Levels, Toxic Effects, and Risk Assessment of Pyrrolizidine Alkaloids in Foods: A Review
Abstract
:1. Introduction
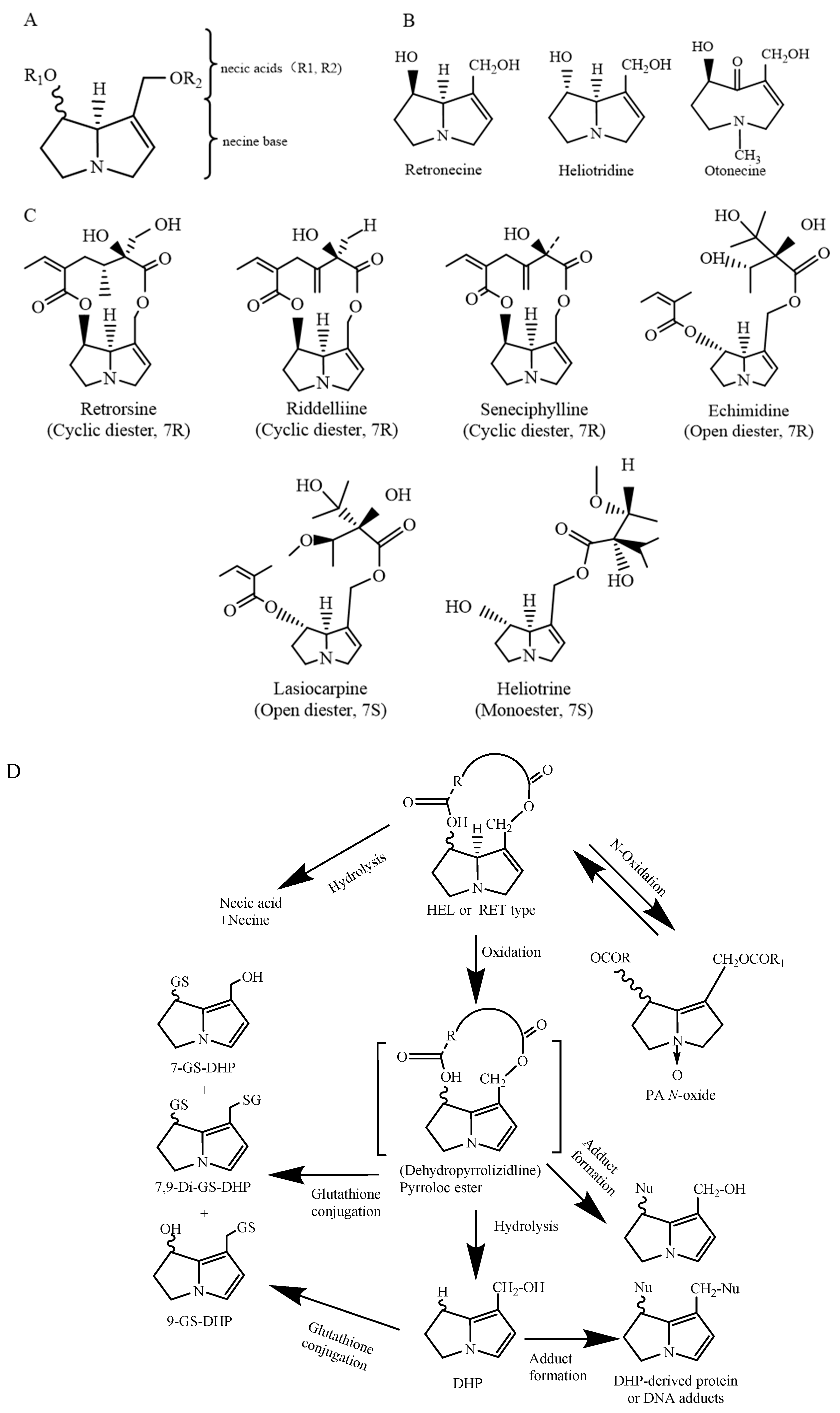
2. Chemical Structure and Toxicity of PAs
3. Status of PA Levels in Foods
| Type of Food | Number of Detected PAs/PANOs | Top Three Abundant PAs/PANOs | Concentration of Total PAs (Average or Range) | Country | Reference |
|---|---|---|---|---|---|
| Rosemary | 21 | Lasiocarpine, senecivernine N-oxide, europine N-oxide | 253 ± 26 | Spain | [19] |
| Basil leaves | 335 ± 29 | ||||
| Thyme leaf | 553 ± 48 | ||||
| Provence mixed herbs (calendula, rosemary, basil, oregano, etc.) | 258 ± 18 | France | |||
| Cumin | 21 | Europine N-oxide, heliotrine N-oxide, lasiocarpine N-oxide | 8515.0 | Belgium | [20] |
| Fennel | 1653.1 | ||||
| Melissa tea | 17 | Seneciphylline-N-oxide, retrorsine-N-oxide, intermedine | 649.6 | Germany | [21] |
| Fennel tea | 51.6 | ||||
| Chamomile | 439.7 | ||||
| Peppermint tea | 134.2 | ||||
| Green tea | 109 | ||||
| Black tea | 255.9 | ||||
| Rooibos | 1856.4 | ||||
| Fennel tea | 9 | Seneciphylline, senecionine, retrorsine | ND | Switzerland | [31] |
| Peppermint tea | 1.0 | ||||
| Chamomile | 1.9 | ||||
| Green tea | ND | ||||
| Black tea | ND | ||||
| Rooibos | 1.5 | ||||
| Black tea | 14 | Crotaline N-oxide, senecionine N-oxide, seneciphylline N-oxide | 19 | Ireland | [32] |
| Oolong tea | ND | ||||
| Green tea | ND | ||||
| Organic Pu’er tea | ND | ||||
| Digestive tea | 1733 | ||||
| Camomile and spearmint tea | 1438 | ||||
| Black tea | 14 | Jacobine, jacobine-N-oxide, seneciphylline | ND-1.91 | China | [33] |
| Green tea | ND-14.3 | ||||
| Dark tea | ND-151.3 | ||||
| Chrysanthemum | ND-5.2 | ||||
| Mixed herbal tea | 27 | Echimidine, enchinatine N-oxide, intermedine | 5.8–215 | Latvia | [39] |
| Honey | 16 | Monocrotaline, echimidine, lycopsamine | 0.04–288.1 | China | [40] |
| 17 | Lycopsamine, lycopsamine N-oxide, monocrotaline | 1.5–323.4 | Ethiopia | [24] | |
| 27 | Echimidine, lycopsamine, senecionine | 0.14–74 | Latvia | [39] | |
| 10 | Echimidine, lycopsamine, intermedine | 2.9 | Poland | [25] | |
| 17 | Seneciphylline-N-oxide, retrorsine-N-oxide, intermedine | 6.1 | Austria | [21] | |
| Total PAs | -- | 283 | Ghana | [41] | |
| 8 | Senecionine, senecionine N-oxide, monocrotaline | 50.5 | Brazil | [26] | |
