]
- Das, A.K.; Anjaneyulu, A.S.R.; Verma, A.K.; Kondaiah, N. Physicochemical, textural, sensory characteristics and storage stability of goat meat patties extended with full-fat soy paste and soy granules. Int. J. Food Sci. Technol. 2008, 43, 383–392. [Google Scholar] [CrossRef]
- Ma, P.; Wang, Y.; Li, B.; Hou, H. Cross-linking effects of carbodiimide, oxidized chitosan oligosaccharide and glutaraldehyde on acellular dermal matrix of balsa fish (Pangasius bocourti). Int. J. Biol. Macromol. 2020, 164, 677–686. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, K.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, L.; Otte, J. Optimization of hydrolysis conditions for production of angiotensin-converting enzyme inhibitory peptides from Balsa fish skin using response surface methodology. J. Aquat. Food Prod. Technol. 2016, 25, 684–693. [Google Scholar] [CrossRef]
- Gao, Y.; Qiu, Y.; Nan, H.; Wang, L.; Yang, D.; Zhang, L.; Yu, Q. Ultra-high pressure-assisted preparation of cowhide gelatin as a promising fat substitute: Improve the nutrition ratio and antioxidant capacity of beef patties. Food Res. Int. 2022, 157, 111260. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A. Free radical biology: Xenobiotics, cancer, and aging. Ann. N. Y. Acad. Sci. 1982, 393, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Paglarini, C.S.; Vidal, V.A.; Ribeiro, W.; Ribeiro, A.P.B.; Bernardinelli, O.D.; Herrero, A.M.; Pollonio, M.A.R. Using inulin-based emulsion gels as fat substitute in salt reduced Bologna sausage. J. Sci. Food Agric. 2021, 101, 505–517. [Google Scholar] [CrossRef]
- Jo, Y.; An, K.A.; Arshad, M.S.; Kwon, J.H. Effects of e-beam irradiation on amino acids, fatty acids, and volatiles of smoked duck meat during storage. Innov. Food Sci. Emerg. Technol. 2018, 47, 101–109. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- AOCS. Official method Ce 2-66. In Preparations of Methyl Esters of Fatty Acids; American Oil Chemists’ Society: Champaign, IL, USA, 1997. [Google Scholar]
- Liu, X.; Qu, H.; Gou, M.; Guo, H.; Wang, L.; Yan, X. Application of Weissella cibaria X31 or Weissella confusa L2 as a starter in low nitrite dry-fermented sausages. Int. J. Food Eng. 2020, 16. [Google Scholar] [CrossRef]
- Półtorak, A.; Marcinkowska-Lesiak, M.; Lendzion, K.; Moczkowska, M.; Onopiuk, A.; Wojtasik-Kalinowska, I.; Wierzbicka, A. Evaluation of the antioxidant, anti-inflammatory and antimicrobial effects of catuaba, galangal, roseroot, maca root, guarana and polyfloral honey in sausages during storage. LWT—Food Sci. Technol. 2018, 96, 364–370. [Google Scholar] [CrossRef]
