Improving the Rheological Properties of Dough Obtained by Partial Substitution of Wheat Flour with Freeze-Dried Olive Pomace
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Characterization of Olive Pomace
2.3. Elemental Analysis of FD-OP and Bread Samples
2.3.1. Elemental Analysis of FD-OP and Bread Samples
2.3.2. Instrumentation
2.3.3. Sample Preparation
2.3.4. Sample Analysis
2.4. Dough Preparation
2.5. Dough Measurements
2.5.1. Dough Extensibility and Stickiness
2.5.2. Rheofermentometer Analysis
2.5.3. Small Deformation Characteristics
2.5.4. Rapid Visco Analyser (RVA)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Olive Pomace
3.2. Dough Preparation and Breadmaking %
The Pasting Profile of Dough
3.3. Dough Measurements
3.3.1. Extensibility and Stickiness of the Dough
3.3.2. Rheofermentometer Analysis
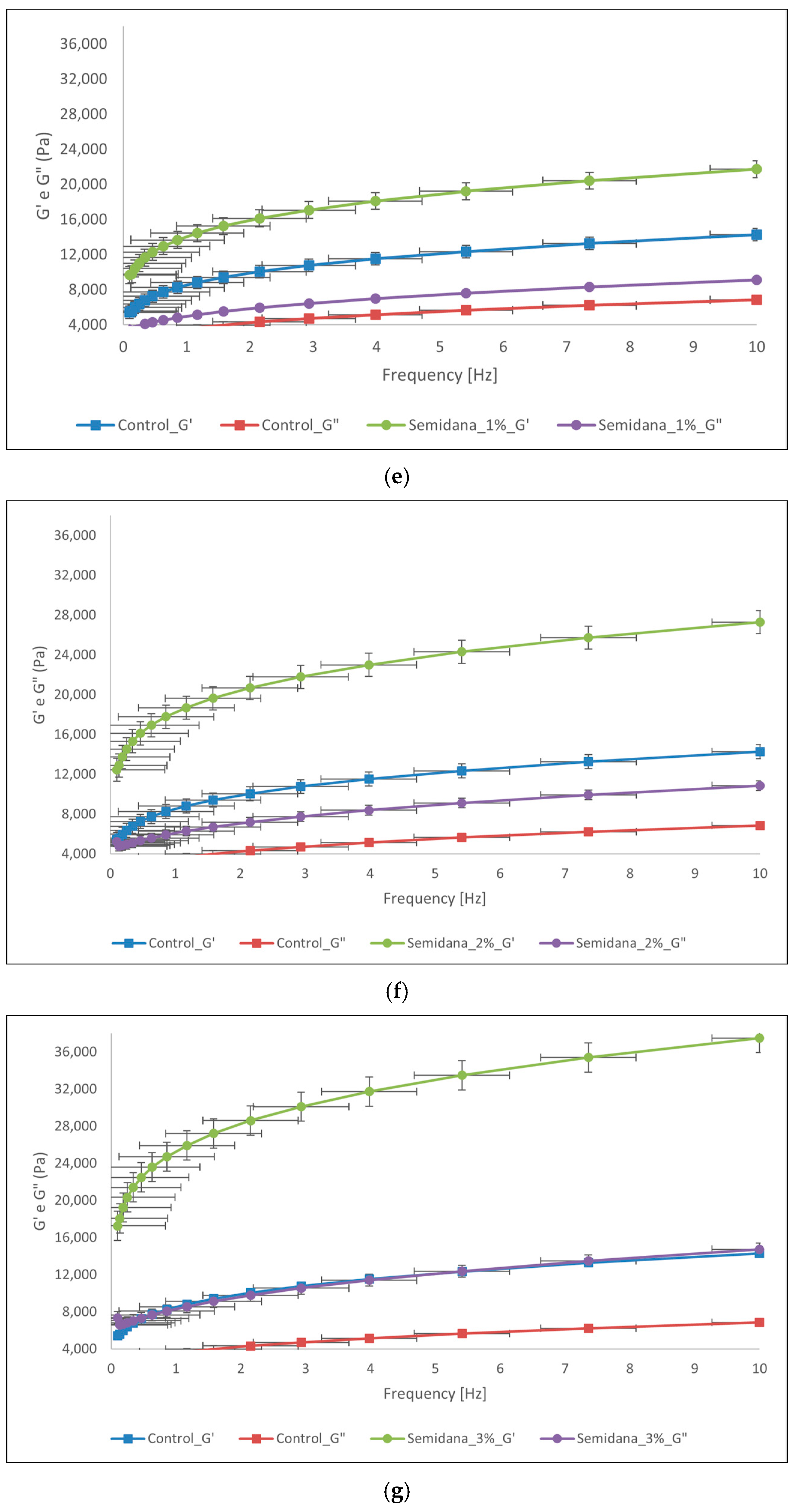
3.3.3. Small Deformation Characteristics (Rheometer Analysis)
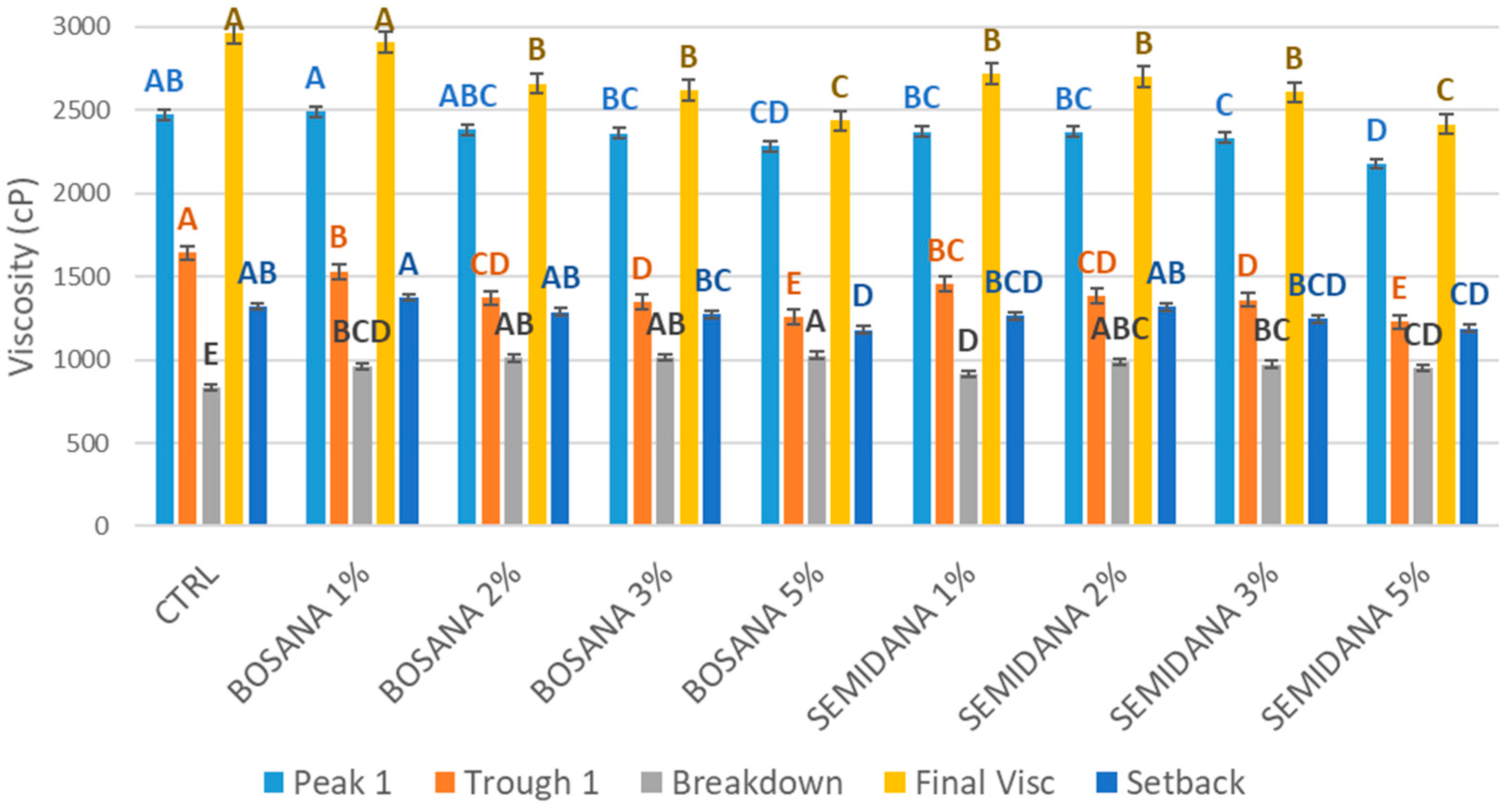
3.3.4. Rapid Visco Analyser (RVA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Bruno, A.; Romeo, R.; Piscopo, A.; Poiana, M. Antioxidant quantification in different portions obtained during olive oil extraction process in an olive oil press mill. J. Sci. Food Agric. 2021, 101, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, J.; Cao, X. Effects of orange peel powder on rheological properties of wheat dough and bread aging. Food Sci. Nutr. 2021, 9, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Conte, P.; Pulina, S.; Del Caro, A.; Fadda, C.; Urgeghe, P.P.; De Bruno, A.; Difonzo, G.; Caponio, F.; Romeo, R.; Piga, A. Gluten-Free Breadsticks Fortified with Phenolic-Rich Extracts. Foods 2021, 10, 923. [Google Scholar] [CrossRef]
- Iuga, M.; Mironeasa, S. Potential of grape byproducts as functional ingredients in baked goods and pasta. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2473–2505. [Google Scholar] [CrossRef]
- Cedola, A.; Cardinali, A.; Del Nobile, M.A.; Conte, A. Enrichment of Bread with Olive Oil Industrial By-Product. J. Agric. Sci. Technol. B 2019, 9, 119–127. [Google Scholar] [CrossRef]
- Bartkiene, E.; Vizbickiene, D.; Bartkevics, V.; Pugajeva, I.; Krungleviciute, V.; Zadeike, D.; Zavistanaviciute, P.; Juodeikiene, G. Application of Pediococcus acidilactici LUHS29 immobilized in apple pomace matrix for high value wheat-barley sourdough bread. LWT 2017, 83, 157–164. [Google Scholar] [CrossRef]
- Majzoobi, M.; Ghavi, F.S.; Farahnaky, A.; Jamalian, J.; Mesbahi, G. Effect of tomato pomace powder on the physicochemical properties of flat bread (barbari bread). J. Food Process. Preserv. 2011, 35, 247–256. [Google Scholar] [CrossRef]
- Gül, H.; Şen, H. Effects of Rosehip Seed Flour on the Rheological Properties of Bread Dough. Sci. Bull. Ser. F Biotechnol. 2017, XXI, 330–335. [Google Scholar]
- Tańska, M.; Zadernowski, R.; Konopka, I. The quality of wheat bread supplemented with dried carrot pomace. Polish J. Nat. Sci. 2007, 22, 126–136. [Google Scholar] [CrossRef]
- Meral, R.; Doǧan, I.S. Grape seed as a functional food ingredient in bread-making. Int. J. Food Sci. Nutr. 2013, 64, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Saeed, F.; Niaz, B.; Afzaal, M.; Ikram, A.; Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Anjum, F.M. Biochemical and nutritional profile of maize bran-enriched flour in relation to its end-use quality. Food Sci. Nutr. 2021, 9, 3336–3345. [Google Scholar] [CrossRef]
