Effects of Wooden Breast Myopathy on Meat Quality Characteristics of Broiler Pectoralis Major Muscle and Its Changes with Intramuscular Connective Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Broiler Breast Fillets Collection
2.2. Histological Evaluation
2.3. Chemical Composition Analysis
2.4. Meat Quality Measurement
2.5. Texture Profile Analyses
2.6. NMR Transverse Relaxation (T2) Measurements
2.7. Collagen Profiles Measurements
2.8. Determination of Hydroxylysyl Pyridoxine, Glycosaminoglycan and Decorin
2.9. Extraction of Intramuscular Connective Tissue
2.10. Differential Scanning Calorimetry Analysis
2.11. Fourier Transform Infrared Spectroscopy Analysis
2.12. Statistical Analysis
3. Results and Discussion
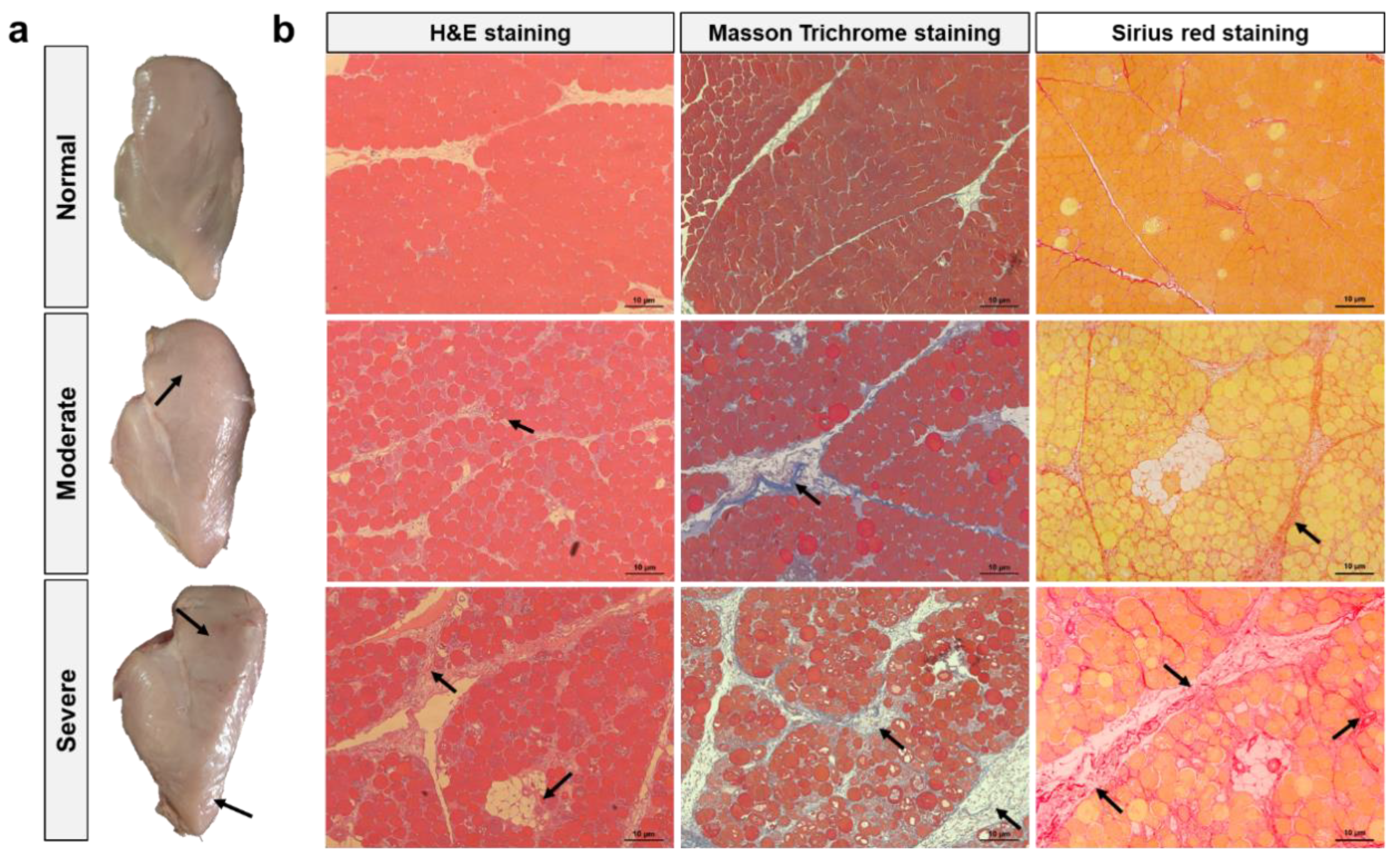
3.1. Macroscopic Appearance and Histology of WB
3.2. Chemical Composition
3.3. Meat Quality Traits
3.4. Textural Properties
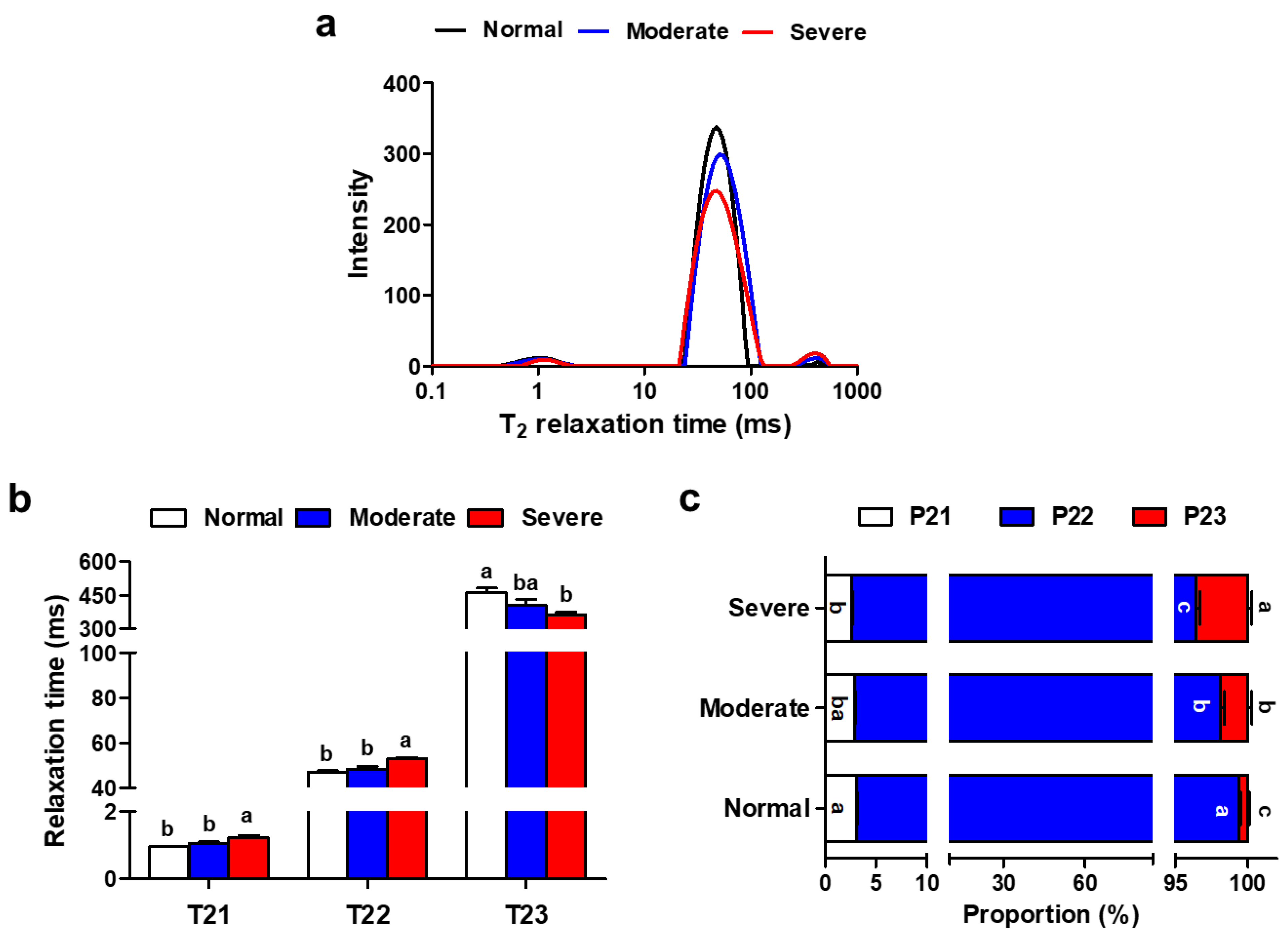
3.5. Water Mobility and Distribution
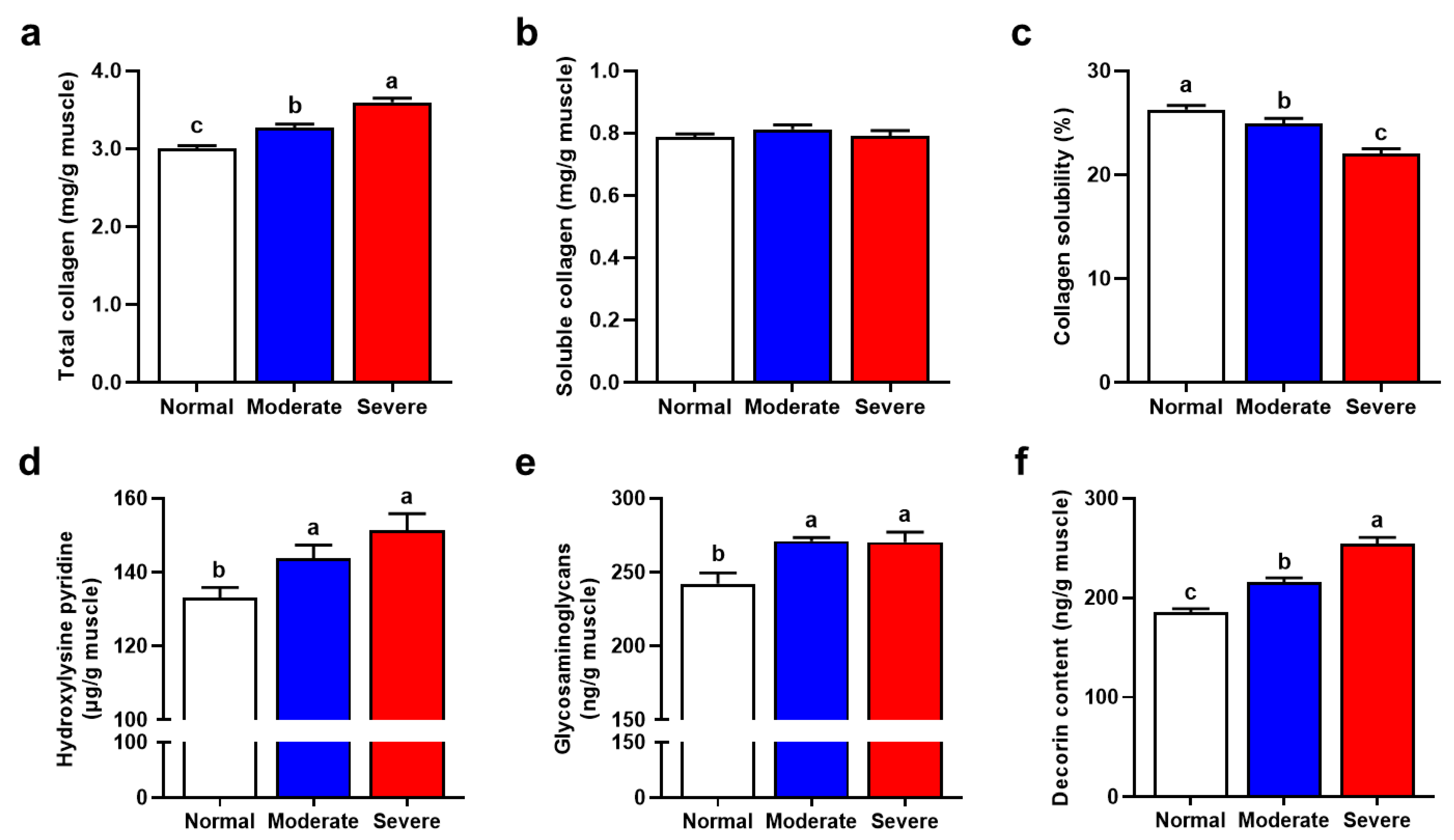
3.6. Collagen Profile
3.7. Major IMCT Components
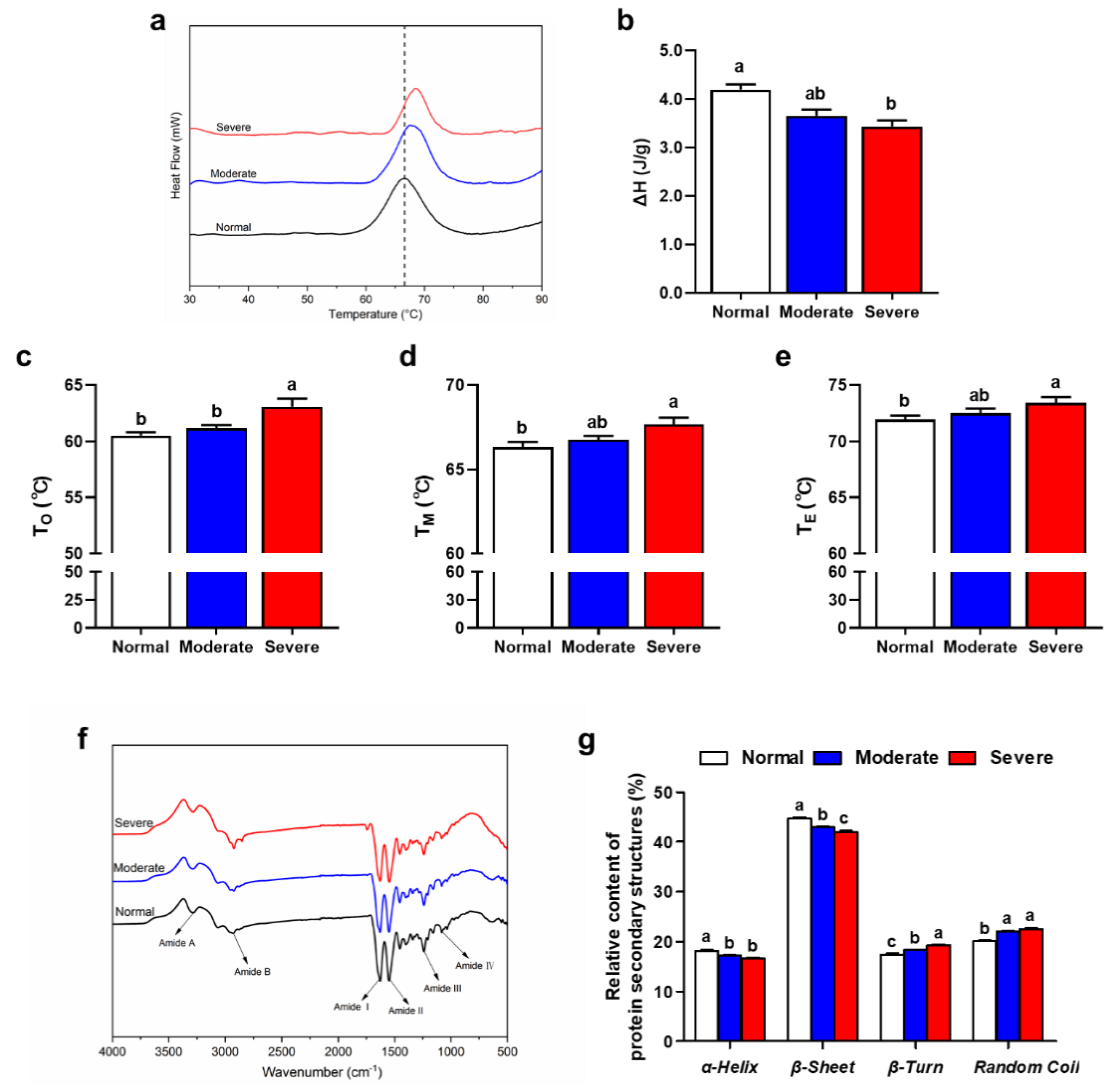
3.8. Thermal Properties of IMCT
3.9. Secondary Structure of IMCT
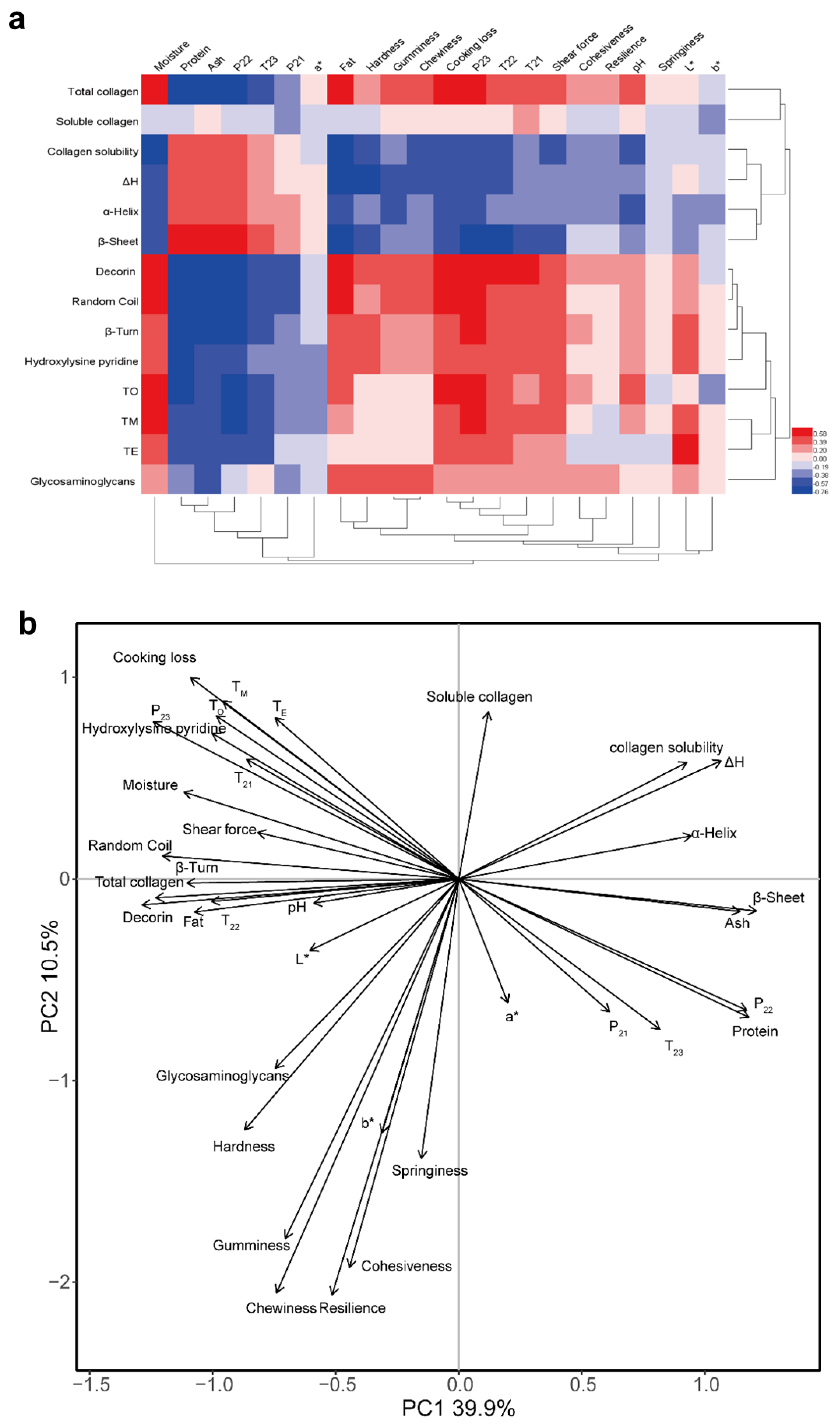
3.10. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petracci, M.; Soglia, F.; Madruga, M.; Carvalho, L.; Ida, E.; Estévez, M. Wooden-Breast, White Striping, and Spaghetti Meat: Causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. Food Saf. 2019, 18, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Sihvo, H.; Immonen, K.; Puolanne, E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014, 51, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Tijare, V.V.; Yang, F.L.; Kuttappan, V.A.; Alvarado, C.Z.; Coon, C.N.; Owens, C.M. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 2016, 95, 2167–2173. [Google Scholar] [CrossRef]