- Ayyash, M.; Al-Nuaimi, A.K.; Al-Mahadin, S.; Liu, S.Q. In vitro investigation of anticancer and ACE-inhibiting activity, α-amylase and α-glucosidase inhibition, and antioxidant activity of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. Food Chem. 2018, 239, 588–597. [Google Scholar] [CrossRef]
- Biffin, T.E.; Smith, M.A.; Bush, R.D.; Collins, D.; Hopkins, D.L. The effect of electrical stimulation and tenderstretching on colour and oxidation traits of alpaca (Vicunga pacos) meat. Meat Sci. 2019, 156, 125–130. [Google Scholar] [CrossRef]
- Marti-Quijal, F.J.; Zamuz, S.; Tomašević, I.; Gómez, B.; Rocchetti, G.; Lucini, L.; Lorenzo, J.M. Influence of different sources of vegetable, whey and microalgae proteins on the physicochemical properties and amino acid profile of fresh pork sausages. LWT—Food Sci. Technol. 2019, 110, 316–323. [Google Scholar] [CrossRef]
- Özünlü, O.; Ergezer, H. Possibilities of using dried oyster mushroom (Pleurotus ostreatus) in the production of beef salami. J. Food Process. Preserv. 2021, 45, e15117. [Google Scholar] [CrossRef]
- Yahya, F.; Ting, H.T. Effect of Different Ratios of Chicken Meat to Fresh Osyter Mushroom (Pleurotus sajor-caju) on the Physicochemical Properties and Sensory Acceptability of Sausages. Int. J. Food Agric. Nat. Resour. 2020, 1, 7–14. [Google Scholar] [CrossRef]
- Souza, C.V.B.; Bellucci, E.R.B.; Lorenzo, J.M.; Barretto, A.C.D.S. Low-fat Brazilian cooked sausage-Paio–with added oat fiber and inulin as a fat substitute: Effect on the technological properties and sensory acceptance. Food Sci. Technol. 2019, 39, 295–303. [Google Scholar] [CrossRef]
- Camila, D.S.P.; de Figueiredo Furtado, G.; Honório, A.R.; Mokarzel, L.; da Silva Vidal, V.A.; Ribeiro, A.P.B.; Pollonio, M.A.R. Functional emulsion gels as pork back fat replacers in Bologna sausage. Food Struct. 2019, 20, 100105. [Google Scholar] [CrossRef]
- Garcia-santos, M.D.S.L.; Conceicao, F.S.; Villas Boas, F.; Salotti De Souza, B.M.; Barretto, A.C.D.S. Effect of the addition of resistant starch in sausage with fat reduction on the physicochemical and sensory properties. Food Sci. Technol. 2019, 39, 491–497. [Google Scholar] [CrossRef]
- Camila De Souza Paglarini, A.; Vidal, V.A.S.; Dos Santos, M.; Coimbra, L.O.; Esmerino, E.A.; Cruz, A.G.; Pollonio, M.A.R. Using dynamic sensory techniques to determine drivers of liking in sodium and fat-reduced Bologna sausage containing functional emulsion gels. Food Res. Int. 2020, 132, 109066. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, P.; Cheng, J.; Chen, Z.; Liu, X. Use of straw mushrooms (Volvariella volvacea) for the enhancement of physicochemical, nutritional and sensory profiles of Cantonese sausages. Meat Sci. 2018, 146, 18–25. [Google Scholar] [CrossRef]
