Effect of Pleurotus eryngii on the Characteristics of Pork Patties during Freezing and Thawing Cycles
Abstract
:1. Introduction
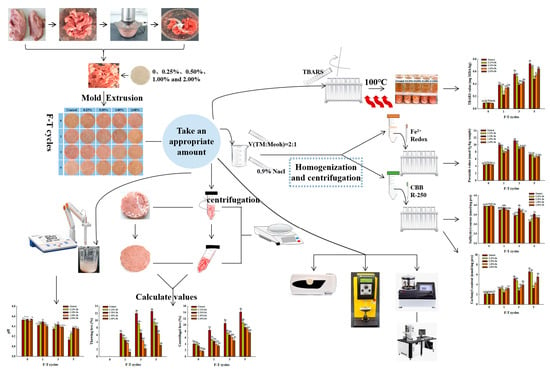
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pe Powder
2.3. Preparation of Pork Patties
2.4. pH Value
2.5. Water Holding Capacity
2.5.1. Centrifugal Loss
2.5.2. Thawing Loss
2.6. Color and Texture
2.7. Lipid Oxidation and Protein Oxidation
2.7.1. Lipid Oxidation
2.7.2. Protein Oxidation
2.8. SEM Determination
2.9. Statistical Analysis
3. Results and Discussion
3.1. pH Value
3.2. Water Holding Capacity
3.3. Color Value
3.4. Texture
3.5. Appearance
3.6. Lipid Oxidation
3.7. Protein Oxidation
3.8. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, N.; Dong, C.; Du, X.; Kong, B.H.; Xia, X.F. Effect of freeze-thaw cycles on the quality of quick-frozen pork patty with different fat content by consumer assessment and instrument-based detection. Meat Sci. 2020, 172, 108313. [Google Scholar] [CrossRef]
- Kim, H.W.; Miller, D.K.; Lee, Y.J.; Kim, Y.H.B. Effects of soy hull pectin and insoluble fiber on physicochemical and oxidative characteristics of fresh and frozen/thawed beef patties. Meat Sci. 2016, 117, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.H.Z.; Hamid, N.; Liu, T.T.; Sarojini, V. Effect of antifreeze peptide pretreatment on ice crystal size, drip loss, texture, and volatile compounds of frozen carrots. J. Agric. Food Chem. 2016, 64, 4327–4335. [Google Scholar] [CrossRef] [PubMed]
- Utrera, M.; Parra, V.; Estevez, M. Protein oxidation during frozen storage and subsequent processing of different beef muscles. Meat Sci. 2014, 96, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.Y.; Yang, C.; Bruce, H.L.; Roy, B.C.; Li, X.; Zhang, C.H. Effects of alternating electric field during freezing and thawing on beef quality. J. Agric. Food Chem. 2023, 419, 135987. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zhang, M.; Jiang, Q.Y.; Law, C.L. A novel infrared and microwave alternate thawing method for frozen pork: Effect on thawing rate and products quality. Meat Sci. 2023, 198, 109084. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Overview of plant extracts as natural preservatives in meat. Food Sci. Technol. 2022, 46, e16796. [Google Scholar] [CrossRef]
- Zahid, M.A.; Eom, J.U.; Parvin, R.; Seo, J.K.; Yang, H.S. Changes in quality traits and oxidation stability of syzygium aromaticum extract-added cooked ground beef during frozen storage. Antioxidants 2022, 11, 534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Li, X.L.; Sun, S.S.; Wang, Y.T.; Li, Z.Y.; Kang, H.B.; Peng, X.Y. Effects of carboxymethyl chitosan on the oxidation stability and gel properties of myofibrillar protein from frozen pork patties. Int. J. Biol. Macromol. 2023, 234, 123710. [Google Scholar] [CrossRef]
- Van, B.J.B.; Puga, K.J.; Hoffman, K.C.; Nasados, J.A.; Bass, P.D.; Colle, M.J. Water binders in beef patties increase yield and extend shelf life. Transl. Anim. Sci. 2023, 7, txad091. [Google Scholar]
