Minced Beef Meat Paste Characteristics: Gel Properties, Water Distribution, and Microstructures Regulated by Medium Molecular Mass of γ-Poly-Glutamic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Ingredients
2.2. Preparation of Different Molecular Weights of γ-PGA
2.3. Molecular Weight Analysis
2.4. Moisturizing Evaluation
2.5. Minced Meat Paste Preparation
2.6. Rheological Properties
2.7. Puncture Test of Gel
2.8. Texture Profile Analysis (TPA)
2.9. Cooking Loss and Water-Holding Capacity (WHC)
2.10. Distributions of Water Molecules
2.11. Color Evaluation
2.12. Scanning Electron Microscopy (SEM)
2.13. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Different Molecular Weights of γ-PGA
3.2. Selection of Degradation Products of γ-PGA with Strong Moisturizing Property
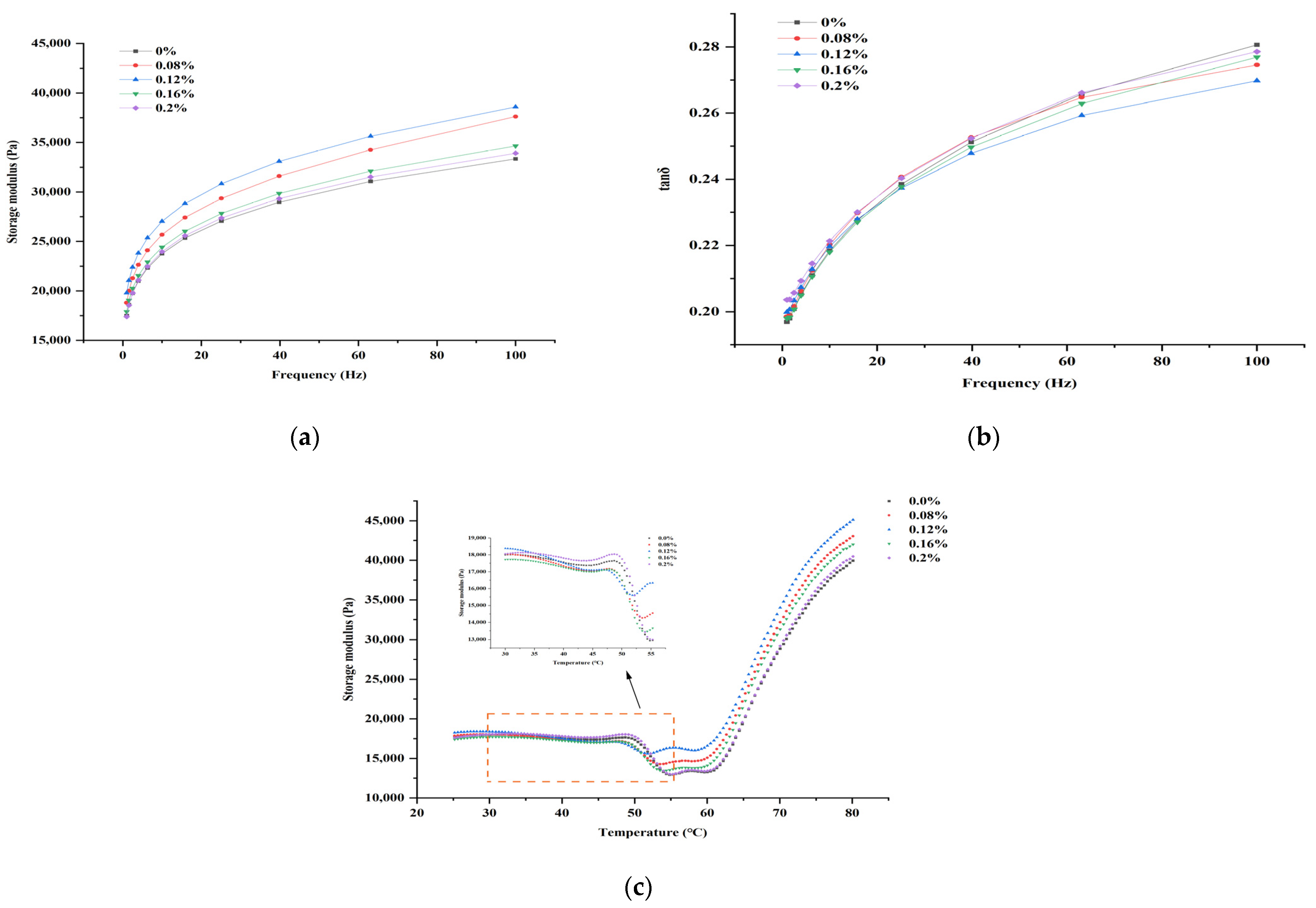
3.3. Rheological Properties during the Gelation Process
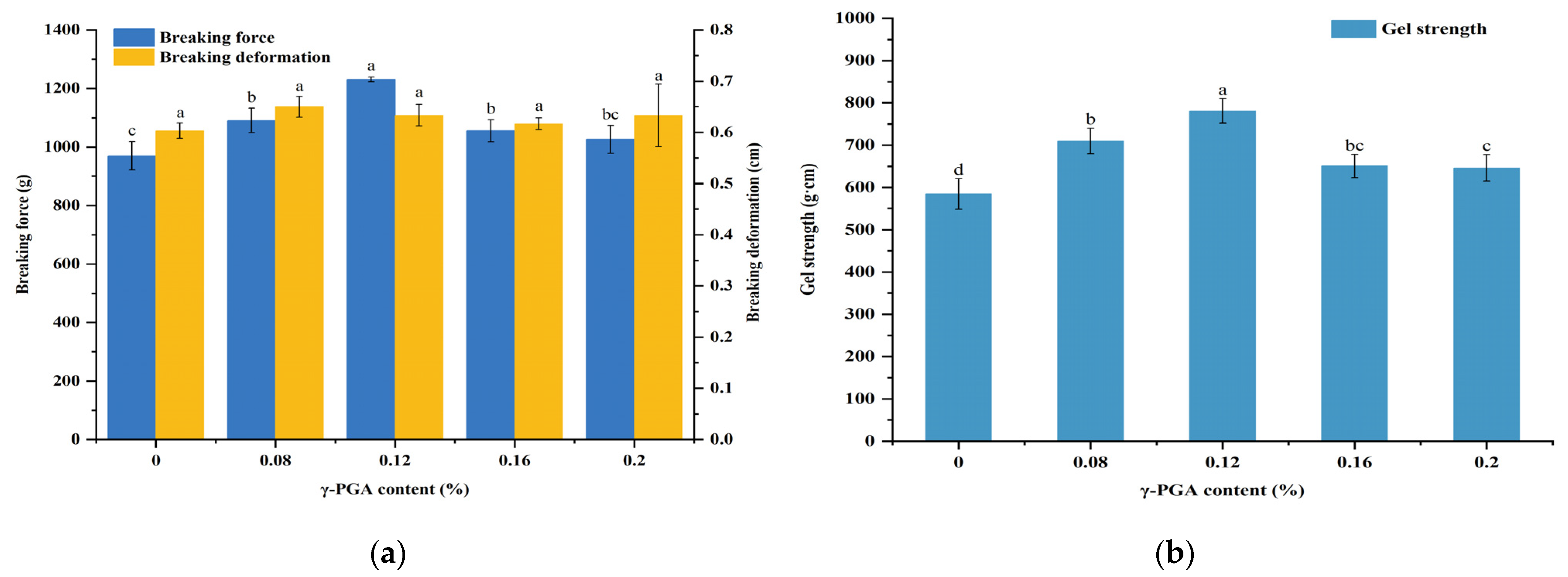
3.4. Influence of the m-γ-PGA Content on Gel Strength
3.5. Effect of m-γ-PGA Content on Gel Texture Properties
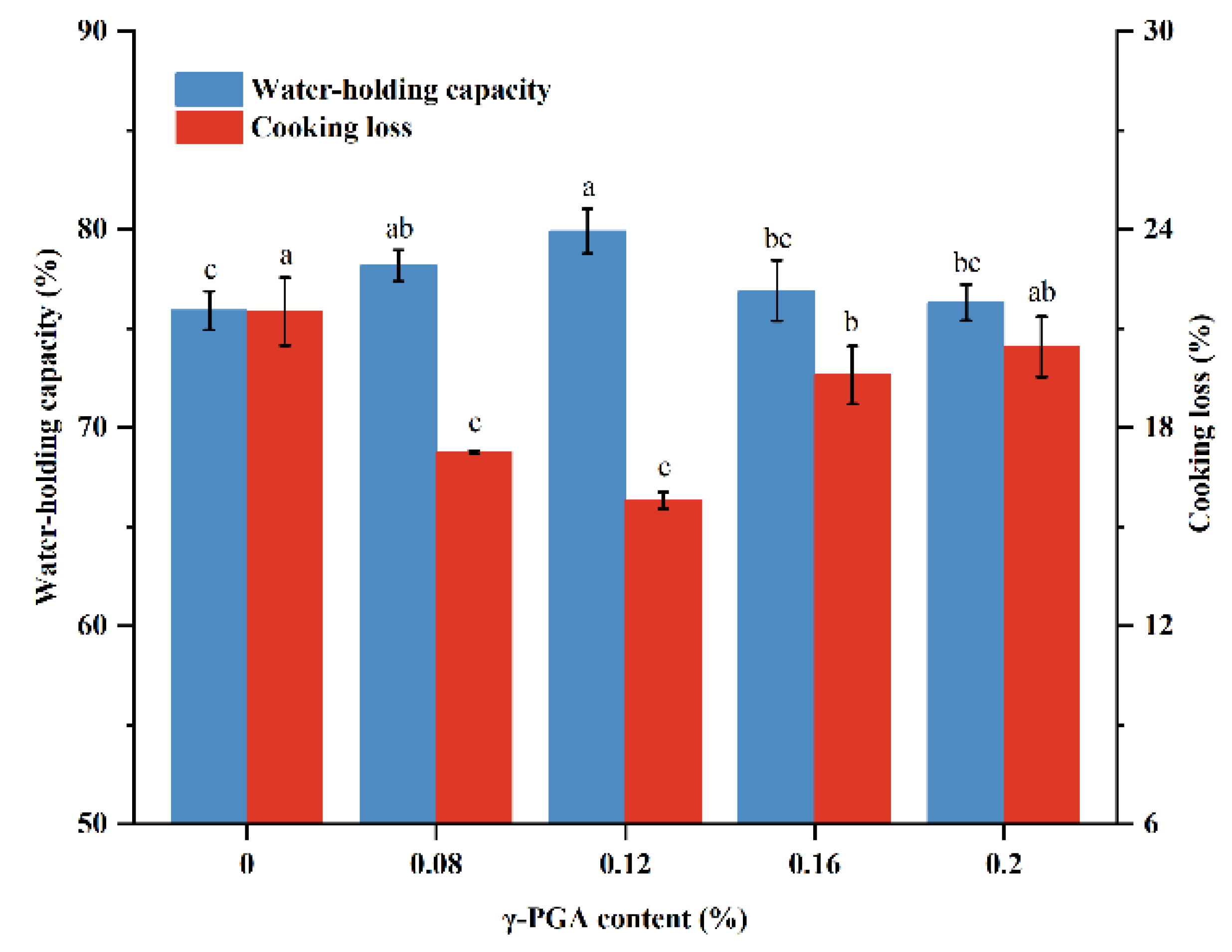
3.6. Cooking Loss and WHC
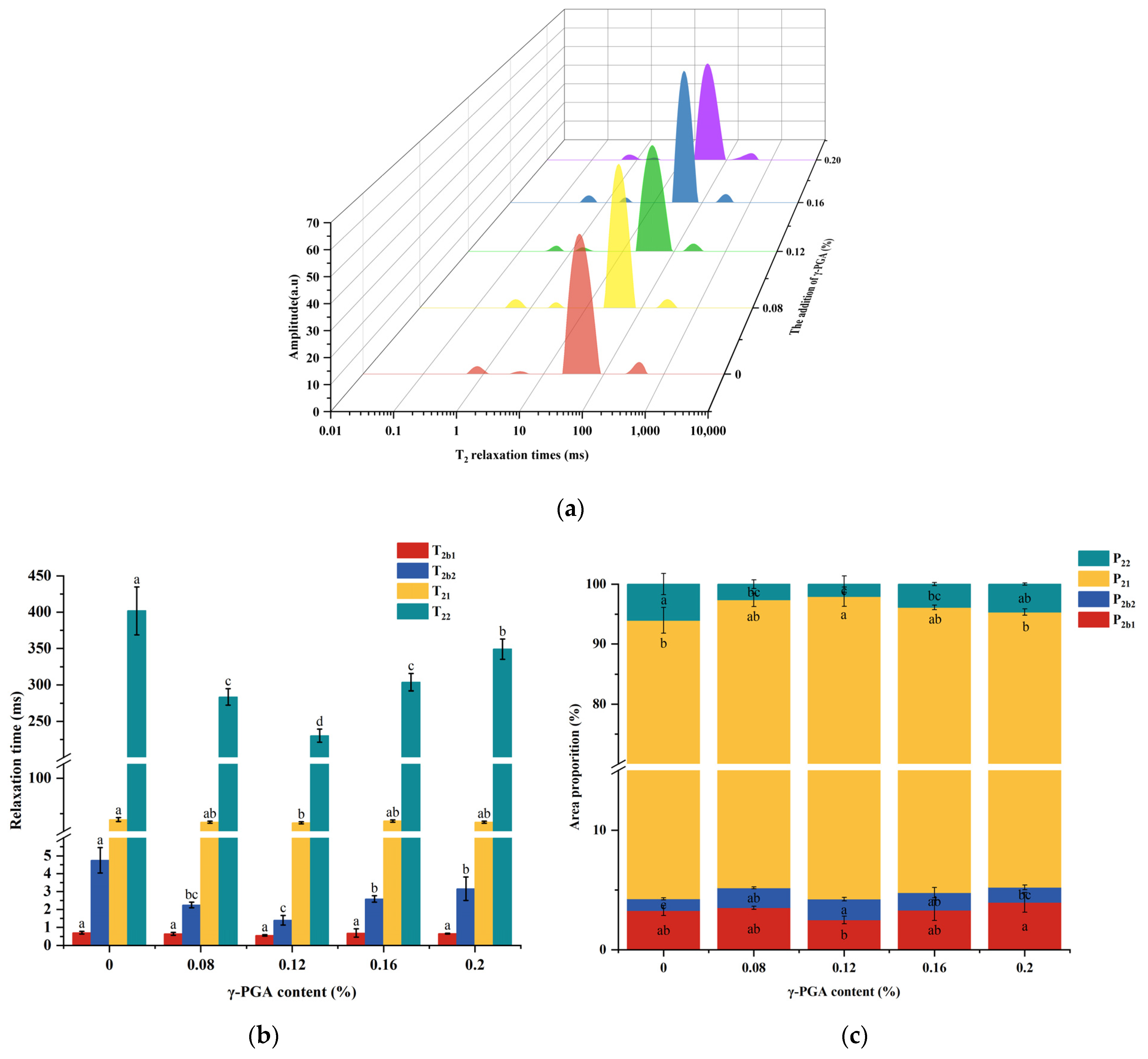
3.7. Distribution of Water in Gel
3.8. Color Evaluation
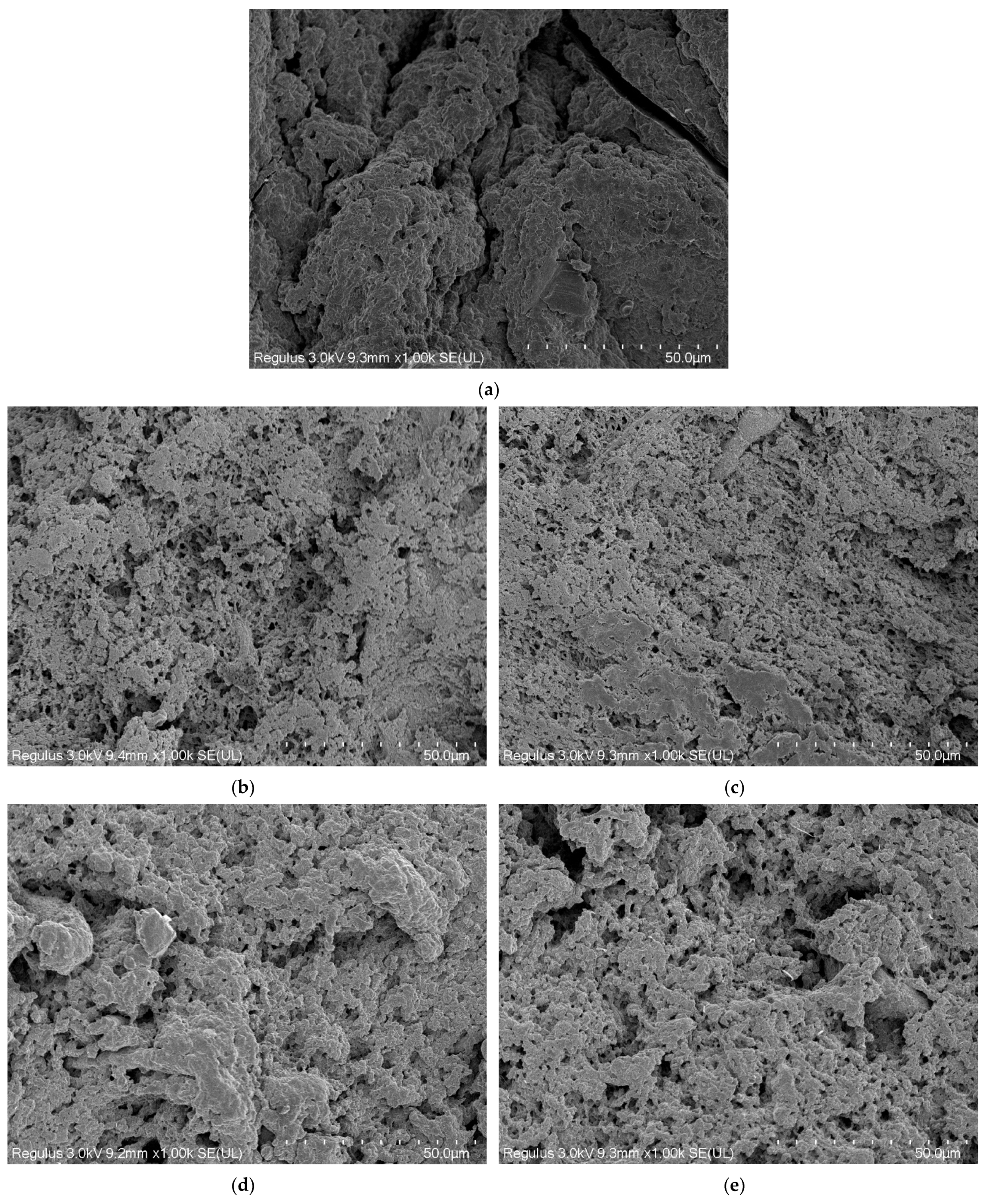
3.9. Microstructural Properties
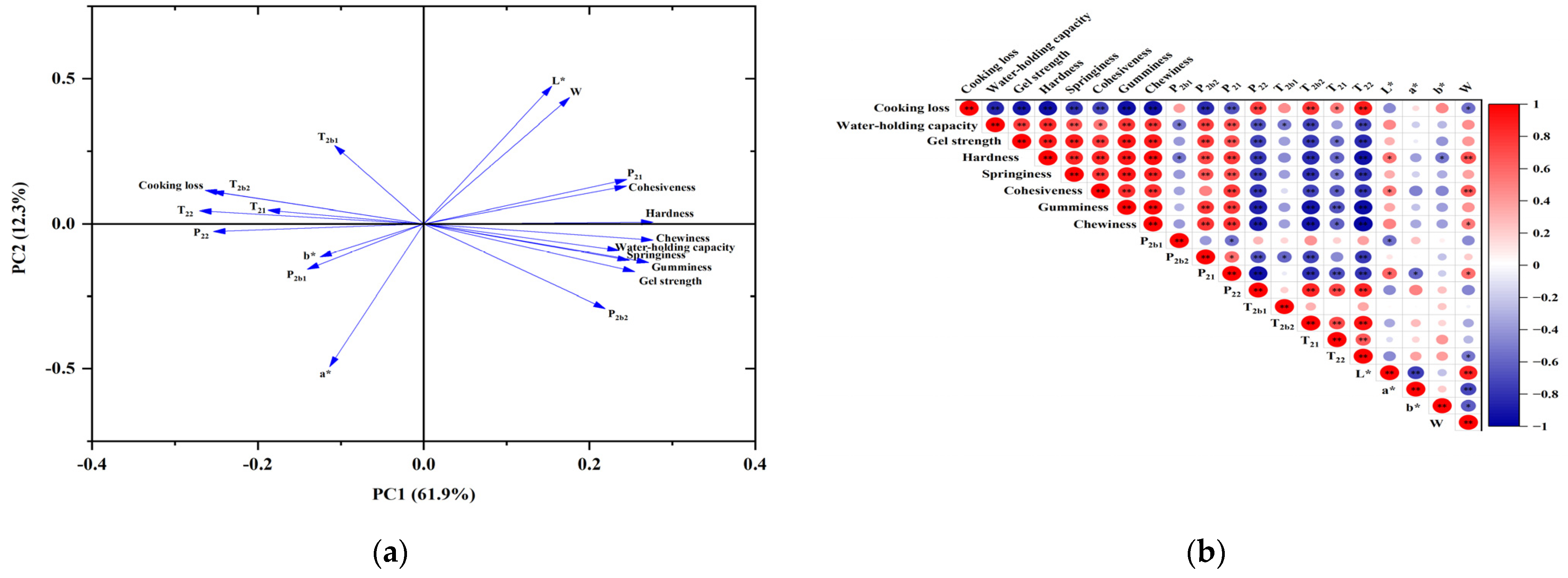
3.10. Principal Component Analysis (PCA) and Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hawley, A.L.; Liang, X.; Børsheim, E.; Wolfe, R.R.; Salisbury, L.; Hendy, E.; Wu, H.; Walker, S.; Tacinelli, A.M.; Baum, J.I. The potential role of beef and nutrients found in beef on outcomes of wellbeing in healthy adults 50 years of age and older: A systematic review of randomized controlled trials. Meat Sci. 2022, 189, 108830. [Google Scholar] [CrossRef]
- Van Wezemael, L.; Caputo, V.; Nayga, R.M.; Chryssochoidis, G.; Verbeke, W. European consumer preferences for beef with nutrition and health claims: A multi-country investigation using discrete choice experiments. Food Policy 2014, 44, 167–176. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, K.; Wang, Y.; Xie, Y.; Wang, Z.; Li, P.; Xu, B. Insight into the mechanism of textural deterioration of myofibrillar protein gels at high temperature conditions. Food Chem. 2020, 330, 127186. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhou, C.; Xia, Q.; Wang, Y.; Geng, F.; He, J.; Sun, Y.; Pan, D.; Cao, J. The effect of fibrin on rheological behavior, gelling properties and microstructure of myofibrillar proteins. LWT 2022, 153, 112457. [Google Scholar] [CrossRef]