| Total PAs | -- | 105 | Uruguay | [42] | |
| 53–76 | Central and South American countries | ||||
| 8 | Guatemala | ||||
| Bee pollen | 18 | Echivulgarine, echivulgarine N-oxide europine | 576 | Switzerland | [29] |
| 17 | Echimidine, echimidine N-oxide, senecionine | 142–3356 | Italy | [43] | |
| Cow milk | 28 | Senecionine, seneciphylline, retrorsine | 0.17 | Germany, The Netherlands, Spain, France, Italy and Greece | [18] |
| Goat milk | 0.11 | ||||
| Cheese | ND | ||||
| Fresh egg | 0.12 | ||||
| Maize (Zea mays L.) | Total PAs | -- | 0.9–2.0 | The Volta region of Ghana | [17] |
| Wheat | 67 | Seneciphylline, seneciphylline N-oxide, senecionine | 0–320 | The Netherlands | [8] |
| Corn | 0–302 | ||||
| Millet | 0–302 | ||||
| Rapeseed | 9–308 | ||||
| Pea | 16–315 | ||||
| Carrot | 0–302 |
4. PAs Extraction and Detection Methods
| Foods | Number of PAs/PANOs | Sample Preparation | Analysis | LOD/LOQ | Recoveries | Ref. |
|---|---|---|---|---|---|---|
| Thyme, oregano, basil, etc. | 7-O-acetylintermedine, echimidine, jacobine, etc., 44 PA/PANO | SLE with 0.05 M H2SO4 followed by SCX-SPE | HPLC-TQ-MS/MS Column: C18 | LOD: 0.1–2.6 μg/kg | 50–119% | [34] |
| Oregano | Lasiocarpine, lasiocarpine N-oxide, europine, etc., 21 PA/PANO | QuEChERS | UHPLC-IT-MS/MS Column: Polar C18 | LOQ: 0.5–25.0 μg/kg | 77–96% | [56] |
| Sorghum, oregano, and mixed herbal tea | Lycopsamine, echinatine, indicine, etc., 33 PAs/PANOs | SLE with methanol containing 0.4% FA followed by reversed phase SPE | RP-U-HPLC-MS/MS Column: Luna Omega C18 | LOQ: 0.5–2.0 μg/kg (sorghum); LOQ: 1.0–5.0 μg/kg (oregano); LOQ: 1.0–10.0 μg/kg mixed herbal tea | Sorghum (82– 115%), oregano (80–106%), and mixed herbal tea (78–117%) for the 50 μg kg−1 spiking level | [58] |
| Echium plantagineum L. honey | Echimidine and echimidine N-oxide | LLE with 0.05 M H2SO4 followed by SCX-SPE. | HPLC-DAD | -- | -- | [44] |
| Monofloral and multifloral honey | Erucifoline, echimidine, echimidine N-oxide, etc., 28 PAs/PANOs | SALLE with acid aqueous solution | UHPLC-HRMS/MS Column: Polar C18 | LOQ: 0.1–2.1 μg/kg | 63.3–103.9% | [51] |
| Honey from the Latvian market | lycopsamine N-oxide, retrorsine N-oxide, retrorsine, etc., 30 PAs/PANOs | QuEChERS (acetonitrile containing 1% FA, MgSO4, etc.) | Nano-LC-Orbitrap MS Column:C18 | LOQ: 0.05–2.5 μg/kg | -- | [39] |