- Wang, X.; Xu, M.; Cheng, J.; Zhang, W.; Liu, X.; Zhou, P. Effect of Flamrnulina velutipes on the physicochemical and sensory characteristics of Cantonese sausages. Meat Sci. 2019, 154, 22–28. [Google Scholar] [CrossRef]
- Choe, J.; Lee, J.; Jo, K.; Jo, C.; Song, M.; Jung, S. Application of winter mushroom powder as an alternative to phosphates in emulsion-type sausages. Meat Sci. 2018, 143, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.N.; Hu, X.L.; Li, W.X. Antioxidant, antitumor and immunostimulatory activities of the polypeptide from Pleurotus eryngii mycelium. Int. J. Biol. Macromol. 2017, 97, 323–330. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Chen, Y.; Liu, T.; Zhang, S.; Fan, H.; Liu, H.; Li, Y. Healthy function and high valued utilization of edible fungi. Food Sci. Hum. Well. 2021, 10, 408–420. [Google Scholar] [CrossRef]
- Wang, L.Y.; Li, C.; Ren, L.L.; Guo, H.Y.; Li, Y. Production of pork sausages using Pleaurotus eryngii with different treatments as replacements for pork back fat. J. Food Sci. 2019, 84, 3091–3098. [Google Scholar] [CrossRef]
- Chung, S.I.; Kim, S.Y.; Nam, Y.J.; Kang, M.Y. Development of surimi gel from king oyster mushroom and cuttlefish meat paste. Food Sci. Biotechnol. 2010, 19, 51–56. [Google Scholar] [CrossRef]
- Li, F.F.; Zhong, Q.; Kong, B.H.; Wang, B.; Pan, N.; Xia, X.F. Deterioration in quality of quick-frozen pork patties induced by changes in protein structure and lipid and protein oxidation during frozen storage. Food Res. Int. 2020, 133, 109142. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, Q.; Xia, X.F.; Kong, B.H.; Xiong, Y.L.L. Oxidative changes and weakened gelling ability of salt-extracted protein are responsible for textural losses in dumpling meat fillings during frozen storage. J. Agric. Food Chem. 2015, 185, 459–469. [Google Scholar] [CrossRef]
- Zhao, M.N.; Li, Y.; Bai, X.; Feng, J.; Xia, X.F.; Li, F.F. Inhibitory effect of guava leaf polyphenols on advanced glycation end products of frozen chicken meatballs (-18 degrees C) and its mechanism analysis. Foods 2022, 11, 2509. [Google Scholar] [CrossRef]
- Qing, Z.; Cheng, J.; Wang, X.; Tang, D.; Liu, X.; Zhu, M. The effects of four edible mushrooms (Volvariella volvacea, Hypsizygus marmoreus, Pleurotus ostreatus and Agaricus bisporus) on physicochemical properties of beef paste. LWT 2021, 135, 110063. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Ubbink, J. Effects of pH and aging on the texture and physicochemical properties of extruded pea protein isolate. Food Hydrocoll. 2023, 140, 108639. [Google Scholar] [CrossRef]
- Shi, S.; Xu, X.W.; Feng, J.; Ren, Y.M.; Bai, X.; Xia, X.F. Preparation of NH3- and H2S-sensitive intelligent pH indicator film from sodium alginate/black soybean seed coat anthocyanins and its use in monitoring meat freshness. Food Packag. Shelf 2023, 35, 100994. [Google Scholar] [CrossRef]
- Torres-Martinez, B.D.; Vargas-Sanchez, R.D.; Torrescano-Urrutia, G.R.; Gonzalez-Avila, M.; Rodriguez-Carpena, J.G.; Huerta-Leidenz, N.; Perez-Alvarez, J.A.; Fernandez-Lopez, J.; Sanchez-Escalante, A. Use of Pleurotus ostreatus to enhance the oxidative stability of pork patties during storage and In vitro gastrointestinal digestion. Foods 2022, 11, 4075. [Google Scholar] [CrossRef] [PubMed]