- Šporin, M.; Avbelj, M.; Kovač, B.; Možina, S.S. Quality characteristics of wheat flour dough and bread containing grape pomace flour. Food Sci. Technol. Int. 2018, 24, 251–263. [Google Scholar] [CrossRef]
- Neifar, M.; Jaouani, A.; Ayari, A.; Abid, O.; Ben Salem, H.; Boudabous, A.; Najar, T.; Ghorbel, R.E. Improving the nutritive value of Olive Cake by solid state cultivation of the medicinal mushroom Fomes fomentarius. Chemosphere 2013, 91, 110–114. [Google Scholar] [CrossRef]
- López-García, A.B.; Cotes-Oalomino, T.; Uceda-Rodríguez, M.; Moreno-Maroto, J.M.; Cobo-Ceacero, C.J.; Fernanda Andreola, N.M.; Martínez-García, C. Application of life cycle assessment in the environmental study of sustainable ceramic bricks made with ‘alperujo’ (Olive pomace). Appl. Sci. 2021, 11, 2278. [Google Scholar] [CrossRef]
- Torrecilla, J.S.; Aragón, J.M.; Palancar, M.C. Improvement of fluidized-bed dryers for drying solid waste (olive pomace) in olive oil mills. Eur. J. Lipid Sci. Technol. 2006, 108, 913–924. [Google Scholar] [CrossRef]
- Uceda-Rodríguez, M.; López-García, A.B.; Moreno-Maroto, J.M.; Cobo-Ceacero, C.J.; Cotes-Palomino, M.T.; García, C.M. Evaluation of the environmental benefits associated with the addition of olive pomace in the manufacture of lightweight aggregates. Materials 2020, 13, 2351. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Alli, I.; Ereifej, K.; Alhamad, M.; Al-Tawaha, A.R.; Rababah, T. Optimisation, characterisation and quantification of phenolic compounds in olive cake. Food Chem. 2010, 123, 117–122. [Google Scholar] [CrossRef]
- Čepo, D.V.; Radić, K.; Jurmanović, S.; Jug, M.; Rajković, M.G.; Pedisić, S.; Moslavac, T.; Albahari, P. Valorization of olive pomace-based nutraceuticals as antioxidants in chemical, food, and biological models. Molecules 2018, 23, 2070. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Hydroxytyrosol and cytoprotection: A projection for clinical interventions. Int. J. Mol. Sci. 2017, 18, 930. [Google Scholar] [CrossRef] [PubMed]
- Catalán, Ú.; López de las Hazas, M.C.; Rubió, L.; Fernández-Castillejo, S.; Pedret, A.; de la Torre, R.; Motilva, M.J.; Solà, R. Protective effect of hydroxytyrosol and its predominant plasmatic human metabolites against endothelial dysfunction in human aortic endothelial cells. Mol. Nutr. Food Res. 2015, 59, 2523–2536. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ma, A.; Gong, G.; Liu, Z.; Wu, Z.; Guo, B.; Chen, Z. Cracking Streptococcus thermophilus to stimulate the growth of the probiotic Lactobacillus casei in co-culture. Int. J. Food Microbiol. 2015, 210, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chi, W.; Hu, J.; Pan, Q.; Zheng, B.; Zeng, S. Sensory and nutritional properties of chinese olive pomace based high fibre biscuit. Emirates J. Food Agric. 2017, 29, 495–501. [Google Scholar] [CrossRef]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; De Sancti, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocr. Metab. Immune Disord. Drug Targets 2017, 18, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Del-río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, G.; Romano, P. Evolution of the Olive Oil Industry along the Entire Production Chain and Related Waste Management. Energies 2022, 15, 465. [Google Scholar] [CrossRef]