- Meng, X.; Wu, D.; Zhang, Z.; Wang, H.; Wu, P.; Xu, Z.; Gao, Z.; Mintah, B.K.; Dabbour, M. An overview of factors affecting the quality of beef meatballs: Processing and preservation. Food Sci. Nutr. 2022, 10, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Ulu, H. Effects of carrageenam and guar gum on the cooking and textual properties of low fat meatballs. Food Chem. 2006, 95, 600–605. [Google Scholar] [CrossRef]
- García-García, E.; Totosaus, A. Low-fat sodium-reduced sausages: Effect of the interaction between locust bean gum, potato starch and κ-carrageenan by a mixture design approach. Meat Sci. 2008, 78, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Hong, S.; Du, Z.; Chao, M.; O’Quinn, T.; Li, Y. Effect of adding modified pea protein as functional extender on the physical and sensory properties of beef patties. LWT 2022, 154, 112774. [Google Scholar] [CrossRef]
- Yi, H.C.; Cho, H.; Hong, J.J.; Ryu, R.K.; Hwang, K.T.; Regenstein, J.M. Physicochemical and organoleptic characteristics of seasoned beef patties with added glutinous rice flour. Meat Sci. 2012, 92, 464–468. [Google Scholar] [CrossRef]
- Domínguez, R.; Lorenzo, J.M.; Pateiro, M.; Munekata, P.E.S.; Alves dos Santos, B.; Basso Pinton, M.; Cichoski, A.J.; Bastianello Campagnol, P.C. Main animal fat replacers for the manufacture of healthy processed meat products. Crit. Rev. Food Sci. Nutr. 2023, 202, 109215. [Google Scholar] [CrossRef]
- Luo, Z.; Guo, Y.; Liu, J.; Qiu, H.; Zhao, M.; Zou, W.; Li, S. Microbial synthesis of poly-γ-glutamic acid: Current progress, challenges, and future perspectives. Biotechnol. Biofuels 2016, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Navale, G.R.; Dharne, M.S. Poly-gamma-glutamic acid biopolymer: A sleeping giant with diverse applications and unique opportunities for commercialization. Biomass Convers. Biorefin. 2021, 13, 4555–4573. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.R.; Go, T.H.; Lee, S.M.; Jeong, S.Y.; Park, G.T.; Hong, C.O.; Son, H.J. In vitro evaluation of new functional properties of poly-γ-glutamic acid produced by Bacillus subtilis D7. Saudi J. Biol. Sci. 2014, 21, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.R.; Madhan Kumarr, M.M.; Natarajan, G.; Ramana, K.V.; Semwal, A.D. Optimized production of poly (γ-glutamic acid) (γ-PGA) using Bacillus licheniformis and its application as cryoprotectant for probiotics. Biotechnol. Appl. Biochem. 2020, 67, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Halmschlag, B.; Steurer, X.; Putri, S.P.; Fukusaki, E.; Blank, L.M. Tailor-made poly-γ-glutamic acid production. Metab. Eng. 2019, 55, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, A.; Bhat, A.; Irorere, V.U.; Hill, D.; Williams, C.; Radecka, I. Poly-γ-glutamic acid: Production, properties and applications. Microbiology 2015, 161, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, V.; Rahiman, N.; Cabral, H.; Quader, S.; Zirak, M.R.; Taghavizadeh Yazdi, M.E.; Jaafari, M.R.; Alavizadeh, S.H. Poly-γ-glutamic acid nanoparticles as adjuvant and antigen carrier system for cancer vaccination. J. Control. Release 2023, 362, 278–296. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-P.; Chang, Y.-C.; Chen, K.-F.; Wang, C.-H. A field pilot-scale study on heavy metal-contaminated soil washing by using an environmentally friendly agent—Poly-γ-glutamic acid (γ-PGA). Environ. Sci. Pollut. Res. 2019, 27, 34760–34769. [Google Scholar] [CrossRef]
- Guan, E.; Zhang, T.; Wu, K.; Yang, Y.; Bian, K. Physicochemical properties and gluten structures of frozen steamed bread dough under freeze–thaw treatment affected by gamma-polyglutamic acid. Food Hydrocoll. 2023, 137, 108334. [Google Scholar] [CrossRef]
- Lim, S.-M.; Kim, J.; Shim, J.-Y.; Imm, B.-Y.; Sung, M.-H.; Imm, J.-Y. Effect of poly-γ-glutamic acids (PGA) on oil uptake and sensory quality in doughnuts. Food Sci. Biotechnol. 2012, 21, 247–252. [Google Scholar] [CrossRef]
- Sung, M.H.; Park, C.; Kim, C.J.; Poo, H.; Soda, K.; Ashiuchi, M. Natural and edible biopolymer poly-γ-glutamic acid: Synthesis, production, and applications. Chem. Rec. 2005, 5, 352–366. [Google Scholar] [CrossRef]