- Boonsumrej, S.; Chaiwanichsiri, S.; Tantratian, S.; Suzuki, T.; Takai, R. Effects of freezing and thawing on the quality changes of tiger shrimp (Penaeus monodon) frozen by air-blast and cryogenic freezing. J. Food Eng. 2007, 80, 292–299. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Diao, X.; Kong, B.; Xia, X. Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci. 2017, 133, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Oxidative stability of previously frozen ostrich Muscularis iliofibularis packaged under different modified atmospheric conditions. Int. J. Food Sci. Technol. 2011, 46, 1171–1178. [Google Scholar] [CrossRef]
- Kim, T.W.; Kim, C.W.; Yang, M.R.; No, G.R.; Kim, S.W.; Kim, I.S. Pork quality traits according to postmortem pH and temperature in berkshire. Korean J. Food Sci. An. 2016, 36, 29–36. [Google Scholar] [CrossRef]
- Zhou, G.H.; Xu, X.L.; Liu, Y. Preservation technologies for fresh meat—A review. Meat Sci. 2010, 86, 119–128. [Google Scholar] [CrossRef]
- Ceron-Guevara, M.I.; Rangel-Vargas, E.; Manuel Lorenzo, J.; Bermudez, R.; Pateiro, M.; Rodriguez, J.A.; Sanchez-Ortega, I.; Santos, E.M. Effect of the addition of edible mushroom flours (Agaricus bisporus and Pleurotus ostreatus) on physicochemical and sensory properties of cold-stored beef patties. J. Food Process. Pres. 2020, 44, e14351. [Google Scholar] [CrossRef]
- Chaplin, M.F. Fibre and water binding. P. Nutr. Soc. 2003, 62, 223–227. [Google Scholar] [CrossRef]
- Bohrer, B.M.; Izadifar, M.; Barbut, S. Structural and functional properties of modified cellulose ingredients and their application in reduced-fat meat batters. Meat Sci. 2023, 195, 109011. [Google Scholar] [CrossRef]
- Ma, L.K.; Zhang, B.; Deng, S.G.; Xie, C. Comparison of the cryoprotective effects of trehalose, alginate, and its oligosaccharides on peeled shrimp (litopenaeus vannamei) during frozen storage. J. Food Sci. 2015, 80, C540–C546. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, L.; Jiang, X.; Chen, Y.; Zhou, G. The effects of three polysaccharides on the gelation properties of myofibrillar protein: Phase behaviour and moisture stability. Meat Sci. 2020, 170, 108228. [Google Scholar] [CrossRef]
- Li, X.B.; Feng, T.; Zhou, F.; Zhou, S.; Liu, Y.F.; Li, W.; Ye, R.; Yang, Y. Effects of drying methods on the tasty compounds of Pleurotus eryngii. J. Agric. Food Chem. 2015, 166, 358–364. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Kim, G.D.; Yang, H.S.; Joo, S.T. Effect of freeze-thaw cycles on physicochemical properties and color stability of beef semimembranosus muscle. Food Res. Int. 2011, 44, 3222–3228. [Google Scholar] [CrossRef]
- Muramoto, T.; Nakai, M.; Suzuki, Y.; Inoue, S.; Ishida, M.; Kinoshita, K.; Hirata, S. Effect of muscle pH on the physicochemical properties of venison from wild deer. Nihon Chikusan Gakkaiho 2021, 92, 335–341. [Google Scholar] [CrossRef]