- Lanza, B.; Cellini, M.; Di Marco, S.; D’amico, E.; Simone, N.; Giansante, L.; Pompilio, A.; Di Loreto, G.; Bacceli, M.; Del Re, P.; et al. Olive pâté by multi-phase decanter as potential source of bioactive compounds of both nutraceutical and anticancer effects. Molecules 2020, 25, 5967. [Google Scholar] [CrossRef]
- Stempfle, S.; Carlucci, D.; de Gennaro, B.C.; Roselli, L.; Giannoccaro, G. Available pathways for operationalizing circular economy into the olive oil supply chain: Mapping evidence from a scoping literature review. Sustainability 2021, 13, 9789. [Google Scholar] [CrossRef]
- Guerrini, L.; Masella, P.; Angeloni, G.; Migliorini, M.; Parenti, A. Changes in Olive Paste Composition During Decanter Feeding and Effects on Oil Yield. Eur. J. Lipid Sci. Technol. 2017, 119, 1–5. [Google Scholar] [CrossRef]
- Pagano, M.; Tomasone, R.; Cedrola, C.; Fedrizzi, M.; Veneziani, G.; Servili, M. Use of Ultrasound in the Extraction Process of Virgin Olive Oil and Influence on Malaxation Time. In Proceedings of the Innovative Biosystems Engineering for Sustainable Agriculture, Forestry and Food Production, International Mid-Term Conference 2019 of the Italian Association of Agricultural Engineering (AIIA), Matera, Italy, 12–13 September 2019; Coppola, A., Di Renzo, G.C., Altieri, G., D’Antonio, P., Eds.; Springer: Cham, Switzerland, 2020; Volume 67, pp. 703–712. [Google Scholar]
- Foti, P.; Russo, N.; Randazzo, C.L.; Choupina, A.B.; Pino, A.; Caggia, C.; Romeo, F.V. Profiling of phenol content and microbial community dynamics during pâté olive cake fermentation. Food Biosci. 2023, 52, 102358. [Google Scholar] [CrossRef]
- ISO International Organization for Standardization. Animal and Vegetable Fats and Oils—Determination of Moisture and Volatile Matter Content; International Organisation for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Association of Official Analytical Chemists. In Official Methods of Analysis, 18th ed.; AOAC: Gaithersburg, MD, USA, 2006. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Crude Fat in Feeds, Cereal Grains and Forage (Randall/Soxtec/Hexanes Extraction-Submersion Method). In Official Methods of Analysis, 18th ed.; AOAC: Gaithersburg, MD, USA, 2006. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Microchemical Determination of Nitrogen. In Official Methods of Analysis, 18th ed.; AOAC: Washington, DC, USA, 2010. [Google Scholar]
- ISO (International Organization for Standardization). Foodstuffs—Determination of Water Activity; International Organisation for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Protein (Crude) in Animal Feed, Combustion Method. In Official Methods of Analysis, 18th ed.; AOAC: Gaithersburg, MD, USA, 2006. [Google Scholar]
