Effects of Panax notoginseng Saponins Encapsulated by Polymerized Whey Protein on the Rheological, Textural and Bitterness Characteristics of Yogurt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Yogurt
2.3. Rheological Characterization of Yogurt
2.4. Texture Profile Analysis
2.5. Syneresis Measurement
2.6. pH Values
2.7. Electronic Tongue Analysis
2.8. Cryo-Scanning Electron Microscopy
2.9. Statistical Analysis
3. Results and Discussion
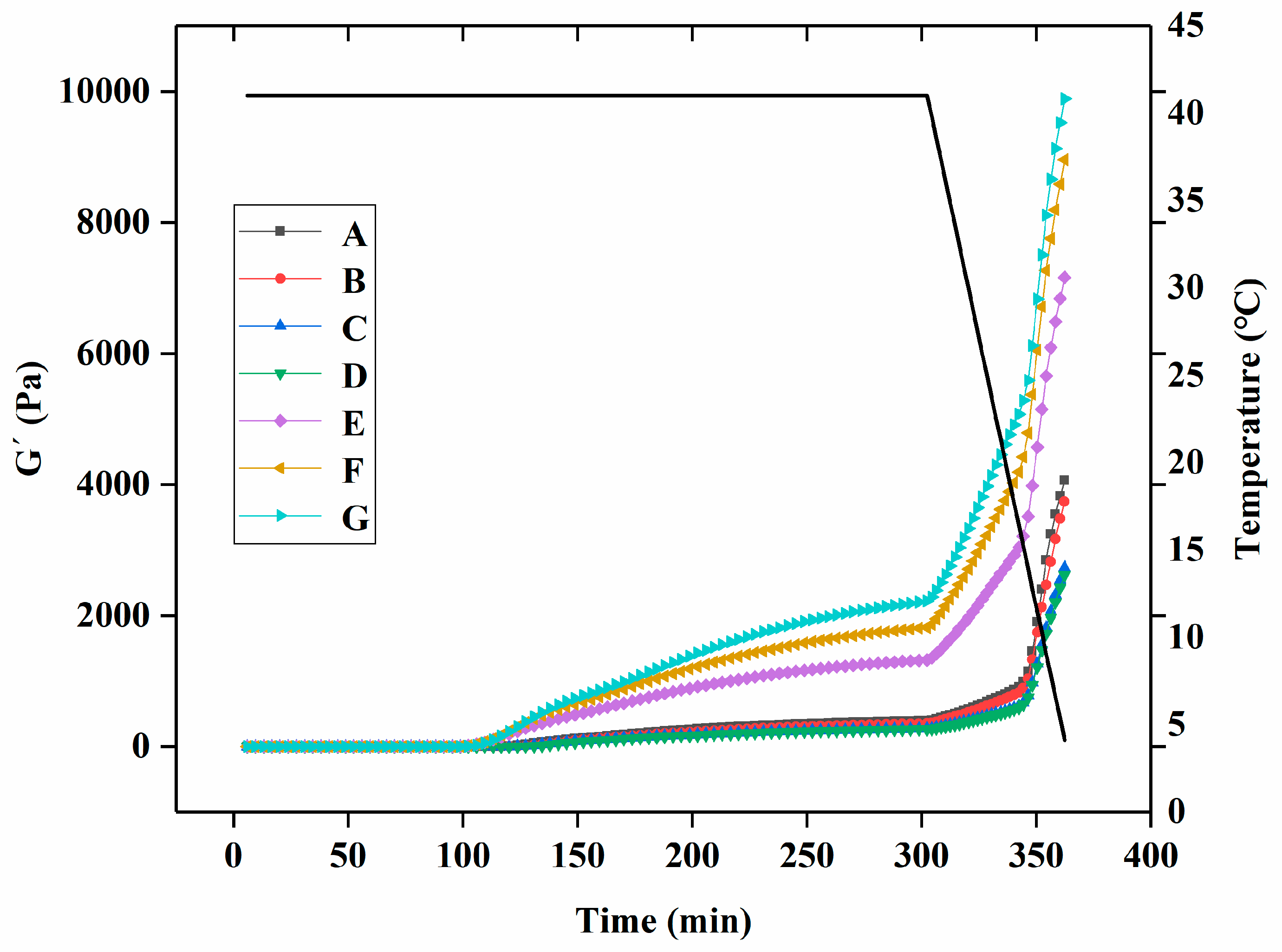
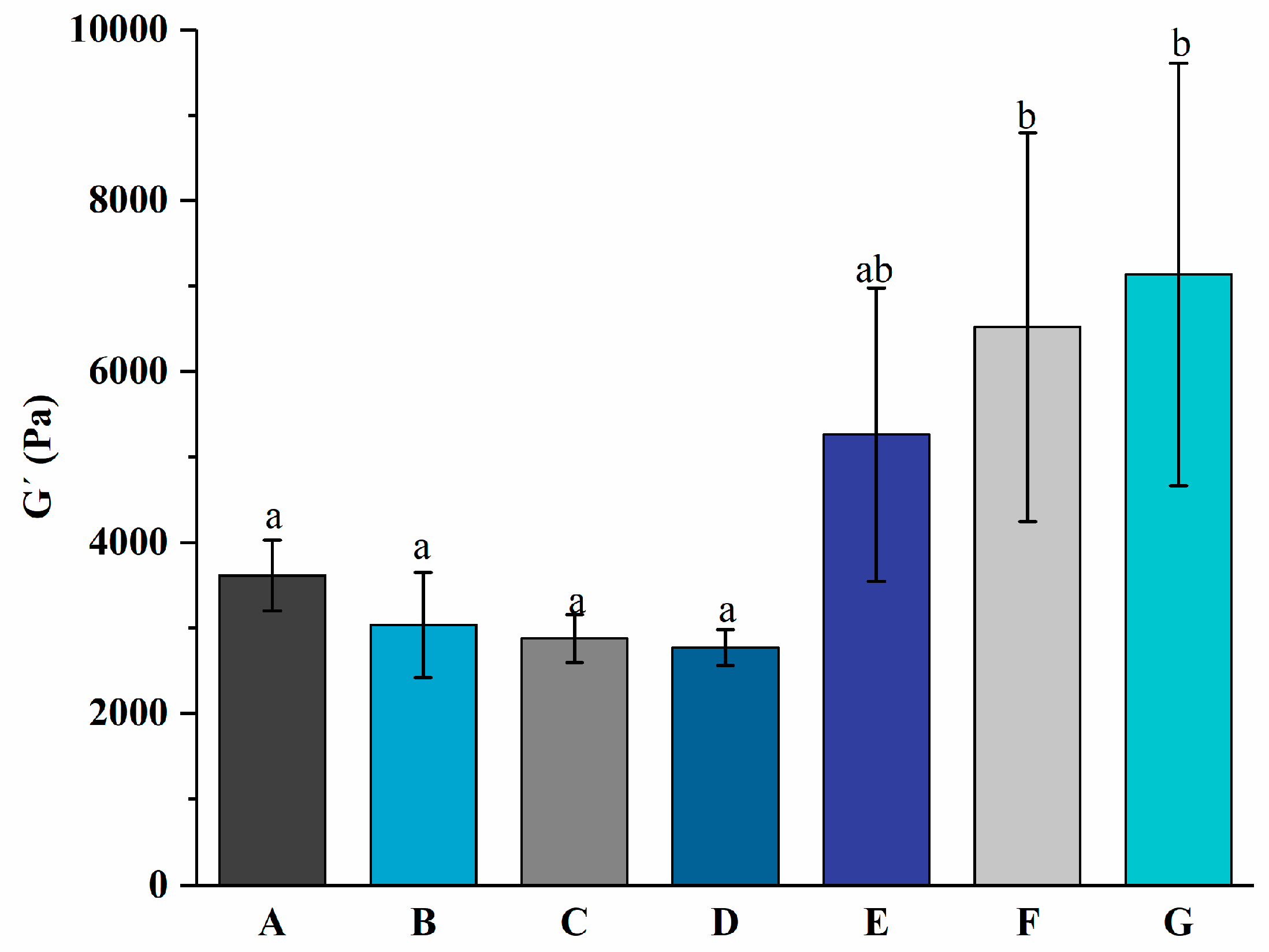
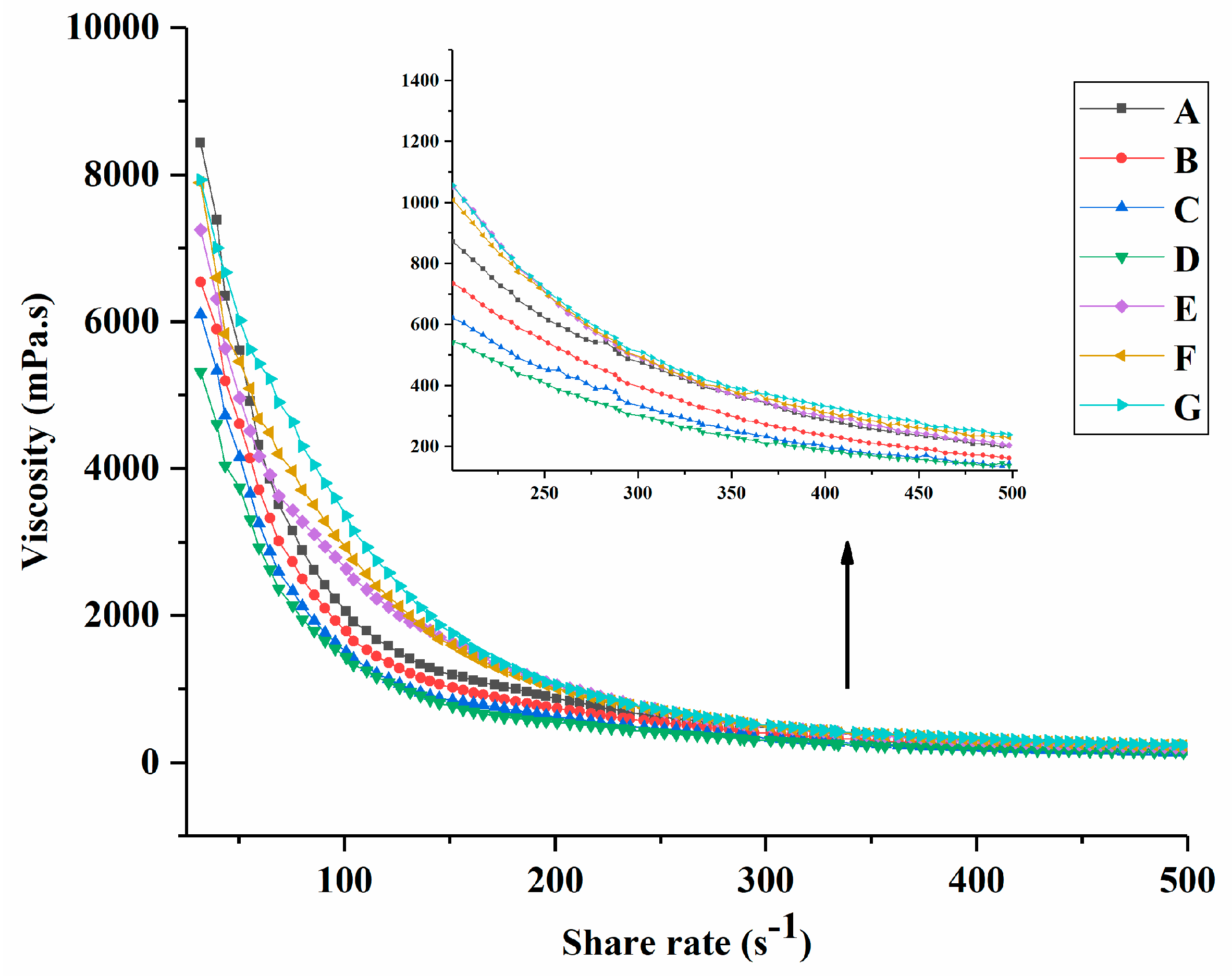
3.1. Rheological Properties of Yogurt during Fermentation
3.2. Texture Profile Analysis
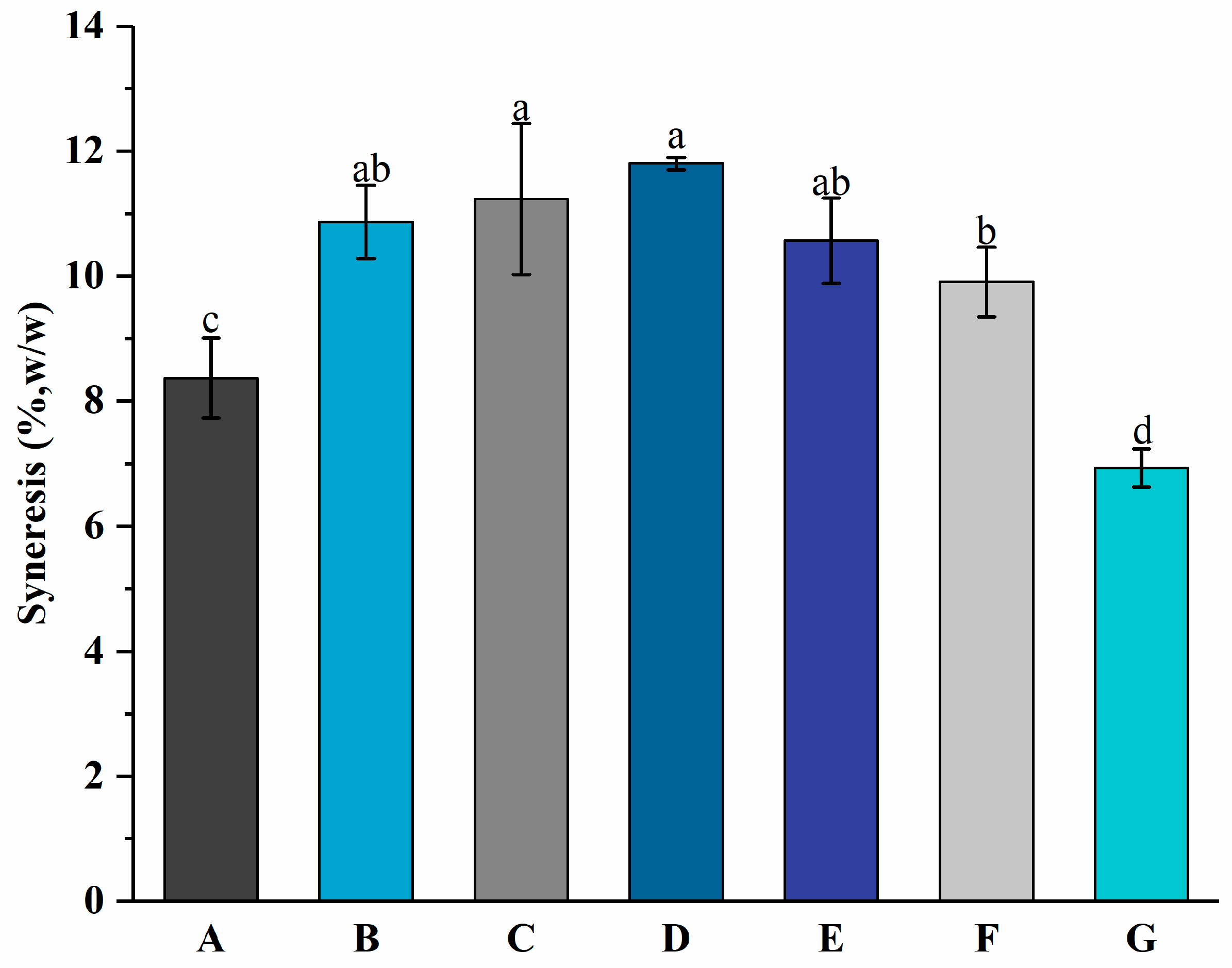
3.3. Syneresis
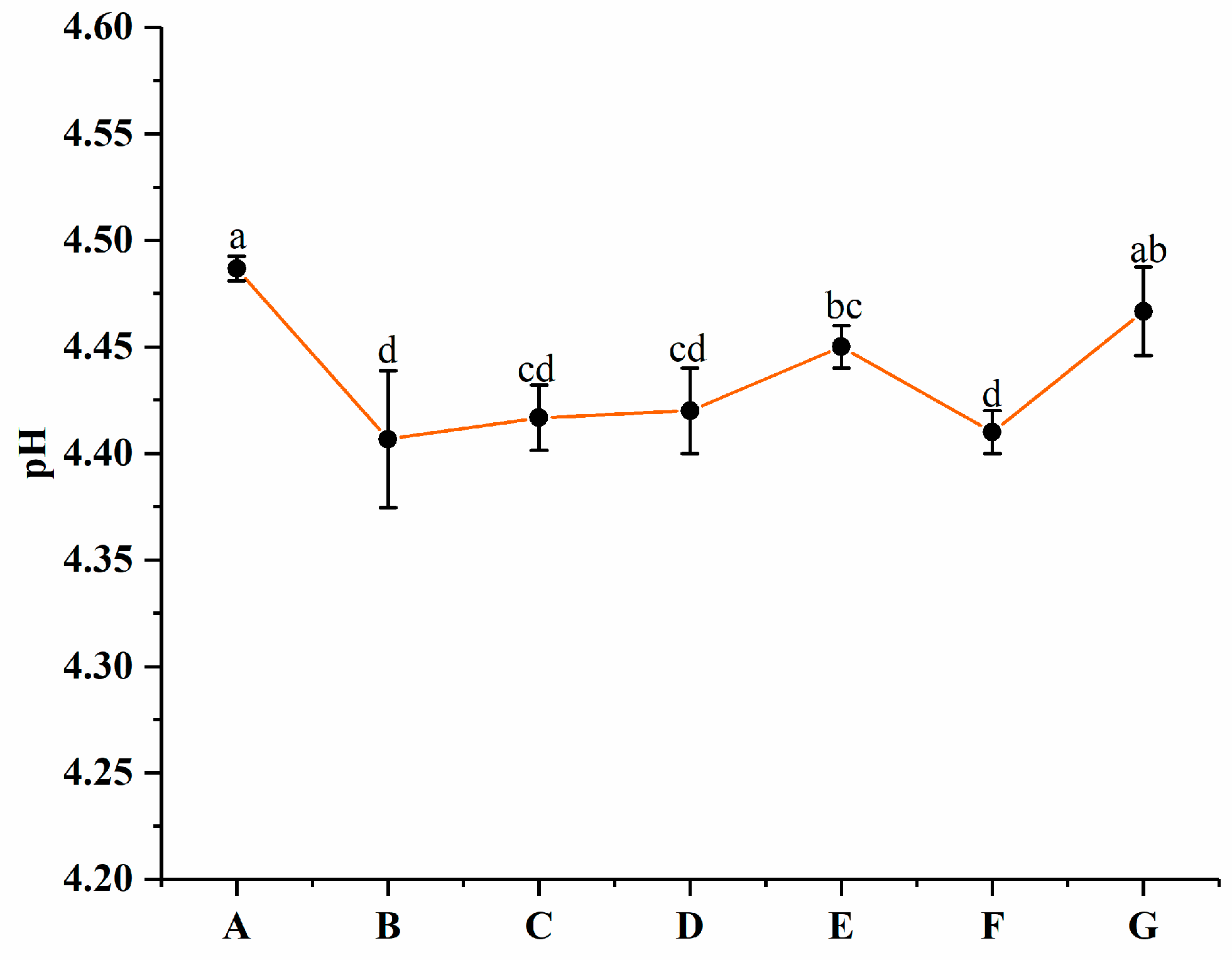
3.4. Changes in pH
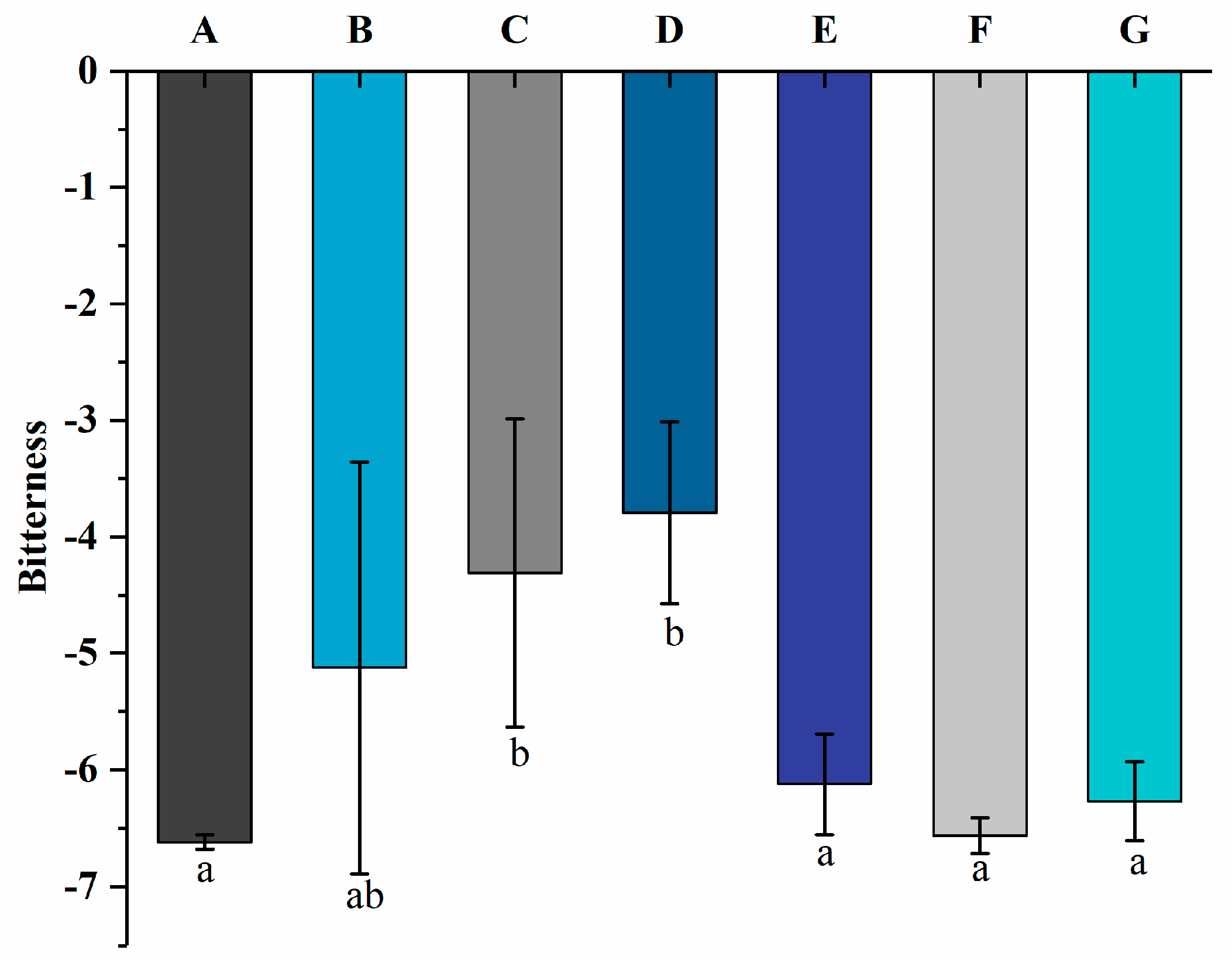
3.5. Bitterness by Electronic Tongue
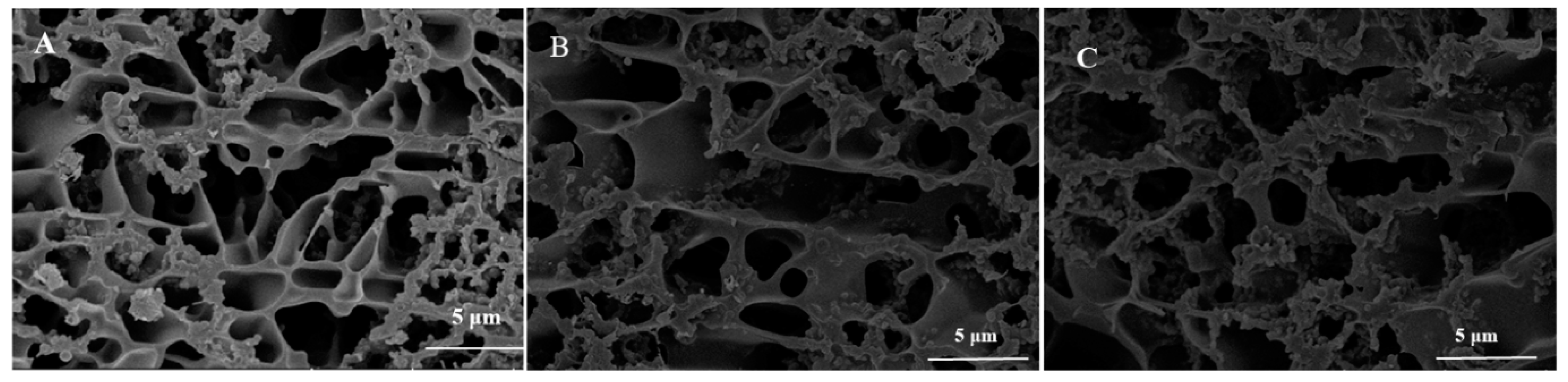
3.6. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, V.; Ferrao, J.; Fernandes, T. Nutritional guidelines and fermented food frameworks. Foods 2017, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Fazilah, N.F.; Ariff, A.B.; Khayat, M.E.; Rios-Solis, L.; Halim, M. Influence of probiotics, prebiotics, synbiotics and bioactive phytochemicals on the formulation of functional yogurt. J. Funct. Foods 2018, 48, 387–399. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligne, B.; Ganzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kaur Sidhu, M.; Lyu, F.; Sharkie, T.P.; Ajlouni, S.; Ranadheera, C.S. Probiotic yogurt fortified with chickpea flour: Physico-chemical properties and probiotic survival during storage and simulated gastrointestinal transit. Foods 2020, 9, 1144. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Sun, Q.; Giovannucci, E.; Mozaffarian, D.; Manson, J.E.; Willett, W.C.; Hu, F.B. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, O.; Meydani, S.N.; Russell, R.M. Yogurt and gut function. Am. J. Clin. Nutr. 2004, 80, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liu, D.; He, C.; Li, X.; He, F. Inhibiting adhesion events by Panax notoginseng saponins and ginsenoside Rb1 protecting arteries via activation of Nrf2 and suppression of p38-VCAM-1 signal pathway. J. Ethnopharmacol. 2016, 192, 423–430. [Google Scholar] [CrossRef]
- Wei, C.; Yue, L.; You, T.; Tao, C. Panax notoginseng saponins alleviate osteoporosis and joint destruction in rabbits with antigen-induced arthritis. Exp. Ther. Med. 2021, 22, 1302. [Google Scholar] [CrossRef]
- Men, S.Y.; Huo, Q.L.; Shi, L.; Yan, Y.; Yang, C.C.; Yu, W.; Liu, B.Q. Panax notoginseng saponins promotes cutaneous wound healing and suppresses scar formation in mice. J. Cosmet. Dermatol. 2020, 19, 529–534. [Google Scholar] [CrossRef]