- Tanimoto, H.; Fox, T.; Eagles, J.; Satoh, H.; Nozawa, H.; Okiyama, A.; Morinaga, Y.; Fairweather-Tait, S.J. Acute effect of poly-γ-glutamic acid on calcium absorption in post-menopausal women. J. Am. Coll. Nutr. 2007, 26, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Wen, J.; He, X.; Shang, Y. Effect of γ-polyglutamic acid on gelation properties of minced chicken breast meat. Food Sci. 2017, 38, 158–164. [Google Scholar] [CrossRef]
- Tao, L.; Tian, L.; Zhang, X.; Huang, X.; Long, H.; Chang, F.; Li, T.; Li, S. Effects of γ-polyglutamic acid on the physicochemical properties and microstructure of grass carp (Ctenopharyngodon idellus) surimi during frozen storage. LWT 2020, 134, 109960. [Google Scholar] [CrossRef]
- Hu, Y.; Shao, Y.; Wu, C.; Yuan, C.; Ishimura, G.; Liu, W.; Chen, S. γ-PGA and MTGase improve the formation of ε-(γ-glutamyl) lysine cross-links within hairtail (Trichiurus haumela) surimi protein. Food Chem. 2018, 242, 330–337. [Google Scholar] [CrossRef]
- Liu, H.; Yan, Q.; Wang, Y.; Li, Y.; Jiang, Z. Efficient production of poly-γ-glutamic acid by Bacillus velezensis via solid-state fermentation and its application. Food Biosci. 2022, 46, 101575. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Z.; He, Y.; Li, C.; Wang, J.; Ma, X. Study on the structure characterization and moisturizing effect of Tremella polysaccharide fermented from GCMCC5.39. Food Sci. Hum. Wellness 2021, 10, 471–479. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, J.J.; Li, K.; Chen, X.; Wang, Y.T.; Bai, Y.H. Evaluation of chickpea protein isolate as a partial replacement for phosphate in pork meat batters: Techno-functional properties and molecular characteristic modifications. Food Chem. 2023, 404, 134585. [Google Scholar] [CrossRef]
- Huang, X.; Sun, L.; Dong, K.; Wang, G.; Luo, P.; Tang, D.; Huang, Q. Mulberry fruit powder enhanced the antioxidant capacity and gel properties of hammered minced beef: Oxidation degree, rheological, and structure. LWT 2022, 154, 112648. [Google Scholar] [CrossRef]
- Yi, S.; Li, Q.; Qiao, C.; Zhang, C.; Wang, W.; Xu, Y.; Mi, H.; Li, X.; Li, J. Myofibrillar protein conformation enhance gel properties of mixed surimi gels with Nemipterus virgatus and Hypophthalmichthys molitrix. Food Hydrocoll. 2020, 106, 105924. [Google Scholar] [CrossRef]