- Lake, J.A.; Abasht, B. Glucolipotoxicity: A Proposed Etiology for Wooden Breast and Related Myopathies in Commercial Broiler Chickens. Front. Physiol. 2020, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Hosotani, M.; Kawasaki, T.; Hasegawa, Y.; Wakasa, Y.; Hoshino, M.; Takahashi, N.; Ueda, H.; Takaya, T.; Iwasaki, T.; Watanabe, T. Physiological and Pathological Mitochondrial Clearance Is Related to Pectoralis Major Muscle Pathogenesis in Broilers With Wooden Breast Syndrome. Front. Physiol. 2020, 11, 579. [Google Scholar] [CrossRef]
- Velleman, S.G.; Clark, D.L.; Tonniges, J.R. Fibrillar collagen organization associated with broiler wooden breast fibrotic myopathy. Avian Dis. 2017, 61, 481–490. [Google Scholar] [CrossRef]
- Xing, T.; Zhao, Z.; Zhao, X.; Xu, X.; Zhang, L.; Gao, F. Enhanced transforming growth factor-beta signaling and fibrosis in the pectoralis major muscle of broiler chickens affected by wooden breast myopathy. Poult. Sci. 2021, 100, 100804. [Google Scholar] [CrossRef]
- Brambila, G.S.; Chatterjee, D.; Bowker, B.; Zhuang, H. Descriptive texture analyses of cooked patties made of chicken breast with the woody breast condition. Poult. Sci. 2017, 96, 3489–3494. [Google Scholar] [CrossRef]
- Chen, H.; Wang, H.; Qi, J.; Wang, M.; Xu, X.; Zhou, G. Chicken breast quality—normal, pale, soft and exudative (PSE) and woody—influences the functional properties of meat batters. Int. J. Food Sci. Technol. 2018, 53, 654–664. [Google Scholar] [CrossRef]
- Velleman, S.G. Pectoralis major (breast) muscle extracellular matrix fibrillar collagen modifications associated with the wooden breast fibrotic myopathy in broilers. Front. Physiol. 2020, 11, 461. [Google Scholar] [CrossRef]
- Purslow, P.P. Contribution of collagen and connective tissue to cooked meat toughness; some paradigms reviewed. Meat Sci. 2018, 144, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Lepetit, J. A theoretical approach of the relationships between collagen content, collagen cross-links and meat tenderness. Meat Sci. 2007, 76, 147–159. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef] [PubMed]