- Wang, Q.; Mu, R.F.; Liu, X.; Zhou, H.M.; Xu, Y.H.; Qin, W.Y.; Yang, C.R.; Wang, L.B.; Li, Z.H.; Xiong, W.Y. Steaming changes the composition of saponins of Panax notoginseng (Burk.) F.H. Chen that function in treatment of hyperlipidemia and obesity. J. Agric. Food Chem. 2020, 68, 4865–4875. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, P.; Cui, J.; Wang, W.; Chen, Y.; Zhang, T. Panax notoginseng saponins attenuate lung cancer growth in part through modulating the level of Met/miR-222 axis. J. Ethnopharmacol. 2016, 193, 255–265. [Google Scholar] [CrossRef]
- Yang, X.; Xiong, X.; Wang, H.; Wang, J. Protective effects of Panax notoginseng saponins on cardiovascular diseases: A comprehensive overview of experimental studies. Evid. Based Complement. Altern. Med. 2014, 2014, 204840. [Google Scholar] [CrossRef]
- Gonzalez-Siso, M.I. The biotechnological utilization of cheese whey: A review. Bioresour. Technol. 1996, 57, 1–11. [Google Scholar] [CrossRef]
- Zandona, E.; Blazic, M.; Rezek Jambrak, A. Whey utilization: Sustainable uses and environmental approach. Food Technol. Biotechnol. 2021, 59, 147–161. [Google Scholar] [CrossRef]
- Yadav, J.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Ghanimah, M.A. Functional and technological aspects of whey powder and whey protein products. Int. J. Dairy Technol. 2018, 71, 454–459. [Google Scholar] [CrossRef]