| Honey | 1,2-unsaturated retronecine-type PAs | LLE with 0.15 M HCL and addition of zinc, followed by MCX-SPE; derivatization with HFBA | GC-MS/MS Column: DB-5MS | LOQ:1 μg/kg | 73.1–93.6% | [49] |
| Bee pollen | Lycopsamine, senecionine, seneciphylline N-oxides, etc., 17 PAs/PANOs | -- | Near-infrared (NIR) spectroscopy | LOQ:0.4 μg/kg | -- | [59] |
| Chamomile tea | Europine, europine N-oxide, heliotrine, etc., 21 PAs/PANOs | C18 μ-SPEed cartridge with two aspiration dispense cycles of 100 μL of MeOH followed by 100 μL of water | UHPLC-IT-MS/MS Column: Luna Omega Polar C18 column | -- | 76–101% | [36] |
| Black tea and green tea | Echimidine, echimidine N-oxide, erucifoline, etc., 28 PAs/PANOs | SALLE with acid aqueous solution | UHPLC-HRMS/MS Column: C18 | LOQ: 1–12 μg/kg | 63.9–116.9% | [51] |
| Fresh tea | Retrorsine, senecionine, jacobine, etc., 15 PAs/PANOs | LLE with 0.1 M H2SO4 followed by PCX SPE | UHPLC–MS/MS Column: HSS T3 | LOQ:1–5 μg/kg | 67.0–111.9% | [60] |
| Herbal tea | Riddelliine, Riddelliine N-oxide, seneciphylline, etc., 34 Pas/PANOs | infusion with boiling water followed by C18-SPE | UHPLC-TQ-MS/MS Column: C18 | LOD:0.2–3.8 μg/kg | 45–122% | [18] |
| Tea from the Latvian market | Lycopsamine, lycopsamine N-oxide, retrorsine, etc., 30 PAs/PANOs | QuEChERS (acetonitrile containing 1% FA, MgSO4, etc.) | Nano-LC-MS-Orbitrap MS Column: C18 | LOQ: 0.5–20 μg/kg | -- | [39] |
| Milk | LOQ: 0.5–20 μg/kg | -- | ||||
| Fresh milk | Senecionine, senkirkine, seneciphylline, etc., 6 PAs/PANOs | LLE with 0.5% FA and dichloromethane | DART-IT-MS | LOQ:1.83–2.82 ng/mL | 89–112% | [45] |
| Milk, yogurt, cheese | Riddelliine, riddelliine N-oxide, seneciphylline, etc., 34 PAs/PANOs | LLE or SLE with hexane containing 0.2% FA followed by reversed-phase SPE | UHPLC-TQ-MS/MS Column: C18 | LOD: 0.03–0.05 μg/L (milk and yogurt); LOD: 0.05–0.15 μg/kg (cheese) | 74–107% | [18] |
| Eggs, pork meat, beef, liver | LOD: 0.05–0.15 μg/kg (egg, pork, and meat); LOD: 0.1–0.25 μg/kg (beef and liver) | Egg (56–103%); meat product (63–91%) | ||||
| Eggs and meat | Senecionine, seneciphylline, riddelliine, etc., 51 PAs/PANOs | SLE with hexane containing 0.2% FA followed by reversed phase SPE | UHPLC-TQ-MS/MS Column: C18 | LOQ:0.1–1 μg/kg | -- | [61] |
| Maize | 1,2-unsaturated RET/HEL-type PAs | SLE with 0.05 M H2SO4 followed by SCX-SPE | HPLC-QTRAP-MS/MS Column: C12 | -- | -- | [17] |