- Alvarenga, T.I.R.C.; Hopkins, D.L.; Ramos, E.M.; Almeida, A.K.; Geesink, G. Ageing-freezing/thaw process affects blooming time and myoglobin forms of lamb meat during retail display. Meat Sci. 2019, 153, 19–25. [Google Scholar] [CrossRef]
- Zakrys, P.I.; Hogan, S.A.; O’Sullivan, M.G.; Allen, P.; Kerry, J.P. Effects of oxygen concentration on the sensory evaluation and quality indicators of beef muscle packed under modified atmosphere. Meat Sci. 2008, 79, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Gudbjornsdottir, B.; Jonsson, A.; Hafsteinsson, H.; Heinz, V. Effect of high-pressure processing on Listeria spp. and on the textural and microstructural properties of cold smoked salmon. LWT 2010, 43, 366–374. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Madane, P.; Biswas, S.; Das, A.; Zhang, W.; Lorenzo, J.M. A comprehensive review on antioxidant dietary fibre enriched meat-based functional foods. Trends Food Sci. Technol. 2020, 99, 323–336. [Google Scholar] [CrossRef]
- Wang, X.P.; Zhou, P.F.; Cheng, J.R.; Chen, Z.Y.; Liu, X.M. Use of straw mushrooms (Volvariella volvacea) for the enhancement of physicochemical, nutritional and sensory profiles of Cantonese sausages. Meat Sci. 2018, 146, 18–25. [Google Scholar] [CrossRef]
- Kim, Y.H.B.; Liesse, C.; Kemp, R.; Balan, P. Evaluation of combined effects of ageing period and freezing rate on quality attributes of beef loins. Meat Sci. 2015, 110, 40–45. [Google Scholar] [CrossRef]
- Carneiro, C.d.S.; Marsico, E.T.; Resende Ribeiro, R.d.O.; Conte Junior, C.A.; Alvares, T.S.; Oliveira de Jesus, E.F. Studies of the effect of sodium tripolyphosphate on frozen shrimp by physicochemical analytical methods and low field nuclear magnetic resonance (LF H-1 NMR). LWT 2013, 50, 401–407. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, J.; Huang, F.; Huang, M.; Zhou, G. Influence of oxidation on the susceptibility of purified desmin to degradation by mu-calpain, caspase-3 and-6. J. Agric. Food Chem. 2014, 150, 220–226. [Google Scholar]
- Anese, M.; Manzocco, L.; Panozzo, A.; Beraldo, P.; Foschia, M.; Nicoll, M.C. Effect of radiofrequency assisted freezing on meat microstructure and quality. Food Res. Int. 2012, 46, 50–54. [Google Scholar] [CrossRef]
- Kuribayashi, T.; Kaise, H.; Uno, C.; Hara, T.; Hayakawa, T.; Joh, T. Purification and characterization of lipoxygenase from Pleurotus ostreatus. J. Agr. Food Chem. 2002, 50, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Perez-Montes, A.; Rangel-Vargas, E.; Manuel Lorenzo, J.; Romero, L.; Santos, E.M. Edible mushrooms as a novel trend in the development of healthier meat products. Curr. Opin. Food Sci. 2021, 37, 118–124. [Google Scholar] [CrossRef]
- Jo, I.H.; Kim, J.; An, H.; Lee, H.Y.; So, Y.S.; Ryu, H.; Sung, G.H.; Shim, D.; Chung, J.W. Pseudo-chromosomal genome assembly in combination with comprehensive transcriptome analysis in agaricus bisporus strain KMCC00540 reveals mechanical stimulus responsive genes associated with browning effect. J. Fungi 2022, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.S.H.; Bruheim, I.; Jacobsen, C. Maillard reaction and lipid peroxidation contribute to non-enzymatic browning in krill-based products: A model study on proposed mechanisms. Eur. J. Lipid Sci. Technol. 2015, 117, 421–430. [Google Scholar] [CrossRef]