- Kord Heydari, M.; Assadpour, E.; Jafari, S.M.; Javadian, H. Encapsulation of rose essential oil using whey protein concentrate-pectin nanocomplexes: Optimization of the effective parameters. Food Chem. 2021, 356, 129731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Z.; Dadmohammadi, Y.; Li, Y.; Jaiswal, A.; Abbaspourrad, A. Whey protein improves the stability of C-phycocyanin in acidified conditions during light storage. Food Chem. 2021, 344, 128642. [Google Scholar] [CrossRef]
- Vardhanabhuti, B.; Allen Foegeding, E.; McGuffey, M.K.; Daubert, C.R.; Swaisgood, H.E. Swaisgood. Gelation properties of dispersions containing polymerized and native whey protein isolate. Food Hydrocoll. 2001, 15, 165–175. [Google Scholar] [CrossRef]
- Nicolai, T.; Britten, M.; Schmitt, C. β-Lactoglobulin and WPI aggregates: Formation, structure and applications. Food Hydrocoll. 2011, 25, 1945–1962. [Google Scholar] [CrossRef]
- Wang, C.; Gao, F.; Zhang, T.; Wang, Y.; Guo, M. Physiochemical, textural, sensory properties and probiotic survivability of Chinese Laosuan Nai (protein-fortified set yoghurt) using polymerised whey protein as a co-thickening agent. Int. J. Dairy Technol. 2015, 68, 261–269. [Google Scholar] [CrossRef]