| Herb (atractylodis rhizoma, chrysanthemi flos, leonuri herba, gastrodiae rhizoma, glycyrrhizae radix) | Retrorsine, Senkirkine, Lycopsamine N-oxide, etc., 28 PAs | 0.05 M H2SO4 in 50% MeOH followed by MCX-SPE | LC–MS/MS Column:Shim-pack GIST-C18 | LOQ: 0.1–6.5 μg/kg (Atractylodis Rhizoma); LOQ: 0.1–10.1 μg/kg (Chrysanthmi Flos); LOQ: 0.1–5.5 μg/kg (Leonuri Herba); LOQ: 0.1–9.1 μg/kg (Gastrodiae Rhizoma); LOQ: 0.1–10.5 μg/kg (Glycyrrhizae Radix) | Atractylodis Rhizoma (72.5–123.7%); Chrysanthmi Flos (70.6–151.7%), Leonuri Herba (80.6–130.9%), Gastrodiae Rhizoma (70.3–122.9%), Glycyrrhizae Radix (67.1–106.9%) | [62] |
| Herb (peppermint, chamomile, nettle, and linden) | Echimidine, erucifolin, heliotrine, etc., 30 PAs/PANOs | QuEChERS (acetonitrile containing 1% FA, followed by graphene to clean up) | LC–MS/MS Column: Hypersil Gold | LOD: 0.05–0.15 μg/kg (egg, pork, and meat); LOD: 0.1–0.25 μg/kg (beef and liver) | 61–128% | [57] |
5. Toxic Effects of PAs
5.1. Acute Toxicity
5.2. Cytotoxicity
5.3. Genotoxicity and Carcinogenicity
6. Risk Assessment of PAs
| Food | MOE Value | References |
|---|---|---|
| Herbal tea | 3121 | [30] |
| Tea | 1872 | [30] |
| Peppermint tea | 5400 | [93] |
| Rooibos | 4200 | [93] |
| Black tea | 6000 | [93] |
| Green tea | 6200 | [93] |
| Melissa tea | 3800 | [93] |
| Chamomile tea | 14,100 | [93] |
| Nettle tea | 10,300 | [93] |
| Fennel tea | 47,400 | [93] |
| Anise | 54,000 | [93] |
| Fennel | 1,467,000 | [93] |
| Coriander | 14,100 | [93] |
| Nettle | 304,000 | [93] |
| Honey | 593,000 | [8] |
| Commercial honey in Brazil | 5010 | [95] |
| Pollen-based plant food supplements | 561,000 | [93] |
| Mixed plant extracts | 415,000 | [93] |
7. Challenges in Risk Assessment of PAs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dusemund, B.; Nowak, N.; Sommerfeld, C.; Lindtner, O.; Schafer, B.; Lampen, A. Risk assessment of pyrrolizidine alkaloids in food of plant and animal origin. Food Chem. Toxicol. 2018, 115, 63–72. [Google Scholar] [CrossRef]
- Smith, L.W.; Culvenor, C.C. Plant sources of hepatotoxic pyrrolizidine alkaloids. J. Nat. Prod. 1981, 44, 129–152. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Dietary Exposure Assessment to Pyrrolizidine Alkaloids in the European Population; European Food Safety Authority: Parma, Italy, 2016.
