Optimization and Characterization of Interspecific Hybrid Crude Palm Oil Unaué HIE OxG Nanoparticles with Vegetable By-Products as Encapsulants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
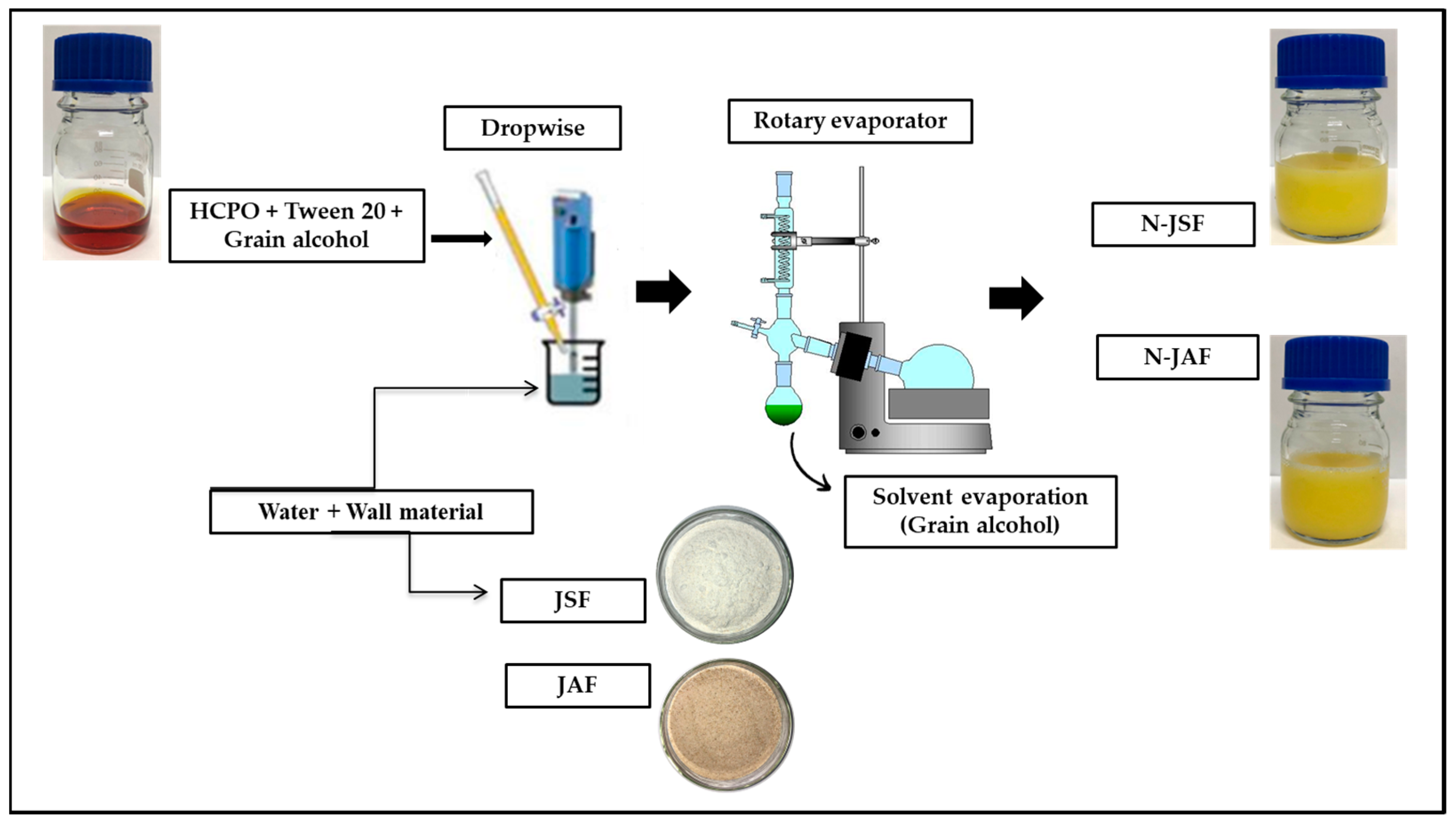
2.2.1. Preparation of Encapsulants
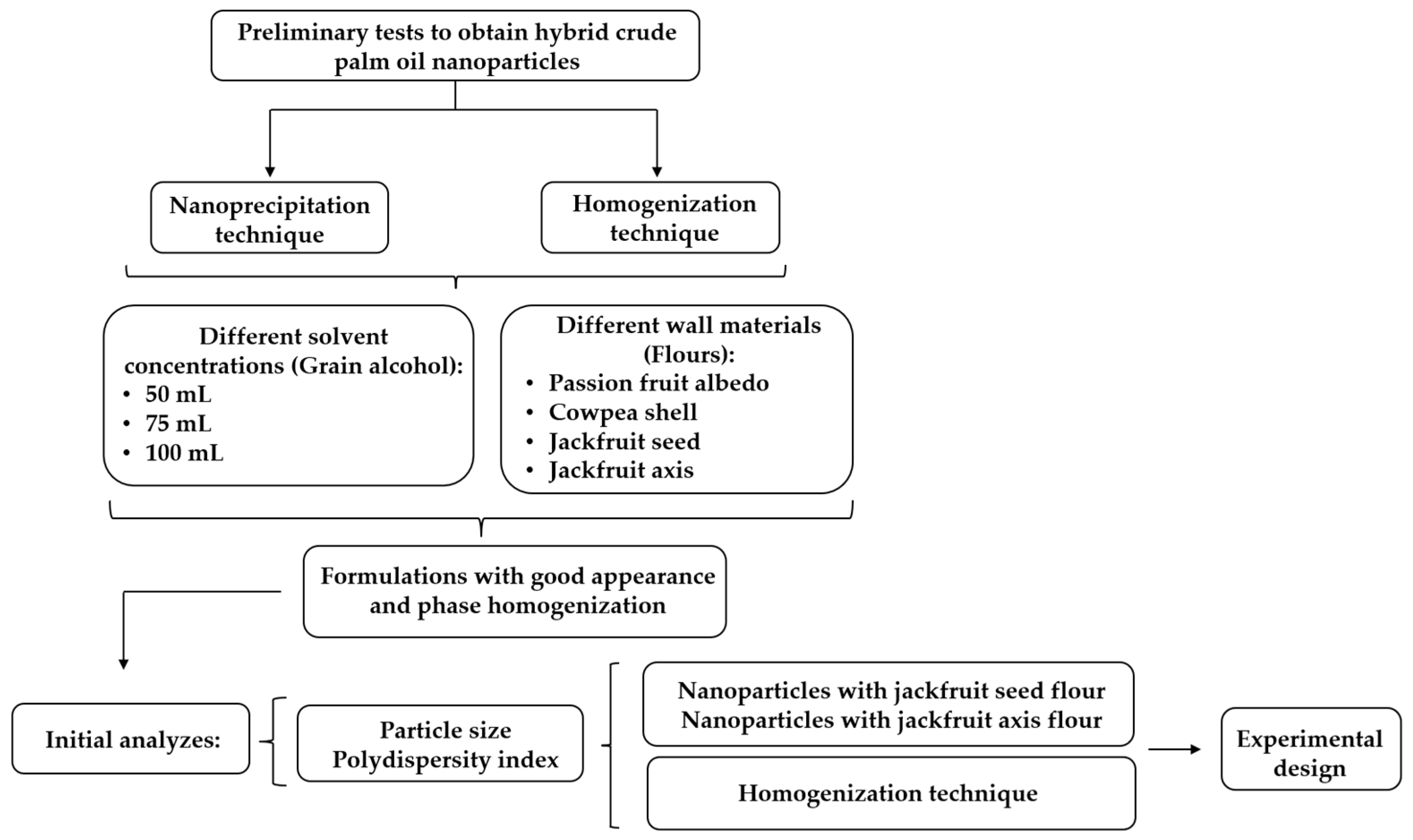
2.2.2. Preliminary Tests for the Formulation of Hybrid Crude Palm Oil Nanoparticles (N-HCPO)
2.2.3. Optimization of the Procedure to Obtain N-OPBH Using 22 Factorial Design