- Wang, M.; Gao, F.; Zheng, H.; Zhang, T.; Guo, M. Microencapsulation of ginsenosides using polymerised whey protein (PWP) as wall material and its application in probiotic fermented milk. Int. J. Food Sci. Technol. 2017, 52, 1009–1017. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, T.; Jiang, N.; Xi, C.; Sun, C.; Zheng, J.; Guo, M. Effects of encapsulated fish oil by polymerized whey protein on the textural and sensory characteristics of low-fat yogurt. Polish J. Food Nutr. Sci. 2016, 66, 189–198. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, W.; Huang, Y. Encapsulation of tartary buckwheat flavonoids and application to yoghurt. J. Microencapsul. 2016, 37, 445–456. [Google Scholar] [CrossRef]
- Zheng, Y.; Fan, C.; Chen, Y.; Quan, J.; Shi, L.; Tian, C.; Shang, X.; Xu, N.; Ye, W.; Yu, L.; et al. Anti-inflammatory, anti-angiogenetic and antiviral activities of dammarane-type triterpenoid saponins from the roots of Panax notoginseng. Food Funct. 2022, 13, 3590–3602. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, X.; Cheng, J.; Ban, Q.; Guo, M. Physicochemical, digestive, and sensory properties of Panax notoginseng saponins encapsulated by polymerized whey protein. Foods 2021, 10, 2942. [Google Scholar] [CrossRef]