- Fu, P.P.; Xia, Q.S.; Lin, G.; Chou, M.W. Pyrrolizidine alkaloids—Genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab. Rev. 2004, 36, 1–55. [Google Scholar] [CrossRef]
- Zhuge, Y.; Liu, Y.; Xie, W.; Zou, X.; Xu, J.; Wang, J.; Chinese Society of Gastroenterology Committee of Hepatobiliary Disease. Expert consensus on the clinical management of pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome. J. Gastroenterol. Hepatol. 2019, 34, 634–642. [Google Scholar] [CrossRef]
- Casado, N.; Casado-Hidalgo, G.; González-Gómez, L.; Morante-Zarcero, S.; Sierra, I. Insight into the Impact of Food Processing and Culinary Preparations on the Stability and Content of Plant Alkaloids Considered as Natural Food Contaminants. Appl. Sci. 2023, 13, 1704. [Google Scholar] [CrossRef]
- Casado, N.; Morante-Zarcero, S.; Sierra, I. The concerning food safety issue of pyrrolizidine alkaloids: An overview. Trends Food Sci. Technol. 2022, 120, 123–139. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Risks for Human Health Related to the Presence of Pyrrolizidine Alkaloids in Honey, Tea, Herbal Infusions and Food Supplements; European Food Safety Authority: Parma, Italy, 2017; p. e04908.
- Merz, K.H.; Schrenk, D. Interim relative potency factors for the toxicological risk assessment of pyrrolizidine alkaloids in food and herbal medicines. Toxicol. Lett. 2016, 263, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Madge, I.; Gehling, M.; Schone, C.; Winterhalter, P.; These, A. Pyrrolizidine alkaloid profiling of four Boraginaceae species from Northern Germany and implications for the analytical scope proposed for monitoring of maximum levels. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2020, 37, 1339–1358. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhao, Y.; Lin, G.; Beland, F.A.; Cai, L.; Fu, P.P. Pyrrolizidine Alkaloid-Protein Adducts: Potential Non-invasive Biomarkers of Pyrrolizidine Alkaloid-Induced Liver Toxicity and Exposure. Chem. Res. Toxicol. 2016, 29, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, D.; Gao, L.; Lin, G.; Mahony, C.; Mulder, P.P.J.; Peijnenburg, A.; Pfuhler, S.; Rietjens, I.; Rutz, L.; Steinhoff, B.; et al. Pyrrolizidine alkaloids in food and phytomedicine: Occurrence, exposure, toxicity, mechanisms, and risk assessment—A review. Food Chem. Toxicol. 2020, 136, 111107. [Google Scholar] [CrossRef] [PubMed]
- Allemang, A.; Mahony, C.; Lester, C.; Pfuhler, S. Relative potency of fifteen pyrrolizidine alkaloids to induce DNA damage as measured by micronucleus induction in HepaRG human liver cells. Food Chem. Toxicol. 2018, 121, 72–81. [Google Scholar] [CrossRef]
- Chou, M.W.; Wang, Y.P.; Yan, J.; Yang, Y.C.; Beger, R.D.; Williams, L.D.; Doerge, D.R.; Fu, P.P. Riddelliine N-oxide is a phytochemical and mammalian metabolite with genotoxic activity that is comparable to the parent pyrrolizidine alkaloid riddelliine. Toxicol. Lett. 2003, 145, 239–247. [Google Scholar] [CrossRef]
- Li, N.; Xia, Q.; Ruan, J.; Fu, P.P.; Lin, G. Hepatotoxicity and tumorigenicity induced by metabolic activation of pyrrolizidine alkaloids in herbs. Curr. Drug Metab. 2011, 12, 823–834. [Google Scholar] [CrossRef]
- Ruan, J.; Yang, M.; Fu, P.; Ye, Y.; Lin, G. Metabolic activation of pyrrolizidine alkaloids: Insights into the structural and enzymatic basis. Chem. Res. Toxicol. 2014, 27, 1030–1039. [Google Scholar] [CrossRef]
- Letsyo, E.; Adams, Z.S.; Dzikunoo, J.; Asante-Donyinah, D. Uptake and accumulation of pyrrolizidine alkaloids in the tissues of maize (Zea mays L.) plants from the soil of a 4-year-old Chromolaena odorata dominated fallow farmland. Chemosphere 2021, 270, 128669. [Google Scholar] [CrossRef]