2.2.4. Particle Size, Polydispersity Index (PDI), and Zeta Potential (ζ)
2.2.5. Encapsulation Efficiency (EE)
2.2.6. Transmission Electron Microscopy (TEM)
2.2.7. Apparent Viscosity, pH, Color Parameters, and Total Carotenoids (TC)
2.2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.9. Statistical Analysis
3. Results and Discussion
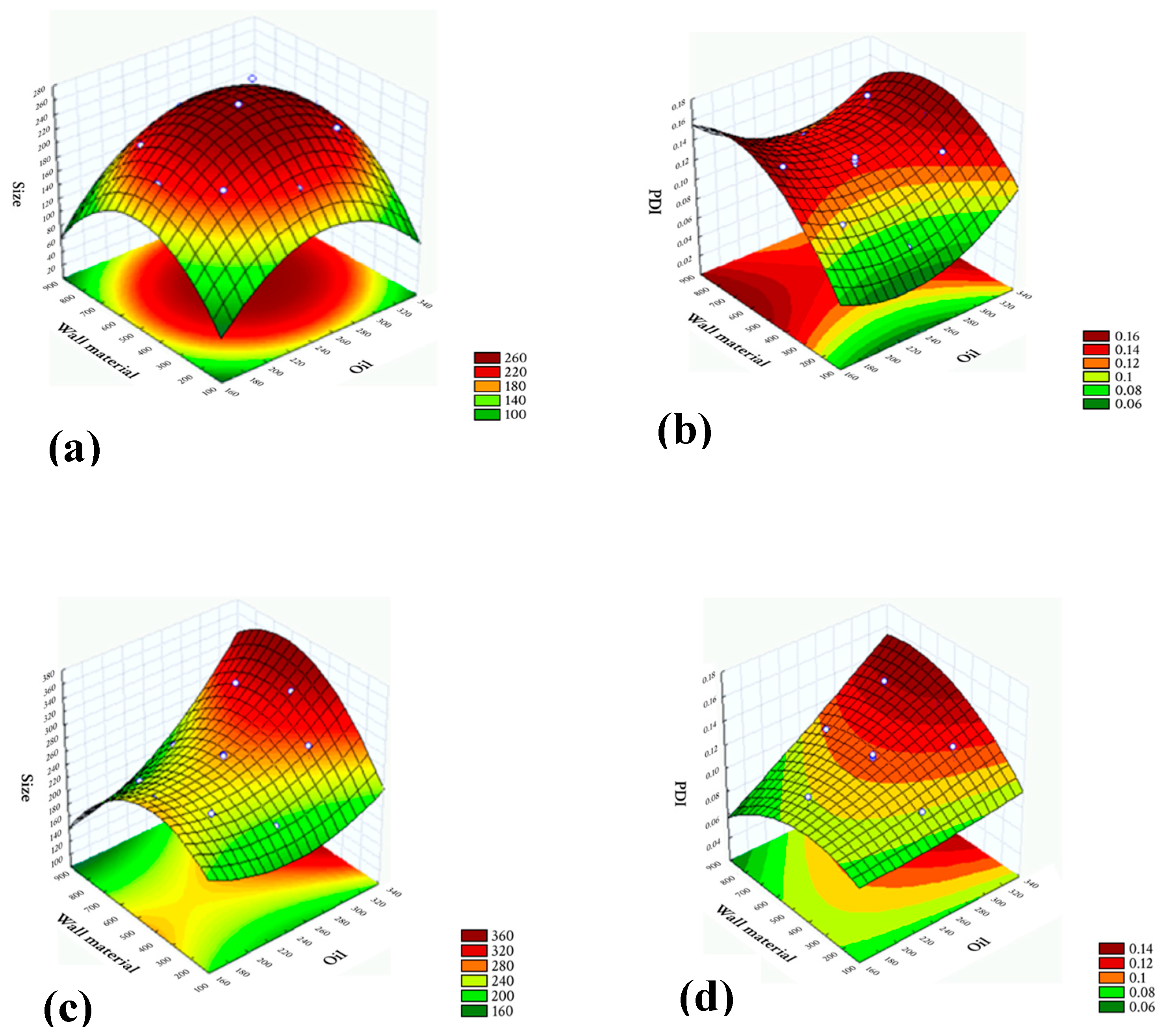
3.1. Optimization of the Procedure for Obtaining N-JSF and N-JAF via 22 Factorial Design