- Song, X.; Sun, X.; Ban, Q.; Cheng, J.; Zhang, S.; Guo, M. Gelation and microstructural properties of a millet-based yogurt-like product using polymerized whey protein and xanthan gum as thickening agents. J. Food Sci. 2020, 85, 3927–3933. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Wang, M.; Guo, M. Chemical, physiochemical, and microstructural properties, and probiotic survivability of fermented goat milk using polymerized whey protein and starter culture kefir mild 01. J. Food Sci. 2017, 82, 2650–2658. [Google Scholar] [CrossRef]
- Córdova-Ramos, J.S.; Gonzales-Barron, U.; Cerrón-Mallqui, L.M. Physicochemical and sensory properties of yogurt as affected by the incorporation of jumbo squid (Dosidicus gigas) powder. LWT 2018, 93, 506–510. [Google Scholar] [CrossRef]
- Ban, Q.; Liu, Z.; Yu, C.; Sun, X.; Jiang, Y.; Cheng, J.; Guo, M. Physiochemical, rheological, microstructural, and antioxidant properties of yogurt using monk fruit extract as a sweetener. J. Dairy Sci. 2020, 103, 10006–10014. [Google Scholar] [CrossRef]
- Wang, W.; Bao, Y.; Hendricks, G.M.; Guo, M. Consistency, microstructure and probiotic survivability of goats’ milk yoghurt using polymerized whey protein as a co-thickening agent. Int. Dairy J. 2012, 24, 113–119. [Google Scholar] [CrossRef]
- Li, J.; Guo, M. Effects of polymerized whey proteins on consistency and water-holding properties of goat’s milk yogurt. J. Food Sci. 2006, 71, C34–C38. [Google Scholar]