- Mulder, P.P.J.; Lopez, P.; Castellari, M.; Bodi, D.; Ronczka, S.; Preiss-Weigert, A.; These, A. Occurrence of pyrrolizidine alkaloids in animal- and plant-derived food: Results of a survey across Europe. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 118–133. [Google Scholar] [CrossRef]
- Izcara, S.; Casado, N.; Morante-Zarcero, S.; Perez-Quintanilla, D.; Sierra, I. Miniaturized and modified QuEChERS method with mesostructured silica as clean-up sorbent for pyrrolizidine alkaloids determination in aromatic herbs. Food Chem. 2022, 380, 132189. [Google Scholar] [CrossRef] [PubMed]
- Willocx, M.; Van der Beeten, I.; Asselman, P.; Delgat, L.; Baert, W.; Janssens, S.B.; Leliaert, F.; Picron, J.F.; Vanhee, C. Sorting out the plants responsible for a contamination with pyrrolizidine alkaloids in spice seeds by means of LC-MS/MS and DNA barcoding: Proof of principle with cumin and anise spice seeds. Food Chem. Mol. Sci. 2022, 4, 100070. [Google Scholar] [CrossRef] [PubMed]
- Bodi, D.; Ronczka, S.; Gottschalk, C.; Behr, N.; Skibba, A.; Wagner, M.; Lahrssen-Wiederholt, M.; Preiss-Weigert, A.; These, A. Determination of pyrrolizidine alkaloids in tea, herbal drugs and honey. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Shimshoni, J.A.; Duebecke, A.; Mulder, P.P.; Cuneah, O.; Barel, S. Pyrrolizidine and tropane alkaloids in teas and the herbal teas peppermint, rooibos and chamomile in the Israeli market. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Brugnerotto, P.; Seraglio, S.K.T.; Schulz, M.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Pyrrolizidine alkaloids and beehive products: A review. Food Chem. 2021, 342, 128384. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Long, Y.; Zhang, C.; Ma, J.; Ke, C.; Tang, C.; Ye, Y.; Lin, G. Dietary alcohol exacerbates the hepatotoxicity induced by pyrrolizidine alkaloids: Hazard from food contamination. J. Hazard. Mater. 2022, 424, 127706. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, E.; Kwiatek, K. Pyrrolizidine Alkaloids in Honey: Determination with Liquid Chromatography-mass Spectrometry Method. J. Vet. Res. 2018, 62, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Valese, A.C.; Molognoni, L.; Ploencio, L.A.D.; de Lima, F.G.; Gonzaga, L.V.; Gorniak, S.L.; Daguer, H.; Barreto, F.; Costa, A.C.O. A fast and simple LC-ESI-MS/MS method for detecting pyrrolizidine alkaloids in honey with full validation and measurement uncertainty. Food Control 2016, 67, 183–191. [Google Scholar] [CrossRef]
- Pinto, B.N.S.; Moura, G.A.; Demuner, A.J.; Alvarenga, E.S. Structural elucidation of a novel pyrrolizidine alkaloid isolated from Crotalaria retusa L. J. Mol. Struct. 2022, 1254, 132394. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Z.T.; Wong, L.L.; He, Y.S.; Zhao, Z.Z.; Ye, Y.; Fu, P.P.; Lin, G. Contamination of hepatotoxic pyrrolizidine alkaloids in retail honey in China. Food Control 2018, 85, 484–494. [Google Scholar] [CrossRef]
- Kast, C.; Kilchenmann, V.; Reinhard, H.; Bieri, K.; Zoller, O. Pyrrolizidine Alkaloids: The Botanical Origin of Pollen Collected during the Flowering Period of Echium vulgare and the Stability of Pyrrolizidine Alkaloids in Bee Bread. Molecules 2019, 24, 2214. [Google Scholar] [CrossRef]
- Bundesinstitut für Risikobewertung (BfR). Pyrrolizidine Alkaloids in Herbal Teas and Teas; Bundesinstitut für Risikobewertung: Berlin, Germany, 2013. [Google Scholar]
- Mathon, C.; Edder, P.; Bieri, S.; Christen, P. Survey of pyrrolizidine alkaloids in teas and herbal teas on the Swiss market using HPLC-MS/MS. Anal. Bioanal. Chem. 2014, 406, 7345–7354. [Google Scholar] [CrossRef]
- Griffin, C.T.; Gosetto, F.; Danaher, M.; Sabatini, S.; Furey, A. Investigation of targeted pyrrolizidine alkaloids in traditional Chinese medicines and selected herbal teas sourced in Ireland using LC-ESI-MS/MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 940–961. [Google Scholar] [CrossRef]