3.2. Characterization of N-JSF and N-JAF
3.2.1. Zeta Potential (ζ)
3.2.2. Encapsulation Efficiency (EE)
3.2.3. Morphology
3.2.4. Apparent Viscosity, pH, Color Parameters, and Total Carotenoids
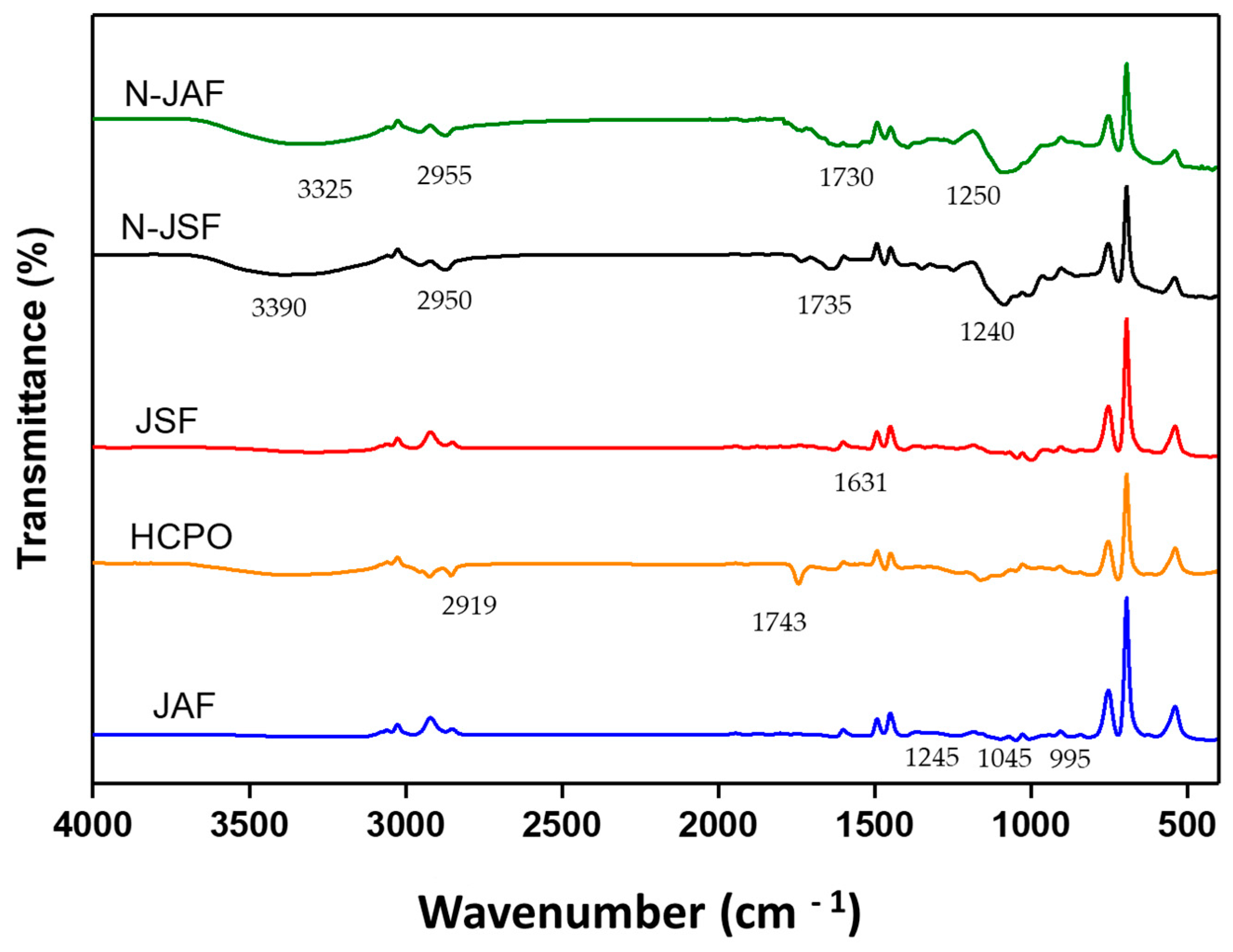
3.2.5. Fourier Transform Infrared Spectroscopy (FTIR)
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT—Crops. Roma. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 November 2023).