- Fang, T.; Guo, M. Physicochemical, texture properties, and microstructure of yogurt using polymerized whey protein directly prepared from cheese whey as a thickening agent. J. Dairy Sci. 2019, 102, 7884–7894. [Google Scholar] [CrossRef] [PubMed]
- Sodini, I.; Remeuf, F.; Haddad, S.; Corrieu, G. The relative effect of milk base, starter, and process on yogurt texture: A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Paseephol, T.; Small, D.M.; Sherkat, F. Rheology and texture of set yogurt as affected by inulin addition. J. Text. Stud. 2008, 39, 617–634. [Google Scholar] [CrossRef]
- Mousavi, M.; Heshmati, A.; Daraei Garmakhany, A.; Vahidinia, A.; Taheri, M. Texture and sensory characterization of functional yogurt supplemented with flaxseed during cold storage. Food Sci. Nutr. 2019, 7, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Lucey, J.A. Cultured dairy products: An overview of their gelation and texture properties. Int. J. Dairy Technol. 2004, 57, 77–84. [Google Scholar] [CrossRef]
- Santiago-García, P.A.; Mellado-Mojica, E.; León-Martínez, F.M.; Dzul-Cauich, J.G.; López, M.G.; García-Vieyra, M.I. Fructans (agavins) from agave angustifolia and agave potatorum as fat replacement in yogurt: Effects on physicochemical, rheological, and sensory properties. LWT 2021, 140, 110846. [Google Scholar] [CrossRef]