- Carvalho, F.A.L.D.; Munekata, P.E.S.; Pateiro, M.; Campagnol, P.C.B.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Effect of replacing backfat with vegetable oils during the shelf-life of cooked lamb sausages. LWT—Food Sci. Technol. 2020, 122, 109052. [Google Scholar] [CrossRef]
- Ferro, A.C.; de Souza Paglarini, C.; Pollonio, M.A.R.; Cunha, R.L. Glyceryl monostearate-based oleogels as a new fat substitute in meat emulsion. Meat Sci. 2021, 174, 108424. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Li, X.; Liu, Y.; Yan, W. Effects of partial replacement of pork back fat by a camellia oil gel on certain quality characteristics of a cooked style Harbin sausage. Meat Sci. 2018, 146, 154–159. [Google Scholar] [CrossRef]
- Monteiro, G.M.; Souza, X.R.; Costa, D.P.B.; Faria, P.B.; Vicente, J. Partial substitution of pork fat with canola oil in Toscana sausage. Innov. Food Sci. Emerg. Technol. 2017, 44, 2–8. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Chen, W.; Yang, Y.; Zhang, J.; Feng, J.; Li, Q. Analysis of volatile compounds of Lentinula edodes grown in different culture substrate formulations. Food Res. Int. 2019, 125, 108517. [Google Scholar] [CrossRef]
- Tagkouli, D.; Bekiaris, G.; Pantazi, S.; Anastasopoulou, M.E.; Koutrotsios, G.; Mallouchos, A.; Kalogeropoulos, N. Volatile Profiling of Pleurotus eryngii and Pleurotus ostreatus Cultivated on Agricultural and Agro-Industrial By-Products. Foods 2021, 10, 1287. [Google Scholar] [CrossRef]
- Sohail, A.; Al-Dalali, S.; Wang, J.; Xie, J.; Shakoor, A.; Asimi, S.; Patil, P. Aroma compounds identified in cooked meat: A review. Food Res. Int. 2022, 157, 111385. [Google Scholar] [CrossRef]
- Mohammad, S.M.; Badrul Hisham, A.A.; Mustapa, N.A.; Chan, K.W.; Zawawi, N. Proximate Analysis, Antioxidant Activity, and Antibacterial Activity of Fish Sausages Fortified with Bee Bread Extract. J. Food Qual. 2021, 2021, 6657553. [Google Scholar] [CrossRef]
- Edgar, C.; Kadri, K. Associations of Volatile Compounds with Sensory Aroma and Flavor: The Complex Nature of Flavor. Molecules 2013, 18, 4887–4905. [Google Scholar] [CrossRef]
- Ramarathnam, N.; Rubin, L.J.; Diosady, L.L. Studies on meat flavor. 3. A novel method for trapping volatile components from uncured and cured pork. J. Agric. Food Chem. 1993, 41, 933–938. [Google Scholar] [CrossRef]
- Wongfhun, P.; Gordon, M.H.; Apichartsrangkoon, A. Flavour characterisation of fresh and processed pennywort (Centella asiatica L.) juices. Food Chem. 2010, 119, 69–74. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Men, H.; Tong, J.; Zhou, J. Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocoll. 2013, 32, 35–41. [Google Scholar] [CrossRef]