- United States Department of Agriculture (USDA). Brazil Palm Oil: Potential and Pitfalls about. In Commodity Intelligence Report; United States Department of Agriculture (USDA): Washington, DC, USA, 2022; Volume 19, pp. 1–9. Available online: https://ipad.fas.usda.gov/highlights/2022/10/Brazil/index.pd (accessed on 12 November 2023).
- Romero, H.M.; Daza, E.; Ayala-Díaz, I.; Ruiz-Romero, R. High-Oleic Palm Oil (HOPO) Production from Parthenocarpic fruits in oil palm interspecific hybrids using naphthalene acetic acid. Agronomy 2021, 11, 290. [Google Scholar] [CrossRef]
- Rodríguez, J.C.; Gómez, D.; Pacetti, D.; Núnnez, O.; Gagliardi, R.; Frega, N.G.; Ojeda, M.L.; Loizzo, M.R.; Tundis, R.; Lucci, P. Effects of the Fruit Ripening Stage on Antioxidant Capacity, Total Phenolics, and Polyphenolic Composition of Crude Palm Oil from Interspecific Hybrid Elaeis oleifera × Elaeis guineensis. J. Agric. Food Chem. 2016, 64, 852–859. [Google Scholar] [CrossRef]
- Pinto, S.S.; Lopes, R.; da Cunha, R.N.V.; dos Santos Filho, L.P.; Moura, J.I.L. Produção e Composição de Cachos e Incidência do Anel Vermelho em Híbridos Interespecíficos de Caiaué com Dendezeiro no Sul da Bahia. Agrotrópica 2019, 31, 5–16. [Google Scholar] [CrossRef]
- da Cunha, R.N.V.; Lopes, R. BRS Manicoré: Híbrido Interespecífico entre o Caiaué e o Dendezeiro Africano Recomendado para Áreas de Incidência de Amarelecimento-Fatal; Techinical Communication; Embrapa Agroindústria de Alimentos: Rio de Janeiro, Brazil, 2010. [Google Scholar]
- Antoniassi, R.; Machado, A.D.F.; Wilhelm, A.E.; Guedes, A.M.M.; Bizzo, H.R.; Oliveira, M.E.C.; Yokoyama, R.; Lopes, R. Óleo de Palma de Alto Oleico Produzido no Brasil no Ano de 2016; Techinical Communication; Embrapa Agroindústria de Alimentos: Rio de Janeiro, Brazil, 2018. [Google Scholar]
- de Almeida, E.S.; Damaceno, D.S.; Carvalho, L.; Victor, P.A.; dos Passos, R.M.; Pontes, P.V.A.; Cunha-Filho, M.; Sampaio, K.A.; Monteiro, S. Thermal and physical Properties of crude palm oil with higher oleic contente. Appl. Sci. 2021, 11, 7094. [Google Scholar] [CrossRef]
- Lucci, P.; Borrero, M.; Ruiz, A.; Pacetti, D.; Frega, N.G.; Diez, O.; Ojeda, M.; Gagliardi, R.; Parra, L.; Angel, M. Palm oil and cardiovascular disease: A randomized trial of the effects of hybrid palm oil supplementation on human plasma lipid patterns. Food Funct. 2016, 7, 347–354. [Google Scholar] [CrossRef]
- Choon, H.T.; Lee, C.J.; Tan, S.N.; Poon, D.T.S.; Chong, C.Y.E.; Pui, L.P. Red Palm Oil: A Review on Processing, Health Benefits and Its Application in Food. J. Oleo Sci. 2021, 70, 1201–1210. [Google Scholar] [CrossRef]
- Sambanthamurthi, R.; Sundram, K.; Tan, Y. Chemistry, and biochemistry of palm oil. Prog. Lipid Res. 2000, 39, 507–558. [Google Scholar] [CrossRef]
- CODEX STAN 210—CXS 210-199; Codex Standard for Named Vegetable Oils. Codex Alimentarius: Rome, Italy, 2023.