- Qian, Z.; Dong, S.; Zhong, L.; Zhan, Q.; Hu, Q.; Zhao, L. Effects of carboxymethyl chitosan on the gelling properties, microstructure, and molecular forces of Pleurotus eryngii protein gels. Food Hydrocoll. 2023, 145, 109158. [Google Scholar] [CrossRef]
- Xing, T.; Xu, Y.; Qi, J.; Xu, X.; Zhao, X. Effect of high intensity ultrasound on the gelation properties of wooden breast meat with different NaCl contents. Food Chem. 2021, 347, 129031. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, W.; Li, X.P.; Yi, S.M.; Mi, H.B.; Xu, Y.X.; Li, J.R. Gel properties of blue round scad (Decapterus maruadsi) mince as influenced by the addition of egg white powder. J. Texture Stud. 2022, 53, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhao, X.; Li, X.; Huang, Q. Effects of different recovered sarcoplasmic proteins on the gel performance, water distribution and network structure of silver carp surimi. Food Hydrocoll. 2022, 131, 107835. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, M.; Wang, L. Effects of different curing methods on edible quality and myofibrillar protein characteristics of pork. Food Chem. 2022, 387, 132872. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, Y.; Yuan, X.; Zhao, Y.; Kang, Z.; Zhu, M.; Ma, H. Effect of low-frequency alternating magnetic field on the rheological properties, water distribution and microstructure of low-salt pork batters. LWT 2022, 159, 132872. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, G.; Wang, Q.; Zheng, S.; Shu, L.; Liang, Z. Metabolic studies of temperature control strategy on poly (γ-glutamic acid) production in a thermophilic strain Bacillus subtilis GXA-28. Bioresour. Technol. 2014, 155, 104–110. [Google Scholar] [CrossRef]
- Johnson, L.C.; Akinmola, A.T.; Scholz, C. Poly (glutamic acid): From natto to drug delivery systems. Biocatal. Agric. Biotechnol. 2022, 40, 102292. [Google Scholar] [CrossRef]
- Yan, H.; Cai, B.; Cheng, Y.; Guo, G.; Li, D.; Yao, X.; Ni, X.; Phillips, G.O.; Fang, Y.; Jiang, F. Mechanism of lowering water activity of konjac glucomannan and its derivatives. Food Hydrocoll. 2012, 26, 383–388. [Google Scholar] [CrossRef]