- AACC (American Association of Cereal Chemists). Insoluble, Soluble, and Total Dietary Fiber (Codex Definition) by an Enzymatic-Gravimetric Method and Liquid Chromatography. In Approved Methods of Analysis, 11th ed.; American Association of Cereal Chemists, Ed.; AACC International: St. Paul, MN, USA, 2011. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Total Dietary Fiber in Foods, Enzymatic-Gravimetric Method. In Official Methods of Analysis, 18th ed.; AOAC: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Simonato, B.; Trevisan, S.; Tolve, R.; Favati, F.; Pasini, G. Pasta fortification with olive pomace: Effects on the technological characteristics and nutritional properties. LWT 2019, 114, 108368. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Schäfer, J.; Félix, L.M.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Thymus pulegioides L. as a rich source of antioxidant, anti-proliferative and neuroprotective phenolic compounds. Food Funct. 2018, 9, 3617–3629. [Google Scholar] [CrossRef]
- Caponio, G.R.; Difonzo, G.; de Gennaro, G.; Calasso, M.; De Angelis, M.; Pasqualone, A. Nutritional Improvement of Gluten-Free Breadsticks by Olive Cake Addition and Sourdough Fermentation: How Texture, Sensory, and Aromatic Profile Were Affected? Front. Nutr. 2022, 9, 830932. [Google Scholar] [CrossRef]
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. J. Funct. Foods 2017, 31, 63–70. [Google Scholar] [CrossRef]
- De Gennaro, G.; Difonzo, G.; Summo, C.; Pasqualone, A.; Caponio, F. Olive Cake Powder as Functional Ingredient to Improve the Quality of Gluten-Free Breadsticks. Foods 2022, 11, 552. [Google Scholar] [CrossRef]
- Vizitiu, D.; Danciou, I. Evaluation of Farinograph and Mixolab for prediction of mixing properties of industrial wheat flour. Acta Univ. Cibiniensis Ser. E Food Technol. 2011, 15, 31–38. [Google Scholar]
- AACC (American Association of Cereal Chemists). General Pasting Method for Wheat or Rye Flour of Starch Using the Rapid Visco Analyser. Approved Methods of Analysis, 10th ed.; AACC International: St. Paul, MN, USA, 2000. [Google Scholar]
- Antónia Nunes, M.; Costa, A.S.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid- and water-soluble components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef]
- Portarena, S.; Baldacchini, C.; Brugnoli, E. Geographical discrimination of extra-virgin olive oils from the Italian coasts by combining stable isotope data and carotenoid content within a multivariate analysis. Food Chem. 2017, 215, 1–6. [Google Scholar] [CrossRef]
- Aissaoui, M.H.; Trabelsi, A.B.H.; bensidhom, G.; Ceylan, S.; Leahy, J.J.; Kwapinski, W. Insights into olive pomace pyrolysis conversion to biofuels and biochars: Characterization and techno-economic evaluation. Sustain. Chem. Pharm. 2023, 32, 101022. [Google Scholar] [CrossRef]
- Fernández-González, R.; Martín-Lara, M.A.; Iáñez-Rodríguez, I.; Calero, M. Removal of heavy metals from acid mining effluents by hydrolyzed olive cake. Bioresour. Technol. 2018, 268, 169–175. [Google Scholar] [CrossRef]
- González, J.F.; Román, S.; Engo, G.; Encinar, J.M.; Martínez, G. Reduction of tars by dolomite cracking during two-stage gasification of olive cake. Biomass Bioenergy 2011, 35, 4324–4330. [Google Scholar] [CrossRef]
- Trindade, P.C.O.; de Carvalho Dalfolo, A.; Monteiro, C.S.; Wagner, R.; Dos Santos, B.A.; Nora, F.M.D.; Verruck, S.; da ROSA, C.S. Development and characterization of biscuits with olive pomace. Food Sci. Technol. 2023, 43. [Google Scholar] [CrossRef]
- Júlio, L.R.C. Tratamento, Caracterização Química e Estudo In Vivo Do Bagaço de Azeitona Resultante Da Extração Do Azeite de Oliva; Universidade Federal de Lavras: Lavras, Brazil, 2014; pp. 1–144. [Google Scholar]