- Mozzon, M.; Foligni, R.; Tylewicz, U. Chemical Characteristics and Nutritional Properties of Hybrid Palm Oils. In Palm Oils; Waisundara, V., Ed.; Intechopen: London, UK, 2018; Volume 9, pp. 149–170. [Google Scholar]
- Ferreira Ribeiro, C.D.; Schappo, F.B.; da Silva Sales, I.; Assunção, L.S.; Otero, D.M.; Magalhães-Guedes, K.T.; Machado, B.A.S.; Block, J.M.; Druzian, J.I.; Nunes, I.L. Novel bioactive nanoparticles from crude palm oil and its fractions as foodstuff ingredients. Food Chem. 2022, 373, 131252. [Google Scholar] [CrossRef]
- Voora, V.; Larrea, C.; Bermudez, S.; Baliño, S. Global Market Report: Palm Oil Prices and Sustainability; International Institute for Sustainable Development: Winnipeg, MB, Cananda, 2023. [Google Scholar]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Azizi, M.H. Nanoencapsulation Approach to Improve Antimicrobial and Antioxidant Activity of Thyme Essential Oil in Beef Burgers During Refrigerated Storage. Food Bioprocess Technol. 2016, 9, 1187–1201. [Google Scholar] [CrossRef]
- Sales, I.S.; de Jesus Freitas, T.; Barbosa Schappo, F.; Aparecida Souza Machado, B.; Nunes, I.L.; Duarte Ferreira Ribeiro, C. Edible and essential oils nanoparticles in food: A review on the production, characterization, application, stability, and market scenario. Crit. Rev. Food Sci. Nutr. 2023, 6, 1–28. [Google Scholar] [CrossRef]
- Javanmardi, Z.; Koushesh Saba, M.; Nourbakhsh, H.; Amini, J. Efficiency of nanoemulsion of essential oils to control Botrytis cinerea on strawberry surface and prolong fruit shelf life. Int. J. Food Microbiol. 2023, 384, 109979. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Consoli, G.M.L.; Cafiso, V.; Stefani, S.; Geraci, C. Essential oils encapsulated in polymer-based nanocapsules as potential candidates for application in food preservation. Food Chem. 2018, 269, 286–292. [Google Scholar] [CrossRef]
- Ricaurte, L.; de Jesús Perea-Flores, M.; Méndez-Méndez, J.V.; Santagapita, P.R.; Quintanilla-Carvajal, M.X. Compound distribution, structural analysis and nanomechanical properties of nanofibers loaded with high-oleic palm oil nanoemulsions for packaging application. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128148. [Google Scholar] [CrossRef]
- Ricaurte, L.; Santagapita, P.R.; Díaz, L.E.; Quintanilla-Carvajal, M.X. Edible gelatin-based nanofibres loaded with oil encapsulating high-oleic palm oil emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124673. [Google Scholar] [CrossRef]
- Beltrán, J.D.; Ricaurte, L.; Estrada, K.B.; Quintanilla-Carvajal, M.X. Effect of homogenization methods on the physical stability of nutrition grade nanoliposomes used for encapsulating high oleic palm oil. LWT-Food Sci. Technol. 2020, 118, 108801. [Google Scholar] [CrossRef]
- Ricaurte, L.; Hernández-Carrión, M.; Moyano-Molano, M.; Clavijo-Romero, A.; Quintanilla-Carvajal, M.X. Physical, thermal and thermodynamical study of high oleic palm oil nanoemulsions. Food Chem. 2018, 256, 62–70. [Google Scholar] [CrossRef]
- Hernández-Carrión, M.; Moyano, M.; Quintanilla-Carvajal, M.X. Design of high-oleic palm oil nanoemulsions suitable for drying in refractance windowTM. J. Food Process. Preserv. 2020, 45, e15076. [Google Scholar] [CrossRef]
- Ricaurte, L.; Perea-Flores, M.D.J.; Martinez, A.; Quintanilla-Carvajal, M.X. Production of high-oleic palm oil nanoemulsions by high-shear homogenization (microfluidization). Innov. Food Sci. Emerg. Technol. 2016, 35, 75–85. [Google Scholar] [CrossRef]
- Campo, C.; Santos, P.P.; Costa, T.M.H.; Paese, K.; Guterres, S.S.; de Oliveira Rios, A.; Flôres, S.H. Nanoencapsulation of chia seed oil with chia mucilage (Salvia hispanica L.) as wall material: Characterization and stability evaluation. Food Chem. 2017, 234, 1–9. [Google Scholar] [CrossRef]
- Bezerra, P.Q.M.; de Matos, M.F.R.; Ramos, I.G.; Magalhães-Guedes, K.T.; Druzian, J.I.; Costa, J.A.V.; Nunes, I.L. Innovative functional nanodispersion: Combination of carotenoid from Spirulina and yellow passion fruit albedo. Food Chem. 2019, 285, 397–405. [Google Scholar] [CrossRef]
- Upadhyay, N.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Dubey, N.K. Assessment of nanoencapsulated Cananga odorata essential oil in chitosan nanopolymer as a green approach to boost the antifungal, antioxidant and in situ efficacy. Int. J. Biol. Macromol. 2021, 171, 480–490. [Google Scholar] [CrossRef]
- dos Santos, C.D.; Menezes, R.C.R.; Dal Bosco, S.M. Releitura de pão de queijo: Versão vegetariana com farinha de oliveira. Rev. Verde Agroecol. Desenvolv. Sustent. 2021, 16, 159–163. [Google Scholar] [CrossRef]
- Gharehbeglou, P.; Jafari, S.M.; Hamishekar, H.; Homayouni, A.; Mirzaei, H. Pectin-whey protein complexes vs. small molecule surfactants for stabilization of double nano-emulsions as novel bioactive delivery systems. J. Food Eng. 2019, 245, 139–148. [Google Scholar] [CrossRef]
- Gonçalves, A.; Goufo, P.; Barros, A.; Domínguez-Perles, R.; Trindade, H.; Rosa, E.A.S.; Ferreira, L.; Rodrigues, M. Cowpea (Vigna unguiculata L. Walp), a renewed multipurpose crop for a more sustainable agri-food system: Nutritional advantages and constraints. J. Sci. Food Agric. 2016, 96, 2941–2951. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.; Mogra, R. Assessment of consumption practices of jackfruit (Artocarpus heterophyllus lam.) seeds in villages of Jalalpur block district Ambedarnagar (U.P.) India. Adv. Life Sci. 2016, 29, 73–75. [Google Scholar]
- Mirpoor, S.F.; Giosafatto, C.V.L.; Porta, R. Biorefining of seed oil cakes as industrial co-streams for production of innovative bioplastics. A review. Trends Food Sci. Technol. 2021, 109, 259–270. [Google Scholar] [CrossRef]
- Lau, K.Q.; Sabran, M.R. Utilization of vegetable and fruit by-products as functional ingredient and food. Front. Nutr. 2021, 8, 661693. [Google Scholar] [CrossRef]
- Nanotechnology Products Database. Available online: https://product.statnano.com/industry/food (accessed on 11 January 2024).