- Pahila, J.; Ishikawa, Y.; Ohshima, T. Effects of ergothioneine-rich mushroom extract on the oxidative stability of astaxanthin in liposomes. J. Agr. Food Chem. 2019, 67, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.; Ferreira Zielinski, A.A.; Helm, C.V.; Maciel, G.M.; Pedro, A.C.; Stafussa, A.P.; Avila, S.; Isidoro Haminiuk, C.W. Bio compounds of edible mushrooms: In vitro antioxidant and antimicrobial activities. LWT 2019, 107, 214–220. [Google Scholar] [CrossRef]
- Armenteros, M.; Morcuende, D.; Ventanas, J.; Estevez, M. The application of natural antioxidants via brine injection protects Iberian cooked hams against lipid and protein oxidation. Meat Sci. 2016, 116, 253–259. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef]
- Arduini, A.; Eddy, L.; Hochstein, P. The reduction of ferryl myoglobin by ergothioneine: A novel function for ergothioneine. Arch. Biochem. Biophys. 1990, 281, 41–43. [Google Scholar] [CrossRef]
- Bao, H.N.D.; Ushio, H.; Ohshima, T. Antioxidative activity and antidiscoloration efficacy of ergothioneine in Mushroom (Flammulina velutipes) extract added to beef and fish meats. J. Agr. Food Chem. 2008, 56, 10032–10040. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, K.; Hüseyin, G. Enrichment of meat emulsion with mushroom (Agaricus bisporus) powder: Impact on rheological and structural characteristics. J. Food Eng. 2018, 237, 128–136. [Google Scholar]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein oxidation: Basic principles and implications for meat quality. Crit. Rev. Food Sci. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Soladoye, O.P.; Juarez, M.L.; Aalhus, J.L.; Shand, P.; Estevez, M. Protein oxidation in processed meat: Mechanisms and potential implications on human health. Compr. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Reyes, K.; Villalobos-Carvajal, R.; Beldarrain-Iznaga, T. Fresh mushroom preservation techniques. Foods 2021, 10, 2126. [Google Scholar] [CrossRef]
- Guinard, J.X.; Miller, A.M.; Mills, K.; Wong, T.; Lee, S.M.; Sirimuangmoon, C.; Schaefer, S.E.; Drescher, G. Consumer acceptance of dishes in which beef has been partially substituted with mushrooms and sodium has been reduced. Appetite 2016, 105, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Mariana, U.; David, M.; Mario, E. Temperature of frozen storage affects the nature and consequences of protein oxidation in beef patties. Meat Sci. 2014, 96, 1250–1257. [Google Scholar]
- Dominguez, R.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Bohrer, B.; Lorenzo, J.M. Protein oxidation in muscle foods: A comprehensive review. Antioxidants 2022, 11, 60. [Google Scholar] [CrossRef]