- Simsek, M.; Süfer, Ö. Olive pomace from olive oil processing as partial flour substitute in breadsticks: Bioactive, textural, sensorial and nutritional properties. J. Food Process. Preserv. 2022, 46, e15705. [Google Scholar] [CrossRef]
- Alhamad, M.N.; Rababah, T.M.; Al-u’datt, M.; Ereifej, K.; Esoh, R.; Feng, H.; Yang, W. The physicochemical properties, total phenolic, antioxidant activities, and phenolic profile of fermented olive cake. Arab. J. Chem. 2017, 10, 136–140. [Google Scholar] [CrossRef]
- Nunes, M.A.; Palmeira, J.D.; Melo, D.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Alves, R.C.; Ferreira, H.; Oliveira, M.B.P.P. Chemical composition and antimicrobial activity of a new olive pomace functional ingredient. Pharmaceuticals 2021, 14, 913. [Google Scholar] [CrossRef]
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel processes for the extraction of phenolic compounds from olive pomace and their protection by encapsulation. Molecules 2021, 26, 1781. [Google Scholar] [CrossRef]
- Tsivas, D.; Vlyssides, A.; Vlysidis, A. Monitoring of a III-Phase Olive Pomace Composting Process Using the CIELAB Colorimetric Method. Waste Biomass Valorization 2021, 12, 5029–5039. [Google Scholar] [CrossRef]
- Rodrigues, R.; Alves, R.C.; Oliveira, M.B.P.P. Exploring Olive Pomace for Skincare Applications: A Review. Cosmetics 2023, 10, 35. [Google Scholar] [CrossRef]
- Pošćić, F.; Furdek Turk, M.; Bačić, N.; Mikac, N.; Bertoldi, D.; Camin, F.; Jukić Špika, M.; Žanetić, M.; Rengel, Z.; Perica, S. Removal of pomace residues is critical in quantification of element concentrations in extra virgin olive oil. J. Food Compos. Anal. 2019, 77, 39–46. [Google Scholar] [CrossRef]
- Camin, F.; Larcher, R.; Perini, M.; Bontempo, L.; Bertoldi, D.; Gagliano, G.; Nicolini, G.; Versini, G. Characterisation of authentic Italian extra-virgin olive oils by stable isotope ratios of C, O and H and mineral composition. Food Chem. 2010, 118, 901–909. [Google Scholar] [CrossRef]
- Yazar, G.; Duvarci, O.; Tavman, S.; Kokini, J.L. Non-linear rheological properties of soft wheat flour dough at different stages of farinograph mixing. Appl. Rheol. 2016, 26, 52508. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Zhao, Y.; Wang, D.; Wang, W. Influence of antioxidant dietary fiber on dough properties and bread qualities: A review. J. Funct. Foods 2021, 80, 104434. [Google Scholar] [CrossRef]
- Farbo, M.G.; Fadda, C.; Marceddu, S.; Conte, P.; Del Caro, A.; Piga, A. Improving the quality of dough obtained with old durum wheat using hydrocolloids. Food Hydrocoll. 2020, 101, 105467. [Google Scholar] [CrossRef]
- Struck, S.; Straube, D.; Zahn, S.; Rohm, H. Interaction of wheat macromolecules and berry pomace in model dough: Rheology and microstructure. J. Food Eng. 2018, 223, 109–115. [Google Scholar] [CrossRef]
- Fakhfakh, N.; Jdir, H.; Jridi, M.; Rateb, M.; Belbahri, L.; Ayadi, M.A.; Nasri, M.; Zouari, N. The mallow, Malva aegyptiaca L. (Malvaceae): Phytochemistry analysis and effects on wheat dough performance and bread quality. LWT 2017, 75, 656–662. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; O’Shea, N.; Gallagher, E. Rheological properties of wheat dough supplemented with functional by-products of food processing: Brewer’s spent grain and apple pomace. J. Food Eng. 2013, 116, 362–368. [Google Scholar] [CrossRef]