- Fang, Z.; Lin, P.; Ha, M.; Warner, R.D. Effects of incorporation of sugarcane fibre on the physicochemical and sensory properties of chicken sausage. Int. J. Food Sci. Technol. 2019, 54, 1036–1044. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Xu, B. Phenolic profiles, antioxidant capacities and metal chelating ability of edible mushrooms commonly consumed in China. LWT—Food Sci. Technol. 2016, 72, 423–431. [Google Scholar] [CrossRef]
- Yu, D.; Feng, M.Q.; Sun, J. Influence of mixed starters on the degradation of proteins and the formation of peptides with antioxidant activities in dry fermented sausages. Food Control 2021, 123, 107743. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Fang, Y.; Ma, N.; Pei, F.; Zhao, L.; Hu, Q. Antioxidant and cytotoxicites of pleurotus eryngii residue polysaccharides obtained by ultrafiltration. LWT—Food Sci. Technol. 2016, 73, 108–116. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, M.; Liu, F.; Feng, X.; Ibrahim, S.A.; Cheng, L.; Huang, W. Effects of freeze drying and hot-air drying on the physicochemical properties and bioactivities of polysaccharides from Lentinula edodes. J. Food Biochem. 2020, 145, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Capasso, G.; Rando, A.; Perna, A.M. Antioxidant Activity of Beef, Pork and Chicken Burgers before and after Cooking and after In Vitro Intestinal Digestion. Foods 2023, 12, 4100. [Google Scholar] [CrossRef] [PubMed]
| Levels | |||
|---|---|---|---|
| Variables | −1 | 0 | 1 |
| Lentinula edodes (L. edodes) (%, x1) | 30 | 35 | 40 |
| Balsa fish skin gelatin (BFSG) (%, x2) | 0.75 | 1 | 1.25 |
| Dry starch (DS) (%, x3) | 9 | 10 | 11 |
| Isolated soy protein (SPI) (%, x4) | 8 | 9 | 10 |
| Run | Independent and Code Variables | Response | |||
|---|---|---|---|---|---|
| Lentinula edodes (L. edodes) (%, x1) | Balsa Fish Skin Gelatin (BFSG) (%, x2) | Dry Starch (DS) (%, x3) | Isolated Soy Protein (SPI) (%, x4) | Actual Value | |
| 1 | 0 (35%) | 0 (1%) | 0 (10%) | 0 (9%) | 9.35 |
| 2 | −1 (30%) | 0 (1%) | −1 (9%) | 0 (9%) | 8.7 |
| 3 | −1 (30%) | 0 (1%) | 0 (10%) | 1 (10%) | 9.09 |
| 4 | 0 (35%) | 0 (1%) | 1 (11%) | −1 (8%) | 9.15 |
| 5 | 1 (40%) | 0 (1%) | 0 (10%) | −1 (8%) | 8.55 |
| 6 | 0 (35%) | 0 (1%) | 1 (11%) | 1 (10%) | 9.41 |
| 7 | 1 (40%) | 0 (1%) | 0 (10%) | 1 (10%) | 8.83 |
| 8 | 0 (35%) | 1 (1.25%) | 0 (10%) | −1 (8%) | 9.18 |
| 9 | 0 (35%) | −1 (0.75%) | −1 (9%) | 0 (9%) | 8.74 |
| 10 | −1 (30%) | −1 (0.75%) | 0 (10%) | 0 (9%) | 8.87 |
| 11 | 0 (35%) | 0 (1%) | −1 (9%) | 1 (10%) | 9.05 |
| 12 | 0 (35%) | −1 (0.75%) | 1 (11%) | 0 (9%) | 9.05 |