- Xu, X.; Yan, H.; Chen, J.; Zhang, X. Bioactive proteins from mushrooms. Biotechnol. Adv. 2011, 29, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Utrera, M.; Estevez, M. Oxidation of myofibrillar proteins and impaired functionality: Underlying mechanisms of the carbonylation pathway. J. Agr. Food Chem. 2012, 60, 8002–8011. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.W.; Wu, D.; Wang, L.M.; Song, G.Y.; Chi, R.S.; Ma, J.; Li, Z.S.; Wang, L.; Sun, W.Q. Insoluble dietary fibers from Lentinus edodes stipes improve the gel properties of pork myofibrillar protein: A water distribution, microstructure and intermolecular interactions study. Foods 2023, 411, 135386. [Google Scholar] [CrossRef] [PubMed]
- Herranz, B.; Martínez, A.; Alvarez, M.D. Influence of fiber addition on white sauces made with corn starch: Effect on their freezing/thawing stability. J. Food Sci. 2018, 84, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Bai, X.; Li, Y.; Kong, B.H.; Nuerjiang, M.; Wu, K.R.; Li, Z.H.; Xia, X.F. Improvement on gel properties of myofibrillar protein from chicken patty with potato dietary fiber: Based on the change in myofibrillar protein structure and water state. Int. J. Biol. Macromol. 2023, 230, 123228. [Google Scholar] [CrossRef]






| Treatments | Color Value | ||||
|---|---|---|---|---|---|
| L* | a* | b* | |||
| F–T cycles | 0 | Control | 42.82 ± 0.78 Da | 10.39 ± 0.49 Aab | 15.28 ± 0.09 Bb |
| 0.25% | 42.71 ± 0.06 Ca | 9.46 ± 0.04 Ac | 14.33 ± 0.03 Bc | ||
| 0.50% | 41.75 ± 0.12 Dab | 10.61 ± 0.05 Aa | 14.24 ± 0.02 Cc | ||
| 1.00% | 41.00 ± 0.09 Cbc | 9.82 ± 0.09 Abc | 15.30 ± 0.07 ABb | ||
| 2.00% | 40.16 ± 0.09 Dc | 9.24 ± 0.06 Ac | 16.00 ± 0.23 Ba | ||
| 1 | Control | 45.04 ± 0.35 Ca | 8.45 ± 0.01 Ba | 15.61 ± 0.1 Ac | |
| 0.25% | 43.81 ± 0.02 Bb | 8.91 ± 0.42 ABa | 15.30 ± 0.07 Ac | ||
| 0.50% | 42.36 ± 0.07 Cc | 8.81 ± 0.39 Ba | 17.92 ± 0.27 Aa | ||
| 1.00% | 42.35 ± 0.17 Bc | 8.91 ± 0.09 Ba | 16.32 ± 0.15 Ab | ||
| 2.00% | 41.87 ± 0.14 Cc | 9.10 ± 0.13 Aa | 17.71 ± 0.06 Aa | ||
| 3 | Control | 46.35 ± 0.18 Ba | 7.69 ± 0.65 BCb | 13.68 ± 0.07 Cb | |
| 0.25% | 44.74 ± 0.08 Bb | 8.61 ± 0.08 Bab | 13.89 ± 0.10 Bab | ||
| 0.50% | 43.46 ± 0.11 Bc | 8.66 ± 0.23 Bab | 12.59 ± 0.36 Dc | ||
| 1.00% | 43.14 ± 0.05 Bcd | 8.82 ± 0.05 Ba | 13.83 ± 0.18 Cb | ||
| 2.00% | 42.80 ± 0.18 Bd | 8.54 ± 0.11 Bab | 14.37 ± 0.13 Ca | ||
| 5 | Control | 54.13 ± 0.07 Aa | 6.95 ± 0.03 Cab | 15.62 ± 0.11 Aa | |
| 0.25% | 53.68 ± 0.83 Aab | 6.98 ± 0.1 Cab | 14.51 ± 0.41 Bb | ||
| 0.50% | 51.96 ± 0.33 Abc | 7.25 ± 0.16 Ca | 15.06 ± 0.29 Bab | ||
| 1.00% | 50.19 ± 0.89 Acd | 7.12 ± 0.33 Cab | 14.49 ± 0.66 BCb | ||
| 2.00% | 48.50 ± 0.66 Ad | 6.68 ± 0.69 Cb | 15.84 ± 0.07 Ba | ||
| Treatments | Hardness (g) | Adhesion (g) | Cohesiveness | Elasticity (mm) | Gumminess (g) | Chewiness (mJ) | ||
|---|---|---|---|---|---|---|---|---|
| F–T (cycles) | 0 | Control | 2721.00 ± 275.15 Aa | 199.00 ± 0.82 Aa | 0.22 ± 0.04 Bb | 3.02 ± 0.45 Bab | 604.00 ± 49.56 Ba | 17.90 ± 2.78 Ca |
| 0.25% | 2361.33 ± 170.87 Aab | 256.33 ± 58.90 Aa | 0.21 ± 0.02 Cb | 2.48 ± 0.06 Bb | 520.33 ± 2.62 Ba | 12.60 ± 0.43 Ba | ||
| 0.50% | 2121.00 ± 168.12 Abc | 251.67 ± 97.11 Aa | 0.25 ± 0.02 Bb | 2.58 ± 0.31 ABab | 503.00 ± 26.99 Aa | 12.90 ± 2.16 Aa | ||