- Latorre, M.E.; Palacio, M.I.; Velazquez, D.E.; Purslow, P.P. Specific effects on strength and heat stability of intramuscular connective tissue during long time low temperature cooking. Meat Sci. 2019, 153, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T. Role of extracellular matrix in development of skeletal muscle and postmortem aging of meat. Meat Sci. 2015, 109, 48–55. [Google Scholar] [CrossRef]
- Kuttappan, V.; Owens, C.; Coon, C.; Hargis, B.; Vazquez-Añon, M. Incidence of broiler breast myopathies at 2 different ages and its impact on selected raw meat quality parameters. Poult. Sci. 2017, 96, 3005–3009. [Google Scholar] [CrossRef]
- Zhu, X.; Puolanne, E.; Ertbjerg, P. Changes of raw texture, intramuscular connective tissue properties and collagen profiles in broiler wooden breast during early storage. Foods 2023, 12, 1530. [Google Scholar] [CrossRef]
- Li, C.B.; Zhou, G.H.; Xu, X.L. Dynamical changes of beef intramuscular connective tissue and muscle fiber during heating and their effects on beef shear force. Food Bioprocess Technol. 2010, 3, 521–527. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC, 17th ed.; 2nd revision; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Xing, T.; Zhao, X.; Zhang, L.; Li, J.L.; Zhou, G.H.; Xu, X.L.; Gao, F. Characteristics and incidence of broiler chicken wooden breast meat under commercial conditions in China. Poult. Sci. 2020, 99, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Zhao, X.; Xu, X.; Li, J.; Zhang, L.; Gao, F. Physiochemical properties, protein and metabolite profiles of muscle exudate of chicken meat affected by wooden breast myopathy. Food Chem. 2020, 316, 126271. [Google Scholar] [CrossRef] [PubMed]
- Dondero, M.; Figueroa, V.; MoraleS, X.; Curotto, E. Transglutaminase effects on gelation capacity of thermally induced beef protein gels. Food Chem. 2006, 99, 546–554. [Google Scholar] [CrossRef]
- Xing, T.; Zhao, X.; Han, M.; Cai, L.; Deng, S.; Zhou, G.; Xu, X. A Comparative study of functional properties of normal and wooden breast broiler chicken meat with nacl addition. Poult. Sci. 2017, 96, 3473–3481. [Google Scholar] [CrossRef]