| 13 | 0 (35%) | 0 (1%) | 0 (10%) | 0 (9%) | 9.33 |
| 14 | 0 (35%) | 0 (1%) | −1 (9%) | −1 (8%) | 8.86 |
| 15 | −1 (30%) | 0 (1%) | 1 (11%) | 0 (9%) | 9.07 |
| 16 | 0 (35%) | −1 (0.75%) | 0 (10%) | 1 (10%) | 9.14 |
| 17 | 0 (35%) | 1 (1.25%) | −1 (9%) | 0 (9%) | 8.83 |
| 18 | 1 (40%) | 0 (1%) | −1 (9%) | 0 (9%) | 8.45 |
| 19 | 0 (35%) | 1 (1.25%) | 0 (10%) | 1 (10%) | 9.27 |
| 20 | 0 (35%) | −1 (0.75%) | 0 (10%) | −1 (8%) | 8.87 |
| 21 | 1 (40%) | −1 (0.75%) | 0 (10%) | 0 (9%) | 8.42 |
| 22 | −1 (30%) | 0 (1%) | 0 (10%) | −1 (8%) | 8.89 |
| 23 | 1 (40%) | 1 (1.25%) | 0 (10%) | 0 (9%) | 8.73 |
| 24 | 0 (35%) | 1 (1.25%) | 1 (11%) | 0 (9%) | 9.33 |
| 25 | 0 (35%) | 0 (1%) | 0 (10%) | 0 (9%) | 9.34 |
| 26 | 1 (40%) | 0 (1%) | 1 (11%) | 0 (9%) | 8.75 |
| 27 | −1 (30%) | 1 (1.25%) | 0 (10%) | 0 (9%) | 8.93 |
| Source of Variation | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 2.02 | 14 | 0.1442 | 199.05 | <0.0001 | Significant |
| x1 | 0.2760 | 1 | 0.2760 | 381.10 | <0.0001 | ** |
| x2 | 0.1160 | 1 | 0.1160 | 160.20 | <0.0001 | ** |
| x3 | 0.3781 | 1 | 0.3781 | 521.98 | <0.0001 | ** |
| x4 | 0.1387 | 1 | 0.1387 | 191.46 | <0.0001 | ** |
| x1x2 | 0.0156 | 1 | 0.0156 | 21.57 | 0.0006 | ** |
| x1x3 | 0.0012 | 1 | 0.0012 | 1.69 | 0.2179 | |
| x1x4 | 0.0016 | 1 | 0.0016 | 2.21 | 0.1630 | |
| x2x3 | 0.0090 | 1 | 0.0090 | 12.46 | 0.0041 | ** |
| x2x4 | 0.0081 | 1 | 0.0081 | 11.18 | 0.0058 | ** |
| x3x4 | 0.0012 | 1 | 0.0012 | 1.69 | 0.2179 | |
| x12 | 1.00 | 1 | 1.00 | 1382.68 | <0.0001 | ** |
| x22 | 0.16 | 1 | 0.16 | 221.23 | <0.0001 | ** |
| x32 | 0.15 | 1 | 0.15 | 211.76 | <0.0001 | ** |
| x42 | 0.02 | 1 | 0.02 | 23.99 | 0.0004 | ** |
| Residual | 0.0087 | 12 | 0.0007 | |||
| Lack of fir | 0.0085 | 10 | 0.0008 | 8.49 | 0.1099 | Not significant |
| Sum | 2.03 | 26 | ||||
| R2 = 0.9957 | R2Adj = 0.9907 | |||||
| Parameters | Control | Novel Sausage |
|---|---|---|
| Fat | 18.33 ± 0.19 b | 3.68 ± 0.08 a |
| Protein | 12.58 ± 0.29 a | 16.21 ± 0.25 b |
| Water | 63.95 ± 0.23 a | 66.91 ± 0.84 b |
| Ash | 3.02 ± 0.06 a | 2.99 ± 0.06 a |
| Energy value | 223.77 ± 0.26 b | 138.69 ± 0.18 a |
| External color | ||
| L* | 46.03 ± 0.18 b | 44.32 ± 0.21 a |
| a* | 18.45 ± 0.20 b | 18.02 ± 0.15 a |
| b* | 18.72 ± 0.09 a | 19.20 ± 0.12 b |
| Internal color | ||
| L* | 55.32 ± 0.13 b | 53.25 ± 0.16 a |
| a* | 16.64 ± 0.06 b | 16.12 ± 0.10 a |
| b* | 14.28 ± 0.05 a | 14.73 ± 0.08 b |
| Parameters | Control | Novel Sausage |
|---|---|---|
| Essential | ||
| Valine | 0.89 ± 0.05 b | 0.84 ± 0.00 a |
| Methionine | 0.06 ± 0.00 a | 0.14 ± 0.00 b |
| Isoleucine | 0.74 ± 0.05 b | 0.72 ± 0.03 a |