- Uthayakumaran, S.; Gras, P.W.; Stoddard, F.L.; Bekes, F. Effect of varying protein content and glutenin-to-gliadin ratio on the functional properties of wheat dough. Cereal Chem. 1999, 76, 389–394. [Google Scholar] [CrossRef]
- Shewry, P.R.; Popineau, Y.; Lafiandra, D.; Belton, P. Wheat glutenin subunits and dough elasticity. Trends Food Sci. Technol. 2000, 11, 433–441. [Google Scholar] [CrossRef]
- Zin, M.H.; Abdan, K.; Norizan, M.N. The Effect of Different Fiber Loading on Flexural and Thermal Properties of Banana/Pineapple Leaf (PALF)/Glass Hybrid Composite; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780081022917. [Google Scholar]
- Kimbell, G.; Azad, M.A. 3D Printing: Bioinspired Materials for Drug Delivery. In Bioinspired and Biomimetic Materials for Drug Delivery; Nurunnabi, M., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 295–318. ISBN 978-0-12-821352-0. [Google Scholar]
- Franck, A.; Germany, T. AN004; TA Instruments: New Castle, DE, USA, 1993. [Google Scholar]




| ICP-MS NexION 300X Perkin Elmer Settings | |||
|---|---|---|---|
| RF power generator (W) | 1300 | KED mode cell entrance voltage (V) | −8.0 |
| Ar plasma flow (dm3 min−1) | 18.0 | KED mode cell exit voltage (V) | −25.0 |
| Ar auxiliary flow (dm3 min−1) | 1.20 | Resolution (Da) | 0.7 |
| Ar nebuliser flow (dm3 min−1) | 0.91 | Scan mode | Peak hopping |
| Nebuliser | Meinhardt®, glass | Detector mode | Dual |
| Spray chamber | Cyclonic, glass | Dwell time (ms) | 50 |
| Skimmer and sampling cones | Nickel | Number of points per peak | 3 |
| Sampling depth (mm) | 0 | Acquisition time (s) | 6 |
| Deflector voltage (V) | −8.00 | Acquisition dead time (ns) | 35 |
| Analog stage voltage (V) | −1750 | KED gas | Helium 99.999%, flow 4.2 cm3 min−1 |
| Pulse stage voltage | +1350 | Masses of optimization | 7Li, 115In, and 205Tl |
| Element | Isotope | Abundance (%) | Analysis Mode | Linearity (R2) | Calibration Range | LOD (µg kg−1) | LOQ (µg kg−1) | Repeatability (RSD%) |
|---|---|---|---|---|---|---|---|---|
| Li | 7.016 | 92.50 | STD | 0.99976 | 0.1–100 | 0.844 | 2.785 | 5% |
| Rb | 84.912 | 72.17 | STD | 0.99996 | 0.1–200 | 0.429 | 1.415 | 5% |
| Sr | 87.906 | 82.58 | STD | 0.99994 | 0.1–200 | 0.146 | 0.483 | 5% |
| Cs | 132.905 | 100.00 | STD | 0.99985 | 0.1–100 | 0.108 | 0.355 | 4% |
| Ba | 137.905 | 71.70 | KED | 0.99994 | 0.1–200 | 0.194 | 0.642 | 4% |
| Pb | 207.977 | 52.40 | STD | 0.99985 | 0.1–100 | 0.009 | 0.031 | 4% |
| Bi | 208.980 | 100.00 | STD | 0.99986 | 0.01–10 | 0.019 | 0.061 | 6% |
| Sc | 44.956 | 100.00 | KED | 0.99998 | 0.1–100 | 1.302 | 4.297 | 21% |
| V | 50.944 | 100.00 | KED | 0.99998 | 0.1–100 | 0.764 | 2.522 | 3% |
| Cr | 51.941 | 83.79 | KED | 0.99999 | 0.1–100 | 1.935 | 6.386 | 1% |
| Mn | 54.938 | 100.00 | KED | 0.99997 | 0.1–200 | 6.252 | 20.632 | 1% |
| Fe | 56.935 | 2.20 | KED | 0.99984 | 0.1–200 | 29.264 | 96.570 | 1% |
| Co | 58.933 | 100.00 | KED | 0.99998 | 0.1–200 | 1.870 | 6.171 | 2% |
| Ni | 59.933 | 26.23 | KED | 0.99997 | 0.1–200 | 1.370 | 4.522 | 1% |
| Cu | 62.930 | 69.17 | KED | 0.99998 | 0.1–200 | 1.342 | 4.429 | 1% |
| Zn | 65.926 | 27.90 | KED | 0.99996 | 0.1–200 | 7.605 | 25.095 | 1% |
| As | 74.922 | 100.00 | KED | 0.99999 | 0.1–100 | 3.081 | 10.166 | 11% |
| Se | 81.917 | 8.73 | KED | 0.99961 | 0.1–200 | 2.587 | 8.536 | 17% |
| Y | 88.905 | 100.00 | STD | 0.99995 | 0.1–100 | 2.074 | 6.845 | 5% |