- Latorre, M.E.; Lifschitz, A.L.; Purslow, P.P. New recommendations for measuring collagen solubility. Meat Sci. 2016, 118, 78–81. [Google Scholar] [CrossRef]
- Nishimura, T.; Hattori, A.; Takahashi, K. Structural changes in intramuscular connective tissue during the fattening of japanese black cattle: Effect of marbling on beef tenderization. J. Anim. Sci. 1999, 77, 93–104. [Google Scholar] [CrossRef]
- Du, X.; Li, H.; Nuerjiang, M.; Shi, S.; Kong, B.; Liu, Q.; Xia, X. Application of ultrasound treatment in chicken gizzards tenderization: Effects on muscle fiber and connective tissue. Ultrason. Sonochemistry 2021, 79, 105786. [Google Scholar] [CrossRef]
- Wu, J.-J. Characteristics of Bovine Intramuscular Collagen under Various Postmortem Conditions. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 1978. [Google Scholar]
- Zhang, Y.; Huang, M.; Shao, X.; Zhang, F.; Li, Z.; Bai, Y.; Xu, X.; Wang, P.; Zhao, T. Insights into intramuscular connective tissue associated with wooden breast myopathy in fast-growing broiler chickens. Foods 2023, 12, 2375. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zhang, J.; Zheng, J.; Li, X.; Shao, J.-H. The study of protein conformation and hydration characteristics of meat batters at various phase transition temperatures combined with low-field nuclear magnetic resonance and fourier transform infrared spectroscopy. Food Chem. 2019, 280, 263–269. [Google Scholar] [CrossRef]
- Che, S.; Wang, C.; Iverson, M.; Varga, C.; Barbut, S.; Bienzle, D.; Susta, L. Characteristics of Broiler Chicken Breast Myopathies (Spaghetti Meat, Woody Breast, White Striping) in Ontario, Canada. Poult. Sci. 2022, 101, 101747. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Li, Z.; Zheng, Z.; Xu, X.; Wang, P.; Zheng, B.; Qi, Z. Correlation between instrumental stress and oral processing property of chicken broiler breast under wooden breast myopathy. Int. J. Food Sci. Technol. 2021, 56, 5518–5532. [Google Scholar] [CrossRef]
- Matarneh, S.K.; Silva, S.L.; Gerrard, D.E. New insights in muscle biology that alter meat quality. Annu. Rev. Anim. Biosci. 2021, 9, 355–377. [Google Scholar] [CrossRef]