- Huang, Q.; Huang, X.; Liu, L.; Song, H.; Geng, F.; Wu, W.; Luo, P. Nano eggshell calcium enhanced gel properties of Nemipterus virgatus surimi sausage: Gel strength, water retention and microstructure. Int. J. Food Sci. Technol. 2021, 56, 5738–5752. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Wang, L.; Zhang, Y.; Tian, H.; Ma, X. Effect of poly-γ-glutamic acid on the stability of set yoghurts. J. Food Sci. Technol. 2018, 55, 4634–4641. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ren, Y.; Zhang, K.; Xiong, Y.L.; Wang, Q.; Shang, K.; Zhang, D. Site-specific incorporation of sodium tripolyphosphate into myofibrillar protein from mantis shrimp (Oratosquilla oratoria) promotes protein crosslinking and gel network formation. Food Chem. 2020, 312, 126113. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Bai, X.; Li, Y.; Kong, B.; Nuerjiang, M.; Wu, K.; Li, Z.; Xia, X. Improvement on gel properties of myofibrillar protein from chicken patty with potato dietary fiber: Based on the change in myofibrillar protein structure and water state. Int. J. Biol. Macromol. 2023, 230, 123228. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wu, X.; Shen, Y.; Song, M.; Xu, C.; Zhang, B.; Aziz, U.; Xu, X. Effect of poly-γ-glutamic acid on hydration and structure of wheat gluten. J. Food Sci. 2020, 85, 3214–3219. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Kunioka, M. Biosynthesis and Hydrolysis of Poly(γ-glutamic acid) from Bacillus subtilis IF03335. Biosci. Biotechnol. Biochem. 2014, 56, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Kim, C.S.; Lee, S.P. Physicochemical properties of poly-γ-glutamic acid produced by a novel Bacillus subtilis HA isolated from Cheonggukjang. Prev. Nutr. Food Sci. 2008, 13, 354–361. [Google Scholar] [CrossRef][Green Version]
- Lee, C.Y.; Kuo, M.I. Effect of γ-polyglutamate on the rheological properties and microstructure of tofu. Food Hydrocoll. 2011, 25, 1034–1040. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Liu, J.; Yu, X. Effects of rice bran feruloyl oligosaccharides on gel properties and microstructure of grass carp surimi. Food Chem. 2023, 407, 135003. [Google Scholar] [CrossRef]
- Luo, L.; Deng, Y.; Liu, G.; Zhou, P.; Zhao, Z.; Li, P.; Zhang, M. Enhancing solubility and reducing thermal aggregation in pea proteins through protein glutaminase-mediated deamidation. Foods 2023, 12, 4130. [Google Scholar] [CrossRef]