| Mo | 97.906 | 24.13 | STD | 0.99996 | 0.01–10 | 0.528 | 1.742 | 2% |
| Cd | 110.904 | 12.80 | STD | 0.99995 | 0.01–10 | 2.245 | 7.408 | 7% |
| Sn | 117.902 | 24.22 | STD | 0.99996 | 0.01–10 | 0.711 | 2.347 | 3% |
| Sb | 120.904 | 57.36 | STD | 0.99995 | 0.01–10 | 1.627 | 5.368 | 6% |
| La | 138.906 | 99.91 | KED | 1.00000 | 0.01–10 | 0.715 | 2.358 | 1% |
| Ce | 139.905 | 88.45 | KED | 1.00000 | 0.01–10 | 3.291 | 10.860 | 1% |
| Pr | 140.907 | 100.00 | KED | 1.00000 | 0.01–10 | 0.473 | 1.560 | 2% |
| Nd | 141.908 | 27.20 | KED | 1.00000 | 0.01–10 | 3.550 | 11.714 | 7% |
| Sm | 151.920 | 26.75 | KED | 1.00000 | 0.01–10 | 0.515 | 1.698 | 6% |
| Eu | 152.929 | 52.19 | KED | 1.00000 | 0.01–10 | 0.117 | 0.386 | 14% |
| Gd | 157.924 | 24.84 | KED | 1.00000 | 0.01–10 | 0.271 | 0.896 | 4% |
| Tb | 158.925 | 100.00 | KED | 1.00000 | 0.01–10 | 0.072 | 0.238 | 14% |
| Dy | 163.929 | 28.18 | KED | 1.00000 | 0.01–10 | 0.243 | 0.802 | 10% |
| Ho | 164.930 | 100.00 | KED | 1.00000 | 0.01–10 | 0.084 | 0.279 | 4% |
| Er | 165.930 | 33.61 | KED | 1.00000 | 0.01–10 | 0.114 | 0.378 | 6% |
| Tm | 168.934 | 100.00 | KED | 1.00000 | 0.01–10 | 0.082 | 0.270 | 5% |
| Yb | 173.939 | 31.83 | KED | 1.00000 | 0.01–10 | 0.133 | 0.439 | 5% |
| Lu | 174.941 | 97.41 | KED | 1.00000 | 0.01–10 | 0.108 | 0.356 | 20% |
| Tl | 204.975 | 70.48 | STD | 1.00000 | 0.01–10 | 0.211 | 0.697 | 2% |
| Hg | 201.971 | 29.86 | STD | 0.99752 | 0.1–100 | 0.345 | 1.139 | 5% |
| U | 238.050 | 99.28 | STD | 0.99981 | 0.01–10 | 0.011 | 0.036 | 3% |
| Sample | Water (%) | Yeast (%) | Salt (%) |
|---|---|---|---|
| Control (CTRL) | 54.0 | 2 | 1.8 |
| Bosana FD-OP (1%) | 54.0 | 2 | 1.8 |
| Bosana FD-OP (2%) | 53.5 | 2 | 1.8 |
| Bosana FD-OP (3%) | 53.0 | 2 | 1.8 |
| Bosana FD-OP (5%) | 51.8 | 2 | 1.8 |
| Semidana FD-OP (1%) | 54.5 | 2 | 1.8 |
| Semidana FD-OP (2%) | 54.2 | 2 | 1.8 |
| Semidana FD-OP (3%) | 53.5 | 2 | 1.8 |
| Semidana FD-OP (5%) | 53.0 | 2 | 1.8 |
| Samples | Dry Matter % (DM) ROP | Fat/DM % ROP | Protein/DM % ROP | Ash/DM % ROP | Water Activity aw (ROP) |
|---|---|---|---|---|---|
| Bosana | 25.36 a ± 0.22 | 17.57 a ± 0.16 | 6.14 a ± 0.11 | 5.29 a ± 1.05 | 0.98 a ± 0.01 |
| Semidana | 19.67 b ± 1.30 | 12.5 b ± 1.22 | 5.29 b ± 0.09 | 4.29 b ± 0.33 | 0.97 a ± 0.01 |
| Samples | DM% FD-OP | TDF/DM % FD-OP | C/DM% FD-OP | H/DM% FD-OP | N/DM% FD-OP | TPC (g GAE kg−1 DM FD-OP) | DPPH (µmol TE g−1 DM FD-OP) |
|---|---|---|---|---|---|---|---|
| Bosana | 98.49 a ± 0.07 | 61.1 b ± 0.99 | 56.23 a ± 1.60 | 7.68 a ± 0.21 | 0.98 a ± 0.02 | 42.67 a ± 1.14 | 227.07 a ± 90.86 |
| Semidana | 98.31 a ± 0.46 | 66.7 a ± 0.52 | 54.51 a ± 0.35 | 7.19 a ± 0.07 | 0.85 b ± 0.01 | 28.36 b ± 1.73 | 195.36 a ± 70.93 |
| Sample | L* | a* | b* |
|---|---|---|---|
| Bosana | 98.17 a ± 0.92 | 0.33 a ± 0.19 | −2.45 a ± 1.46 |
| Semidana | 98.68 a ± 5.46 | −0.11 a ± 1.19 | 1.88 b ± 2.38 |
| FD-OP Cultivar | Calcium (Ca) | Magnesium (Mg) | Sodium (Na) | Potassium (K) | Phosphorus (P) |
|---|---|---|---|---|---|
| Bosana | 830.8 a ± 34.3 | 601.5 a ± 2.1 | 458.6 a ± 9.0 | 13,269.9 a ± 431.5 | 1353.3 a ± 48.4 |
| Semidana | 759.4 a ± 8.1 | 526.3 b ± 5.6 | 432.4 a ± 20.5 | 11,577.9 b± 195.9 | 1466.0 a ± 37.6 |
| Sample | Dough Development Time (min:s) | Consistency (FE) | Added Water % (w/w) | Stability (min:s) |
|---|---|---|---|---|