- Velleman, S.G.; Clark, D.L. Histopathologic and myogenic gene expression changes associated with wooden breast in broiler breast muscles. Avian Dis. 2015, 59, 410–418. [Google Scholar] [CrossRef]
- Mazzoni, M.; Petracci, M.; Meluzzi, A.; Cavani, C.; Clavenzani, P.; Sirri, F. Relationship between pectoralis major muscle histology and quality traits of chicken meat. Poult. Sci. 2015, 94, 123–130. [Google Scholar] [CrossRef]
- Tasoniero, G.; Cullere, M.; Cecchinato, M.; Puolanne, E.; Zotte, A.D. Technological quality, mineral profile, and sensory attributes of broiler chicken breasts affected by white striping and wooden breast myopathies. Poult. Sci. 2016, 95, 2707–2714. [Google Scholar] [CrossRef]
- Abasht, B.; Mutryn, M.F.; Michalek, R.D.; Lee, W.R. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PLoS ONE 2016, 11, e0153750. [Google Scholar] [CrossRef]
- Soglia, F.; Laghi, L.; Canonico, L.; Cavani, C.; Petracci, M. Functional property issues in broiler breast meat related to emerging muscle abnormalities. Food Res. Int. 2016, 89, 1071–1076. [Google Scholar] [CrossRef]
- Chatterjee, D.; Zhuang, H.; Bowker, B.; Rincon, A.; Sanchez-Brambila, G. Instrumental texture characteristics of broiler pectoralis major with the wooden breast condition1. Poult. Sci. 2016, 95, 2449–2454. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y. Perception and measurement of food texture: Solid Foods. J. Texture Stud. 2018, 49, 160–201. [Google Scholar] [CrossRef]
- Thanatsang, K.V.; Malila, Y.; Arayamethakorn, S.; Srimarut, Y.; Tatiyaborworntham, N.; Uengwetwanit, T.; Panya, A.; Rungrassamee, W.; Visessanguan, W. Nutritional properties and oxidative indices of broiler breast meat affected by wooden breast abnormality. Animals 2020, 10, 2272. [Google Scholar] [CrossRef]
- Maxwell, A.; Bowker, B.; Zhuang, H.; Chatterjee, D.; Adhikari, K. Descriptive sensory analysis of marinated and non-marinated wooden breast fillet portions. Poult. Sci. 2018, 97, 2971–2978. [Google Scholar] [CrossRef] [PubMed]