- Oliveira, L.F.; Nascimento, M.R.F.; Borges, S.V.; Ribeiro, P.C.; Do, N.; Ruback, V.R. Alternative use of yellow passion fruit peel (Passiflora edulis f. Flavicarpa) for sweet production in syrup. Food Sci. Technol. 2002, 22, 259–262. [Google Scholar]
- Jiménez, M.; Domínguez, J.A.; Pascual-Pineda, L.A.; Azuara, E.; Beristain, C.I. Elaboration and characterization of O/W cinnamon (Cinnamomum zeylanicum) and black pepper (Piper nigrum) emulsions. Food Hydrocoll. 2018, 77, 902–910. [Google Scholar] [CrossRef]
- Froiio, F.; Ginot, L.; Paolino, D.; Lebaz, N.; Bentaher, A.; Fessi, H.; Elaissari, A. Essential oils-loaded polymer particles: Preparation, characterization and antimicrobial property. Polymers 2019, 11, 1017. [Google Scholar] [CrossRef]
- Assunção, L.S.; Bezerra, P.Q.M.; Poletto, S.H.V.; de Oliveira Rios, A.; Ramos, I.G.; Ferreira-Ribeiro, C.D.; Machado, B.A.S.; Druzian, J.I.; Costa, J.A.V.; Nunes, I.L. Combination of carotenoids from Spirulina and PLA/PLGA or PHB: New options to obtain bioactive nanoparticles. Food Chem. 2021, 346, 128742. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Assis, D.; Brandão, L.V.; de Sousa Costa, L.A.; Figueiredo, T.V.B.; Sousa, L.S.; Padilha, F.F.; Druzian, J.I. A Study of the Effects of Aeration and Agitation on the Properties and Production of Xanthan Gum from Crude Glycerin Derived from Biodiesel Using the Response Surface Methodology. Appl. Biochem. Biotechnol. 2014, 172, 2769–2785. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, T.; Muniyandy, S.; Chuah, L.H.; Janarthanan, P. Encapsulation of red palm oil in carboxymethyl sago cellulose beads by emulsification and vibration technology: Physicochemical characterization and in vitro digestion. J. Food Eng. 2018, 231, 10–21. [Google Scholar] [CrossRef]
- Antonioli, G.; Fontanella, G.; Echeverrigaray, S.; Delamare, A.P.L.; Pauletti, G.F.; Barcellos, T. Poly (lactic acid) nanocapsules containing lemongrass essential oil for postharvest decay control: In vitro and in vivo evaluation against phytopathogenic fungi. Food Chem. 2020, 326, 126997. [Google Scholar] [CrossRef]
- Quintanar-Guerrero, D.; Allémann, E.; Fessi, H.; Doelker, E. Preparation techniques and mechanisms of formation of biodegradable nanoparticles from preformed polymers. Drug Dev. Ind. Pharm. 1998, 24, 1113–1128. [Google Scholar] [CrossRef]
- Lancheros, R.J.; Beleño, J.Á.; Godoy-Silva, R.D.; Guerrero, C.A. Producción de nanopartículas de PLGA por el método de emulsión y evaporación para encapsular N-Acetilcisteína (NAC). J. Fac. Sci. 2014, 92, 161–168. [Google Scholar] [CrossRef]
- Floury, J.; Desrumaux, A.; Lardières, J. Effect of high-pressure homogenization on droplet size distributions and rheological properties of model oil-in-water emulsions. Innov. Food Sci. Emerg. Technol. 2000, 1, 127–134. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Nunes, I.L. Oil nanoencapsulation: Development, application, and incorporation into the food market. Nanoscale Res. Lett. 2019, 9, 14. [Google Scholar] [CrossRef]
- Hemmatkhah, F.; Zeynali, F.; Almasi, H. Encapsulated cumin seed essential oil-loaded active papers: Characterization and evaluation of the effect on quality attributes of beef hamburger. Food Bioprocess Technol. 2020, 13, 533–547. [Google Scholar] [CrossRef]
- Mohanraj, V.J.; Chen, Y. Nanoparticle—A review. Trop. J. Pharm. 2006, 5, 561–573. [Google Scholar] [CrossRef]
- Li, Z.; Lan, Y.; Miao, J.; Chen, X.; Chen, B.; Liu, G.; Wu, X.; Zhu, X.; Cao, Y. Phytochemicals, antioxidant capacity and cytoprotective effects of jackfruit (Artocarpus heterophyllus Lam.) axis extracts on HepG2 cells. Food Biosci. 2021, 41, 100933. [Google Scholar] [CrossRef]
- Zagtap, U.B.; Bapat, V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. Seed extract and its antibacterial activity. Ind. Crops Prod. 2013, 46, 132–137. [Google Scholar]