- Nakai, S. Structure-function relationships of food proteins: With an emphasis on the importance of protein hydrophobicity. J. Agric. Food Chem. 1983, 31, 676–683. [Google Scholar] [CrossRef]
- Alrosan, M.; Tan, T.C.; Easa, A.M.; Gammoh, S.; Alu’datt, M.H. Molecular forces governing protein-protein interaction: Structure-function relationship of complexes protein in the food industry. Crit. Rev. Food Sci. Nutr. 2021, 62, 4036–4052. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Oiseth, S.K.; Purslow, P.P.; Warner, R.D. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Muriel Mundo, J.L.; Liu, J.; Tan, Y.; Zhou, H.; Zhang, Z.; McClements, D.J. Characterization of electrostatic interactions and complex formation of γ-poly-glutamic acid (PGA) and ε-poly-l-lysine (PLL) in aqueous solutions. Food Res. Int. 2020, 128, 108781. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Q.; Wei, S.; Xia, Q.; Pan, Y.; Liu, S.; Ji, H.; Deng, C.; Hao, J. LF-NMR as a tool for predicting the 3D printability of surimi-starch systems. Food Chem. 2022, 374, 131727. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, H.; Xu, W.; Jia, C.; Peng, Y.; Zhuang, X.; Qi, J.; Xiong, G.; Mei, L.; Xu, X. The effect of water-insoluble dietary fiber from star anise on water retention of minced meat gels. Food Res. Int. 2022, 157, 111425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiong, Y.; Bakry, A.M.; Xiong, S.; Yin, T.; Zhang, B.; Huang, J.; Liu, Z.; Huang, Q. Effect of yeast β-glucan on gel properties, spatial structure and sensory characteristics of silver carp surimi. Food Hydrocoll. 2019, 88, 256–264. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, R.; Li, X.; Wang, X.; Zeng, L.; Wen, X.; Huang, Q. Effect of oil-modified crosslinked starch as a new fat replacer on gel properties, water distribution, and microstructures of pork meat batter. Food Chem. 2023, 409, 135337. [Google Scholar] [CrossRef]
- Rodríguez-Martín, N.M.; Márquez-López, J.C.; Cerrillo, I.; Millán, F.; González-Jurado, J.A.; Fernández-Pachón, M.-S.; Pedroche, J. Production of chickpea protein hydrolysate at laboratory and pilot plant scales: Optimization using principal component analysis based on antioxidant activities. Food Chem. 2024, 437, 137707. [Google Scholar] [CrossRef]

| Samples | Mn (Da) | Mw (Da) | PDI |
|---|---|---|---|