- Bertram, H.; Purslow, P.; Andersen, H. Relationship between meat structure, water mobility, and distribution: A low-field nuclear magnetic resonance study. J. Agric. Food Chem. 2002, 50, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Velleman, S. Spatial influence on breast muscle morphological structure, myofiber size, and gene expression associated with the wooden breast myopathy in broilers. Poult. Sci. 2016, 95, 2930–2945. [Google Scholar] [CrossRef]
- Monsón, F.; Sañudo, C.; Sierra, I. Influence of cattle breed and ageing time on textural meat quality. Meat Sci. 2004, 68, 595–602. [Google Scholar] [CrossRef]
- Li, X.; Ha, M.; Warner, R.D.; Dunshea, F.R. Meta-analysis of the relationship between collagen characteristics and meat tenderness. Meat Sci. 2022, 185, 108717. [Google Scholar] [CrossRef]
- Kong, F.; Tang, J.; Lin, M.; Rasco, B. THermal effects on chicken and salmon muscles: Tenderness, cook loss, area shrinkage, collagen solubility and microstructure. LWT-Food Sci. Technol. 2008, 41, 1210–1222. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, Y.; Xiao, Y.; Chen, H.; Zhao, L.; Huang, M.; Zhou, G. Differences in physicochemical and nutritional properties of breast and thigh meat from crossbred chickens, commercial broilers, and spent hens. Asian-Australas. J. Anim. Sci. 2016, 29, 855–864. [Google Scholar] [CrossRef]
- Zimmerman, S.; Thomas, D.; Velleman, S.; Li, X.; Thomas, R.; McCormick, R. Time course of collagen and decorin changes in rat cardiac and skeletal muscle post-MI. Am. J. Physiol.-Heart Circ. Physiol. 2001, 281, H1816–H1822. [Google Scholar] [CrossRef]
- McCormick, R. The flexibility of the collagen compartment of muscle. Meat Sci. 1994, 36, 79–91. [Google Scholar] [CrossRef]
- Velleman, S.G. Meat science and muscle biology symposium: Extracellular matrix regulation of skeletal muscle formation. J. Anim. Sci. 2012, 90, 936–941. [Google Scholar] [CrossRef]
- Stoilov, I.; Starcher, B.; Mecham, R.; Broekelmann, T. Measurement of elastin, collagen, and total protein levels in tissues. In Methods In Extracellular Matrix Biology; Mecham, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 143, pp. 133–146. ISBN 0091-679X. [Google Scholar]