- He, X.; Zou, Y.; Yoon, W.B.; Park, S.J.; Park, D.S.; Ahn, J. Effects of probiotic fermentation on the enhancement of biological and pharmacological activities of Codonopsis lanceolata extracted by high pressure treatment. J. Biosci. Bioeng. 2011, 112, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Lao, Y.; Zhang, M.; Li, Z.; Bhandari, B. A novel combination of enzymatic hydrolysis and fermentation: Effects on the flavor and nutritional quality of fermented cordyceps militaris beverage. LWT 2020, 120, 108934. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Xiang, H.; Cheng, J.; Ban, Q.; Sun, X.; Guo, M. Effects of Panax notoginseng Saponins Encapsulated by Polymerized Whey Protein on the Rheological, Textural and Bitterness Characteristics of Yogurt. Foods 2024, 13, 486. https://doi.org/10.3390/foods13030486
Zhou Z, Xiang H, Cheng J, Ban Q, Sun X, Guo M. Effects of Panax notoginseng Saponins Encapsulated by Polymerized Whey Protein on the Rheological, Textural and Bitterness Characteristics of Yogurt. Foods. 2024; 13(3):486. https://doi.org/10.3390/foods13030486
Chicago/Turabian StyleZhou, Zengjia, Huiyu Xiang, Jianjun Cheng, Qingfeng Ban, Xiaomeng Sun, and Mingruo Guo. 2024. "Effects of Panax notoginseng Saponins Encapsulated by Polymerized Whey Protein on the Rheological, Textural and Bitterness Characteristics of Yogurt" Foods 13, no. 3: 486. https://doi.org/10.3390/foods13030486