| CTRL | 17:40 a ± 00:59.4 | 502.0 a ± 2.8 | 54.0 | 2:00 b ± 00:02.83 |
| 1% Bosana | 01:25 b ± 00:01.41 | 500.0 a ± 4.2 | 54.0 | 2:01 b ± 00:08.49 |
| 2% Bosana | 01:28 b ± 00:02.83 | 494.5 a ± 6.4 | 53.5 | 1:32 c,d ± 00:02.83 |
| 3% Bosana | 01:12 b ± 00:04.24 | 497.5 a ± 4.9 | 53.0 | 1:17 d ± 00:07.07 |
| 5% Bosana | 01:14 b ± 00:07.07 | 493.0 a ± 2.8 | 51.8 | 1:10 d ± 00:01.41 |
| 1% Semidana | 01:30 b ± 00:04.24 | 498.0 a ± 0.0 | 54.5 | 2:39 a ± 00:09.90 |
| 2% Semidana | 01:19 b ± 00:05.66 | 497.5 a ± 7.8 | 54.2 | 2:02 b ± 00:11.31 |
| 3% Semidana | 01:26 b ± 00:01.41 | 494.5 a ± 4.9 | 53.5 | 1:54 b,c ± 00:02.83 |
| 5% Semidana | 01:19 b ± 00:04.24 | 487.5 a ± 3.5 | 53.0 | 1:27 d ± 00:04.24 |
| Sample | Stickiness (N) | Resistance to Extension (N) |
|---|---|---|
| CTRL | 0.34 ab ± 0.02 | 0.11 f ± 0.00 |
| 1% Bosana | 0.36 a ± 0.03 | 0.34 bc ± 0.03 |
| 2% Bosana | 0.24 d ± 0.01 | 0.27 b ± 0.02 |
| 3% Bosana | 0.19 e ± 0.01 | 0.30 cd ± 0.04 |
| 5% Bosana | 0.15 f ± 0.02 | 0.44 a ± 0.03 |
| 1% Semidana | 0.34 ab ± 0.02 | 0.24 e ± 0.04 |
| 2% Semidana | 0.35 ab ± 0.02 | 0.27 de ± 0.03 |
| 3% Semidana | 0.32 b ± 0.03 | 0.32 c ± 0.03 |
| 5% Semidana | 0.28 c ± 0.03 | 0.33 bc ± 0.04 |
| Sample | Dough Development | Gas Behaviour | |||
|---|---|---|---|---|---|
| Hm (mm) | (Hm-h)/Hm (%) | T1(h) | T’1 (h) | (CR) Vr/Vt: (%) | |
| CTRL | 51.10 a ± 0.85 | 5.50 b,c ± 2.69 | 01:47:15 | 01:54:45 | 90.90 c ± 0.71 |
| 1% Bosana | 31.95 c ± 0.07 | 1.00 d ± 0.14 | 02:35:15 | 02:16:30 | 95.90 b ± 3.25 |
| 2% Bosana | 23.75 d ± 0.35 | 3.65 c ± 0.07 | 02:39:00 | 02:27:45 | 98.80 a ± 0.00 |
| 3% Bosana | 13.00 e ± 3.96 | 8.40 a ± 0.42 | 02:24:00 | 02:26:15 | 99.10 a ± 0.14 |
| 5% Bosana | 12.55 e ± 0.07 | 6.05 b ± 1.34 | 01:46:30 | 02:30:45 | 99.20 a ± 0.14 |
| 1% Semidana | 41.40 b ± 0.57 | 0.65 d ± 0.92 | 02:48:00 | 02:23:00 | 98.85 a ± 0.07 |
| 2% Semidana | 32.10 c ± 1.13 | 0.30 d ± 0.28 | 02:57:45 | 02:30:45 | 98.95 a ± 0.21 |
| 3% Semidana | 25.10 d ± 6.90 | 0.50 d ± 0.25 | 02:53:00 | 02:35:30 | 99.16 a ± 0.21 |
| 5% Semidana | 19.50 de ± 0.28 | 0.80 d ± 0.85 | 02:51:45 | 02:38:15 | 99.20 a ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahdah, P.; Cabizza, R.; Farbo, M.G.; Fadda, C.; Mara, A.; Hassoun, G.; Piga, A. Improving the Rheological Properties of Dough Obtained by Partial Substitution of Wheat Flour with Freeze-Dried Olive Pomace. Foods 2024, 13, 478. https://doi.org/10.3390/foods13030478
Dahdah P, Cabizza R, Farbo MG, Fadda C, Mara A, Hassoun G, Piga A. Improving the Rheological Properties of Dough Obtained by Partial Substitution of Wheat Flour with Freeze-Dried Olive Pomace. Foods. 2024; 13(3):478. https://doi.org/10.3390/foods13030478
Chicago/Turabian StyleDahdah, Patricia, Roberto Cabizza, Maria Grazia Farbo, Costantino Fadda, Andrea Mara, Georges Hassoun, and Antonio Piga. 2024. "Improving the Rheological Properties of Dough Obtained by Partial Substitution of Wheat Flour with Freeze-Dried Olive Pomace" Foods 13, no. 3: 478. https://doi.org/10.3390/foods13030478
APA StyleDahdah, P., Cabizza, R., Farbo, M. G., Fadda, C., Mara, A., Hassoun, G., & Piga, A. (2024). Improving the Rheological Properties of Dough Obtained by Partial Substitution of Wheat Flour with Freeze-Dried Olive Pomace. Foods, 13(3), 478. https://doi.org/10.3390/foods13030478