| Phenylalanine | 0.84 ± 0.04 a | 0.83 ± 0.02 a |
| Lysine | 1.29 ± 0.09 b | 1.02 ± 0.01 a |
| Leucine | 1.12 ± 0.08 b | 1.09 ± 0.01 a |
| Threonine | 0.73 ± 0.05 a | 0.73 ± 0.01 a |
| Total EAAs | 5.67 ± 0.36 a | 5.37 ± 0.08 a |
| Nonessential | ||
| Aspartic | 2.04 ± 0.16 a | 2.42 ± 0.02 b |
| Glutamic | 3.53 ± 0.25 a | 3.93 ± 0.03 b |
| Serine | 0.85 ± 0.04 a | 0.85 ± 0.02 a |
| Histidine | 0.84 ± 0.02 b | 0.75 ± 0.03 a |
| Glycine | 1.01 ± 0.02 b | 0.98 ± 0.00 a |
| Arginine | 0.81 ± 0.04 b | 0.72 ± 0.02 a |
| Alanine | 1.11 ± 0.06 b | 1.03 ± 0.01 a |
| Tyrosine | 0.39 ± 0.03 b | 0.22 ± 0.02 a |
| Cysteine | 0.03 ± 0.01 a | 0.13 ± 0.01 b |
| ∑NEAA | 10.61 ± 0.63 a | 11.03 ± 0.16 a |
| Total FAA | 16.28 ± 0.99 a | 16.4 ± 0.24 a |
| Parameters | Control | Novel Sausage |
|---|---|---|
| C14:0 | 2.71 ± 0.01 a | 1.56 ± 0.07 b |
| C15:0 | ND | 0.19 ± 0.01 |
| C16:0 | 45.46 ± 0.69 b | 48.17 ± 0.23 a |
| C17:0 | 0.41 ± 0.04 a | 0.21 ± 0.03 b |
| C18:0 | 19.87 ± 0.09 a | 16.67 ± 0.09 b |
| C20:0 | ND | 0.31 ± 0.02 |
| C16:1 n5 | 0.34 ± 0.04 a | 0.26 ± 0.04 a |
| C16:1 n7 | 4.55 ± 0.13 a | 3.19 ± 0.07 b |
| C18:1 | 76.69 ± 0.72 b | 82.10 ± 0.70 a |
| C20:1 | 1.97 ± 0.01 a | 0.71 ± 0.05 b |
| C18:2 | 32.83 ± 0.18 b | 121.70 ± 0.67 a |
| C20:4 | 0.52 ± 0.02 b | 4.39 ± 0.13 a |
| SFA | 68.45 ± 0.83 a | 67.11 ± 0.45 b |
| MUFA | 83.55 ± 0.9 b | 86.26 ± 0.86 a |
| PUFA | 33.35 ± 0.20 b | 126.09 ± 0.80 a |
| PUFA/SFA | 0.49 | 1.88 |
| NO. | Compound | Control | Novel Sausage |
|---|---|---|---|
| Aldehydes | |||
| 1 | Hexanal | 1.55 ± 0.09 a | 2.22 ± 0.13 b |
| 2 | Decanal | 1.01 ± 0.06 b | 0.67 ± 0.05 a |
| 3 | Nonanal | 2.01 ± 0.05 b | 1.82 ± 0.10 a |
| 4 | Octanal | ND | 0.87 ± 0.02 |
| 5 | trans-2-Decenal | ND | 0.34 ± 0.01 |
| 6 | Benzaldehyde | ND | 1.12 ± 0.06 |
| 7 | trans-2, trans-4-Decadienal | ND | 0.69 ± 0.06 |
| 8 | (E)-Cinnamaldehyde | 8.97 ± 0.35 b | 7.47 ± 0.15 a |
| Total | 13.53 | 15.21 | |
| Alcohols | |||
| 9 | Syringol | 0.87 ± 0.21 a | 0.98 ± 0.06 b |
| 10 | Cinnamyl alcohol | 3.60 ± 0.33 b | 3.16 ± 0.54 a |
| 11 | Estragole | 0.41 ± 0.06 a | 0.50 ± 0.02 b |
| 12 | Linalool | 2.02 ± 0.18 b | 1.69 ± 0.17 a |
| 13 | 1-Hexanol | ND | 0.51 ± 0.05 |
| 14 | 1-Octen-3-ol | ND | 0.72 ± 0.06 |
| Total | 6.90 | 7.55 | |
| Alkenes | |||
| 15 | 3-Carene | 9.78 ± 0.67 b | 5.53 ± 0.76 a |
| 16 | Muurolene | 1.62 ± 0.28 a | 2.45 ± 0.32 b |
| 17 | Cadinene | 0.70 ± 0.03 a | 1.11 ± 0.16 b |
| 18 | Selinene | 0.55 ± 0.08 b | 0.36 ± 0.03 a |
| 19 | Guaiene | 1.23 ± 0.19 a | 1.38 ± 0.14 b |
| 20 | Caryophyllene | 20.92 ± 1.32 b | 19.34 ± 1.76 a |
| 21 | alpha-Phellandrene | 2.16 ± 0.85 | ND |
| 22 | Ocimene | 1.04 ± 0.11 | ND |