- Danielson, K.; Baribault, H.; Holmes, D.; Graham, H.; Kadler, K.; Iozzo, R. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef]
- Cakir, E.; Vinyard, C.J.; Essick, G.; Daubert, C.R.; Drake, M.; Foegeding, E.A. Interrelations among physical characteristics, sensory perception and oral processing of protein-based soft-solid structures. Food Hydrocoll. 2012, 29, 234–245. [Google Scholar] [CrossRef]
- Kopp, J.; Bonnet, M.; Renou, J. Effect of collagen crosslinking on collagen-water interactions (A DSC Investigation). Matrix 1990, 9, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Velleman, S.G.; Clark, D.L.; Tonniges, J.R. The effect of the wooden breast myopathy on sarcomere structure and organization. Avian Dis. 2018, 62, 28–35. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Xiong, S.; Liu, R.; You, J.; Yin, T.; Hu, Y. THe effect of cross-linking degree on physicochemical properties of surimi gel as affected by MTGase. J. Sci. Food Agric. 2021, 101, 6228–6238. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, P.; Han, D.; Huang, F.; Li, X.; Song, Y.; Wang, H.; Liu, J.; Zheng, J.; Zhang, C. Role of intramuscular connective tissue in water holding capacity of porcine muscles. Foods 2022, 11, 3835. [Google Scholar] [CrossRef]
- Florek, M.; Domaradzki, P.; Skalecki, P.; Ryszkowska-Siwko, M.; Ziomek, M.; Tajchman, K.; Gondek, M.; Pyz-Lukasik, R. Content and solubility of collagen and their relation to proximate composition and shear force of meat from different anatomical location in carcass of european beaver (Castor Fiber). Foods 2022, 11, 1288. [Google Scholar] [CrossRef]
- Lepetit, J. Collagen Contribution to Meat Toughness: Theoretical Aspects. Meat Sci. 2008, 80, 960–967. [Google Scholar] [CrossRef]
| Items | Category | SEM | p Value | ||
|---|---|---|---|---|---|
| Normal | Moderate | Severe | |||
| Chemical composition | |||||
| Moisture (%) | 73.29 c | 74.13 b | 75.26 a | 0.24 | <0.001 |