| 1.00% | 2116.67 ± 7.59 Abc | 196.33 ± 3.77 Aa | 0.34 ± 0.16 Aab | 3.56 ± 1.28 Aab | 638.33 ± 341.93 Aa | 28.97 ± 21.20 Aa | ||
| 2.00% | 1917.33 ± 109.14 Ac | 279.67 ± 111.96 Aa | 0.50 ± 0.12 ABa | 4.86 ± 1.33 Aa | 939.33 ± 190.93 Aa | 46.90 ± 22.43 Aa | ||
| 1 | Control | 2578.33 ± 270.37 Aa | 198.33 ± 0.47 Aa | 0.46 ± 0.04 ABa | 3.20 ± 0.09 Ba | 698.50 ± 29.50 Ba | 21.90 ± 0.30 BCa | |
| 0.25% | 2072.00 ± 130.26 Aab | 182.67 ± 20.29 Aa | 0.46 ± 0.04 Ba | 2.93 ± 0.26 ABab | 496.00 ± 36.00 Bb | 13.60 ± 1.80 Bb | ||
| 0.50% | 1947.00 ± 74.65 Ab | 198.33 ± 0.47 ABa | 0.27 ± 0.05 Bb | 2.98 ± 0.11 Aab | 484.50 ± 39.50 Ab | 14.15 ± 1.65 Ab | ||
| 1.00% | 1810.33 ± 270.04 Ab | 192.00 ± 9.93 Aa | 0.41 ± 0.10 Aab | 2.72 ± 0.16 Ab | 506.00 ± 6.00 Ab | 13.15 ± 0.65 Ab | ||
| 2.00% | 1824.67 ± 126.15 Ab | 197.00 ± 2.16 ABa | 0.41 ± 0.08 ABab | 2.62 ± 0.09 Bb | 501.50 ± 51.50 BCb | 12.60 ± 1.40 Bb | ||
| 3 | Control | 1609.50 ± 492.50 Ba | 129.50 ± 34.50 Aa | 0.46 ± 0.03 ABab | 4.07 ± 0.16 ABa | 1103.00 ± 4.50 Aa | 44.05 ± 1.95 ABa | |
| 0.25% | 969.00 ± 199.00 Bb | 48.00 ± 5.00 Bbc | 0.56 ± 0.05 Aa | 3.04 ± 0.05 ABb | 525.95 ± 85.00 Bb | 15.60 ± 2.30 Bb | ||
| 0.50% | 978.00 ± 172.50 Bb | 96.00 ± 28.00 Bab | 0.51 ± 0.05 Aa | 3.00 ± 0.05 Ab | 444.0 ± 28.00 Abc | 13.05 ± 1.05 Abc | ||
| 1.00% | 977.50 ± 126.50 Bb | 77.50 ± 9.50 Cabc | 0.50 ± 0.03 Aa | 2.91 ± 0.07 Ab | 487.00 ± 88.0 Abc | 13.85 ± 2.15 Abc | ||
| 2.00% | 603.50 ± 7.50 Bc | 36.00 ± 14.00 Cc | 0.34 ± 0.07 Bb | 2.86 ± 0.20 Bb | 359.50 ± 15.50 Cc | 10.05 ± 0.25 Bc | ||
| 5 | Control | 1549.5 ± 25.5 Ba | 133.00 ± 79.00 Aa | 0.76 ± 0.24 Aa | 4.61 ± 0.78 Aa | 1198.00 ± 205.36 Aa | 55.63 ± 17.04 Aa | |
| 0.25% | 941.50 ± 67.50 Bb | 51.00 ± 14.00 Ba | 0.55 ± 0.03 ABa | 3.66 ± 0.56 Aab | 958.33 ± 90.03 Aab | 34.67 ± 7.88 Aab | ||
| 0.50% | 538.00 ± 114.00 Cbc | 73.50 ± 20.50 Ba | 0.48 ± 0.07 Aa | 2.49 ± 0.04 Bb | 540.00 ± 50.84 Ab | 13.30 ± 1.02 Ab | ||
| 1.00% | 519.50 ± 2.50 Cbc | 102.50 ± 7.50 Ba | 0.59 ± 0.01 Aa | 3.15 ± 0.66 Ab | 751.33 ± 240.80 Ab | 24.73 ± 13.22 Ab | ||
| 2.00% | 277.00 ± 92.00 Cc | 80.00 ± 4.00 BCa | 0.60 ± 0.04 Aa | 3.91 ± 0.26 ABab | 742.00 ± 103.04 ABb | 28.23 ± 2.66 ABab | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Chai, Y.; Li, F.; Bao, Y. Effect of Pleurotus eryngii on the Characteristics of Pork Patties during Freezing and Thawing Cycles. Foods 2024, 13, 501. https://doi.org/10.3390/foods13030501
Zhang M, Chai Y, Li F, Bao Y. Effect of Pleurotus eryngii on the Characteristics of Pork Patties during Freezing and Thawing Cycles. Foods. 2024; 13(3):501. https://doi.org/10.3390/foods13030501
Chicago/Turabian StyleZhang, Miaojing, Yangyang Chai, Fangfei Li, and Yihong Bao. 2024. "Effect of Pleurotus eryngii on the Characteristics of Pork Patties during Freezing and Thawing Cycles" Foods 13, no. 3: 501. https://doi.org/10.3390/foods13030501
APA StyleZhang, M., Chai, Y., Li, F., & Bao, Y. (2024). Effect of Pleurotus eryngii on the Characteristics of Pork Patties during Freezing and Thawing Cycles. Foods, 13(3), 501. https://doi.org/10.3390/foods13030501