- Xin, X.; Zhang, H.; Xu, G.; Tan, Y.; Zhang, J.; Lv, X. Influence of CTAB and SDS on the properties of oil-in-water nano-emulsion with paraffin and span 20/tween 20. Colloids Surf. A Physicochem. Eng. Asp. 2013, 418, 60–67. [Google Scholar] [CrossRef]
- Ilyasoglu, H.; El, S.N. Nanoencapsulation of EPA/DHA with sodium caseinate e gum arabic complex and its usage in the enrichment of fruit juice. LWT-Food Sci. Technol. 2014, 56, 461–468. [Google Scholar] [CrossRef]
- Esfahani, R.; Jafari, S.M.; Jafarpour, A.; Dehnad, D. Loading of fish oil into nanocarriers prepared through gelatin-gum Arabic complexation. Food Hydrocoll. 2019, 90, 291–298. [Google Scholar] [CrossRef]
- Samyn, P.; Van Nieuwkerke, D.; Schoukens, G.; Stanssens, D.; Vonck, L.; Van Den Abbeele, H. Hybrid palm-oil/styrene-maleimide nanoparticles synthesized in aqueous dispersion under different conditions. J. Microencapsul. 2015, 32, 336–348. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Characteristics and storage stability of nanoliposomes loaded with shrimp oil as affected by ultrasonication and microfluidization. Food Chem. 2020, 310, 125916. [Google Scholar] [CrossRef] [PubMed]
- Polychniatou, V.; Tzia, C. Study of formulation and stability of co-surfactant free water-in-olive oil nano- and submicron emulsions with food grade non-ionic surfactants. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 79–88. [Google Scholar] [CrossRef]
- de Sousa, A.P.M.; Campos, A.R.N.; de Santana, R.A.C.; Dantas, D.L.; de Macedo, A.D.B.; da Silva, J.R.B.; Malaquias, A.B.; da Nóbrega Albuquerque, T.; da Silva, G.B.; Gomes, J.P. Parâmetros de qualidade física e química do eixo central, mesocarpo e semente de jaca submetidos a diferentes processos de secagem. Res. Soc. Dev. 2022, 11, e34311427328. [Google Scholar] [CrossRef]
- Lobato, K.B.S.; Paese, K.; Forgearini, J.C.; Guterres, S.S.; Jablonski, A.; de Oliveira Rios, A. Evaluation of stability of bixin in nanocapsules in model systems of photosensitization and heating. LWT-Food Sci. Technol. 2015, 60, 8–14. [Google Scholar] [CrossRef]
- Rodas, A.B.M.; Rodrigues, R.M.M.S.; Sakuma, H.; Tavares, L.Z.; Sgarbi, C.R.; Lopes, W.C. Caracterização físico-química histológica e viabilidade de bactérias lácticas em iogurtes com frutas. Ciênc. Tecnol. Aliment. 2001, 21, 304–309. [Google Scholar] [CrossRef]
- Donato, M.; Ribeiro, C.D.F.; Block, J.M.; Nunes, I.L. Efeito da adição de óleo de palma bruto nanoencapsulado na estabilidade oxidativa de molho para salada em teste de oxidação acelerada. Res. Soc. Dev. 2020, 9, e4229107841. [Google Scholar] [CrossRef]
- de Almeida, D.T.; Viana, T.V.; Costa, M.M.; de Santana Silva, C.; Feitosa, S. Effects of different storage conditions on the oxidative stability of crude and refined palm oil, olein and stearin (Elaeis guineensis). Food Sci. Technol. 2019, 39, 211–217. [Google Scholar] [CrossRef]
- Ceballos, C.; Moyano, M.J.; Vicario, I.M.; Alba, J.; Heredia, F.J. Chromatic Evolution of Virgin Olive Oils Submitted to an Accelerated Oxidation Test. J. Am. Oil Chem. Soc. 2003, 80, 3. [Google Scholar] [CrossRef]
- Ousji, O.; Sleno, L. Identification of In Vitro Metabolites of Synthetic Phenolic Antioxidants BHT, BHA, and TBHQ by LC-HRMS/MS. Int. J. Mol. Sci. 2020, 21, 9525. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A. Carotenoids, and other pigments as natural colorants. Pure Appl. Chem. 2006, 78, 1477–1491. [Google Scholar] [CrossRef]
- Souza, W.A.; Vilas-Boas, O.M.G.C. A deficiência de vitamina A no Brasil: Um panorama. Rev. Panam. Salud Publica 2002, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Daugelaite, J.; Shahidi, F. DNA scission and LDL cholesterol oxidation inhibition and antioxidant activities of Bael (Aegle marmelos) flower extracts. J. Tradit. Complement. Med. 2018, 8, 428–435. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Y.; Lu, L.; Shi, C.; Huang, Y.; Mao, Z.; Duan, C.; Guo, Y.; Huang, C. Hydrodynamic cavitation: A feasible approach to intensify the emulsion cross-linking process for chitosan nanoparticle synthesis. Ultrason. Sonochem. 2021, 74, 105551. [Google Scholar] [CrossRef]