| Protein (%) | 23.75 a | 22.77 b | 21.61 c | 0.21 | <0.001 |
| Fat (%) | 1.21 c | 1.74 b | 2.37 a | 0.14 | <0.001 |
| Ash (%) | 1.47 a | 1.39 b | 1.32 c | 0.01 | <0.001 |
| Meat quality parameters | |||||
| Lightness | 51.72 | 52.63 | 53.38 | 0.7 | ns |
| Redness | 4.44 | 3.85 | 4.3 | 0.36 | ns |
| Yellowness | 6.72 | 6.91 | 6.81 | 0.42 | ns |
| pH | 5.91 b | 5.94 ba | 6.00 a | 0.03 | 0.047 |
| Cooking loss (%) | 14.31 c | 17.67 b | 21.58 a | 0.49 | <0.001 |
| Shear force (N) | 32.78 b | 36.37 b | 45.10 a | 2.36 | 0.003 |
| Textural properties | |||||
| Hardness (g) | 2814.96 b | 3164.68 ba | 3576.05 a | 180.6 | 0.019 |
| Springiness (mm) | 0.55 | 0.55 | 0.56 | 0.02 | ns |
| Cohesiveness | 0.48 | 0.48 | 0.52 | 0.02 | ns |
| Gumminess (g) | 1308.92 b | 1807.60 a | 1860.76 a | 155.7 | 0.029 |
| Chewiness (g·mm) | 739.69 b | 1009.53 ba | 1087.39 a | 101.3 | 0.04 |
| Resilience | 0.22 | 0.24 | 0.25 | 0.03 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, T.; Xing, T.; Zhao, X.; Xu, X. Effects of Wooden Breast Myopathy on Meat Quality Characteristics of Broiler Pectoralis Major Muscle and Its Changes with Intramuscular Connective Tissue. Foods 2024, 13, 507. https://doi.org/10.3390/foods13040507
Bian T, Xing T, Zhao X, Xu X. Effects of Wooden Breast Myopathy on Meat Quality Characteristics of Broiler Pectoralis Major Muscle and Its Changes with Intramuscular Connective Tissue. Foods. 2024; 13(4):507. https://doi.org/10.3390/foods13040507
Chicago/Turabian StyleBian, Tianjiao, Tong Xing, Xue Zhao, and Xinglian Xu. 2024. "Effects of Wooden Breast Myopathy on Meat Quality Characteristics of Broiler Pectoralis Major Muscle and Its Changes with Intramuscular Connective Tissue" Foods 13, no. 4: 507. https://doi.org/10.3390/foods13040507
APA StyleBian, T., Xing, T., Zhao, X., & Xu, X. (2024). Effects of Wooden Breast Myopathy on Meat Quality Characteristics of Broiler Pectoralis Major Muscle and Its Changes with Intramuscular Connective Tissue. Foods, 13(4), 507. https://doi.org/10.3390/foods13040507