| l-γ-PGA | 155,484 | 340,963 | 2.19 |
| m-γ-PGA | 565,275 | 731,502 | 1.29 |
| h-γ-PGA | 1,240,501 | 1,303,672 | 1.05 |
| m-γ-PGA Content (%) | Hardness (g) | Springiness | Cohesiveness | Gumminess (g) | Chewiness (g) |
|---|---|---|---|---|---|
| 0 | 6008.31 ± 105.74 e | 0.88 ± 0.01 d | 0.68 ± 0.01 c | 4093.49 ± 62.15 d | 3780.67 ± 71.50 e |
| 0.08 | 7011.90 ± 60.65 b | 0.91 ± 0.01 ab | 0.71 ± 0.02 ab | 5184.90 ± 70.37 b | 4683.89 ± 82.90 b |
| 0.12 | 7995.10 ± 84.85 a | 0.92 ± 0.00 a | 0.73 ± 0.01 a | 5437.57 ± 71.53 a | 4989.21 ± 43.46 a |
| 0.16 | 6564.22 ± 10.26 c | 0.90 ± 0.00 c | 0.69 ± 0.02 bc | 4727.90 ± 58.57 c | 4413.54 ± 58.76 c |
| 0.20 | 6307.70 ± 91.44 d | 0.90 ± 0.01 bc | 0.69 ± 0.01 bc | 4673.07 ± 83.47 c | 4084.28 ± 86.60 d |
| γ-PGA Content (%) | L* | a* | b* | W |
|---|---|---|---|---|
| 0 | 64.45 ± 0.34 ab | 3.00 ± 0.34 a | 13.55 ± 0.36 ab | 61.83 ± 0.45 b |
| 0.08 | 64.40 ± 0.32 ab | 3.08 ± 0.14 a | 13.70 ± 0.28 a | 61.73 ± 0.22 b |
| 0.12 | 64.81 ± 0.15 a | 2.87 ± 0.05 a | 12.50 ± 0.37 b | 62.54 ± 0.20 a |
| 0.16 | 64.13 ± 0.33 b | 3.05 ± 0.30 a | 14.23 ± 0.31 a | 61.29 ± 0.41 bc |
| 0.20 | 63.43 ± 0.26 c | 3.26 ± 0.12 a | 13.32 ± 1.06 ab | 60.94 ± 0.40 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, M.; Zhang, T.; Miao, M. Minced Beef Meat Paste Characteristics: Gel Properties, Water Distribution, and Microstructures Regulated by Medium Molecular Mass of γ-Poly-Glutamic Acid. Foods 2024, 13, 510. https://doi.org/10.3390/foods13040510
Qiao M, Zhang T, Miao M. Minced Beef Meat Paste Characteristics: Gel Properties, Water Distribution, and Microstructures Regulated by Medium Molecular Mass of γ-Poly-Glutamic Acid. Foods. 2024; 13(4):510. https://doi.org/10.3390/foods13040510
Chicago/Turabian StyleQiao, Mengmeng, Tao Zhang, and Ming Miao. 2024. "Minced Beef Meat Paste Characteristics: Gel Properties, Water Distribution, and Microstructures Regulated by Medium Molecular Mass of γ-Poly-Glutamic Acid" Foods 13, no. 4: 510. https://doi.org/10.3390/foods13040510
APA StyleQiao, M., Zhang, T., & Miao, M. (2024). Minced Beef Meat Paste Characteristics: Gel Properties, Water Distribution, and Microstructures Regulated by Medium Molecular Mass of γ-Poly-Glutamic Acid. Foods, 13(4), 510. https://doi.org/10.3390/foods13040510