- Yazid, N.S.M.; Abdullah, N.; Muhammad, N. Comparison of chemical, functional and morphological characteristics of jackfruit (Artocarpus heterophyllus Lam.) (J33) seed starch and commercial native starches. Int. Conf. Biodivers. 2018, 169, 012031. [Google Scholar]
- Adiani, V.; Gupta, S.; Variyar, P. FTIR-based rapid microbial quality estimation of fresh-cut jackfruit (Artocarpus heterophyllus) bulbs. J. Food Meas. Charact. 2022, 16, 1944–1951. [Google Scholar] [CrossRef]
- Assunção, L.S.; Oliveira, T.S.; Santos, I.O.; Leal, I.L.; Barreto, G.A.; Machado, B.A.S.; Nunes, I.L.; Souza, C.O.; Ferreira-Ribeiro, C.D. Nanopartículas de óleo de Palma Bruto Híbrido Unaué HIE OxG (Elaeis guineensis × Elaeis oleifera) Obtidas por Método de Homogeneização. BR Patent nº 10 2022 019533 1, 8 September 2022. [Google Scholar]

| Formulations | Independent Variables | Response Parameters | Response Parameters | |||
|---|---|---|---|---|---|---|
| X1 (HCPO) | X2 (WM) | Size (nm) * | PDI * | Size (nm) ** | PDI ** | |
| 1 | −1 | −1 | 209.33 ± 2.85 | 0.10 ± 0.01 | 250.76 ± 3.27 | 0.092 ±0.00 |
| 2 | −1 | 1 | 204.76 ± 2.98 | 0.13 ± 0.00 | 224.80 ± 1.04 | 0.087 ± 0.00 |
| 3 | 1 | −1 | 234.20 ± 2.50 | 0.13 ± 0.00 | 283.70 ± 4.92 | 0.126 ± 0.02 |
| 4 | 1 | 1 | 241.00 ± 1.49 | 0.15 ± 0.03 | 308.20 ± 5.14 | 0.144 ± 0.01 |
| 5 | −1.41 | 0 | 196.90 ± 4.43 | 0.14 ± 0.01 | 248.43 ± 1.55 | 0.108 ± 0.01 |
| 6 | 1.41 | 0 | 206.50 ± 2.10 | 0.13 ± 0.02 | 317.03 ± 2.61 | 0.128 ± 0.00 |
| 7 | 0 | −1.41 | 194.46 ± 0.72 | 0.07 ± 0.01 | 212.03 ± 1.25 | 0.098 ± 0.00 |
| 8 | 0 | 1.41 | 218.13 ± 2.01 | 0.12 ± 0.01 | 230.03 ± 2.37 | 0.113 ± 0.02 |
| 9 (CP) | 0 | 0 | 264.56 ± 1.12 | 0.12 ± 0.02 | 266.50 ± 1.25 | 0.116 ± 0.01 |
| 10 (CP) | 0 | 0 | 264.40 ± 3.05 | 0.12 ± 0.02 | 266.00 ± 5.57 | 0.117 ± 0.00 |
| 11 (CP) | 0 | 0 | 264.73 ± 4.52 | 0.12 ± 0.01 | 265.70 ± 2.33 | 0.118 ± 0.00 |
| Samples | Apparent Viscosity (cP) | pH | Color Parameters | TC (µg/g) | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| Free oil | 23.04 ± 0.18 a | 3.52 ± 0.08 c | 27.98 ± 0.09 b | 10.98 ± 0.00 a | 11.69 ± 0.01 a | 921.94 ± 28.39 a |
| N-JSF | 22.89 ± 2.38 a | 5.75 ± 0.05 a | 39.01 ± 3.91 a | 0.64 ± 0.09 b | 8.20 ± 2.53 b | 809.76 ± 41.53 b |
| N-JAF | 20.89 ± 0.82 a | 5.49 ± 0.05 b | 41.98 ± 0.03 a | 0.55 ± 0.14 b | 10.40 ± 0.39 ab | 799.94 ± 45.60 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assunção, L.S.; Oliveira de Souza, C.; Shahidi, F.; Santos Oliveira, T.; Assis, D.d.J.; Pereira Santos, L.F.; Nunes, I.L.; Machado, B.A.S.; Ferreira Ribeiro, C.D. Optimization and Characterization of Interspecific Hybrid Crude Palm Oil Unaué HIE OxG Nanoparticles with Vegetable By-Products as Encapsulants. Foods 2024, 13, 523. https://doi.org/10.3390/foods13040523
Assunção LS, Oliveira de Souza C, Shahidi F, Santos Oliveira T, Assis DdJ, Pereira Santos LF, Nunes IL, Machado BAS, Ferreira Ribeiro CD. Optimization and Characterization of Interspecific Hybrid Crude Palm Oil Unaué HIE OxG Nanoparticles with Vegetable By-Products as Encapsulants. Foods. 2024; 13(4):523. https://doi.org/10.3390/foods13040523
Chicago/Turabian StyleAssunção, Larissa Santos, Carolina Oliveira de Souza, Fereidoon Shahidi, Tainara Santos Oliveira, Denilson de Jesus Assis, Luis Fernandes Pereira Santos, Itaciara Larroza Nunes, Bruna Aparecida Souza Machado, and Camila Duarte Ferreira Ribeiro. 2024. "Optimization and Characterization of Interspecific Hybrid Crude Palm Oil Unaué HIE OxG Nanoparticles with Vegetable By-Products as Encapsulants" Foods 13, no. 4: 523. https://doi.org/10.3390/foods13040523