| 23 | Cadina-3,9-diene | 2.09 ± 0.78 a | 4.22 ± 0.98 b |
| 24 | 1R-.alpha.-Pinene | 0.86 ± 0.02 | ND |
| 25 | Copaene | 14.36 ± 1.71 | ND |
| 26 | Humulene | 1.38 ± 0.14 | ND |
| 27 | Terpinen | ND | 3.29 ± 1.12 |
| 28 | Phellandrene | ND | 1.00 ± 0.09 |
| 29 | (+)-Cyclosativene | ND | 0.54 ± 0.02 |
| 30 | alpha-Cubebene | ND | 22.04 ± 1.65 |
| 31 | Eudesmene | ND | 0.60 ± 0.04 |
| 32 | Cubebene | ND | 0.78 ± 0.03 |
| 33 | Isoterpinolene | ND | 0.90 ± 0.06 |
| Total | 56.68 | 63.52 | |
| Ketones | |||
| 34 | 3,4-Dimethylacetophenone | ND | 0.53 ± 0.03 |
| 35 | Phloretin | 0.54 ± 0.03 a | 0.61 ± 0.02 b |
| Total | 0.54 | 1.14 | |
| Ester | |||
| 36 | Hexadecanoic acid, methyl ester | 0.45 ± 0.06 | ND |
| Total | 0.45 | ND | |
| Acids | |||
| 37 | 4-Hydroxyaminobenzoic acid | 0.70 ± 0.06 | ND |
| Total | 0.45 | ND | |
| Others | |||
| 38 | Anethole | 1.86 ± 0.23 a | 2.74 ± 0.25 b |
| 39 | Diallyl disulphide | 12.58 ± 0.73 a | 3.98 ± 0.94 b |
| 40 | o-Cymene | 0.95 ± 0.06 a | 1.15 ± 0.23 b |
| 41 | 4-Methoxy-3-(methoxymethyl)phenol | 0.84 ± 0.04 a | 0.98 ± 0.06 b |
| 42 | 1,3-Dithiane | 0.84 ± 0.05 | ND |
| 43 | Bicyclo [3.1.1]heptane, 6,6-dimethyl-2-methylene-, (1S)- | 2.21 ± 0.87 | ND |
| 44 | Cajeputen | 1.26 ± 0.27 a | 2.55 ± 0.04 b |
| 45 | 2-Amylfuran | ND | 0.85 ± 0.03 |
| 46 | 4-Carvomenthenol | ND | 0.34 ± 0.01 |
| 47 | m-Cymene | 0.66 ± 0.01 | ND |
| Parameters | Control | Novel Sausage |
|---|---|---|
| Total phenolic content (TPC) (mg/g) | 0.21 ± 0.01 a | 2.63 ± 0.08 b |
| DPPH radical scavenging activity (%) | 48.55 ± 0.17 a | 80.39 ± 0.76 b |
| ABTS radical scavenging activity (%) | 5.35 ± 0.11 a | 26.29 ± 0.25 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Yin, J.; Wan, K.; Guo, H.; Jiang, G. Effects of Balsa Fish Skin Gelatin, Lentinula edodes Mushrooms, Soy Protein Isolate, and Starch on the Sensory Quality and Characterization of Physicochemical and Antioxidant Properties of New Sausage. Foods 2024, 13, 465. https://doi.org/10.3390/foods13030465
Wang L, Yin J, Wan K, Guo H, Jiang G. Effects of Balsa Fish Skin Gelatin, Lentinula edodes Mushrooms, Soy Protein Isolate, and Starch on the Sensory Quality and Characterization of Physicochemical and Antioxidant Properties of New Sausage. Foods. 2024; 13(3):465. https://doi.org/10.3390/foods13030465
Chicago/Turabian StyleWang, Liyan, Jiacheng Yin, Kang Wan, Hongyue Guo, and Guochuan Jiang. 2024. "Effects of Balsa Fish Skin Gelatin, Lentinula edodes Mushrooms, Soy Protein Isolate, and Starch on the Sensory Quality and Characterization of Physicochemical and Antioxidant Properties of New Sausage" Foods 13, no. 3: 465. https://doi.org/10.3390/foods13030465