- Han, H.L.; Jiang, C.L.; Wang, C.; Wang, Z.Q.; Chai, Y.F.; Zhang, X.C.; Liu, X.; Lu, C.Y.; Chen, H.P. Development, optimization, validation and application of ultra high performance liquid chromatography tandem mass spectrometry for the analysis of pyrrolizidine alkaloids and pyrrolizidine alkaloid N-oxides in teas and weeds. Food Control 2022, 132, 108518. [Google Scholar] [CrossRef]
- Kaltner, F.; Rychlik, M.; Gareis, M.; Gottschalk, C. Occurrence and Risk Assessment of Pyrrolizidine Alkaloids in Spices and Culinary Herbs from Various Geographical Origins. Toxins 2020, 12, 155. [Google Scholar] [CrossRef]
- Selmar, D.; Wittke, C.; Beck-von Wolffersdorff, I.; Klier, B.; Lewerenz, L.; Kleinwachter, M.; Nowak, M. Transfer of pyrrolizidine alkaloids between living plants: A disregarded source of contaminations. Environ. Pollut. 2019, 248, 456–461. [Google Scholar] [CrossRef]
- Fernández-Pintor, B.; Casado, N.; Morante-Zarcero, S.; Sierra, I. Evaluation of the thermal stability and transfer rate of pyrrolizidine alkaloids during the brewing of herbal infusions contaminated with Echium vulgare and Senecio vulgaris weeds. Food Control 2023, 153, 109926. [Google Scholar] [CrossRef]
- Hoogenboom, L.A.; Mulder, P.P.; Zeilmaker, M.J.; van den Top, H.J.; Remmelink, G.J.; Brandon, E.F.; Klijnstra, M.; Meijer, G.A.; Schothorst, R.; Van Egmond, H.P. Carry-over of pyrrolizidine alkaloids from feed to milk in dairy cows. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Taenzer, J.; Gehling, M.; Klevenhusen, F.; Saltzmann, J.; Danicke, S.; These, A. Rumen Metabolism of Senecio Pyrrolizidine Alkaloids May Explain Why Cattle Tolerate Higher Doses than Monogastric Species. J. Agric. Food Chem. 2022, 70, 10111–10120. [Google Scholar] [CrossRef] [PubMed]
- Jansons, M.; Fedorenko, D.; Pavlenko, R.; Berzina, Z.; Bartkevics, V. Nanoflow liquid chromatography mass spectrometry method for quantitative analysis and target ion screening of pyrrolizidine alkaloids in honey, tea, herbal tinctures, and milk. J. Chromatogr. A 2022, 1676, 463269. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhu, L.; Ma, J.; Wong, L.; Zhao, Z.; Ye, Y.; Fu, P.P.; Lin, G. Comprehensive investigation and risk study on pyrrolizidine alkaloid contamination in Chinese retail honey. Environ. Pollut. 2020, 267, 115542. [Google Scholar] [CrossRef] [PubMed]
- Letsyo, E.; Jerz, G.; Winterhalter, P.; Dubecke, A.; von der Ohe, W.; von der Ohe, K.; Beuerle, T. Pyrrolizidine alkaloids in floral honeys of tropical Ghana: A health risk assessment. Food Addit. Contam. Part B Surveill. 2017, 10, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Dubecke, A.; Beckh, G.; Lullmann, C. Pyrrolizidine alkaloids in honey and bee pollen. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 348–358. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Inacio, L.; Merlanti, R.; Lucatello, L.; Bisutti, V.; Contiero, B.; Serva, L.; Segato, S.; Capolongo, F. Pyrrolizidine alkaloids in bee pollen identified by LC-MS/MS analysis and colour parameters using multivariate class modeling. Heliyon 2020, 6, e03593. [Google Scholar] [CrossRef]
- Moreira, R.; Fernandes, F.; Valentao, P.; Pereira, D.M.; Andrade, P.B. Echium plantagineum L. honey: Search of pyrrolizidine alkaloids and polyphenols, anti-inflammatory potential and cytotoxicity. Food Chem. 2020, 328, 127169. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Li, L.N.; Xiong, F.; Xie, Y.Q.; Xiong, A.Z.; Wang, Z.T.; Yang, L. Rapid identification and determination of pyrrolizidine alkaloids in herbal and food samples via direct analysis in real-time mass spectrometry. Food Chem. 2021, 334, 127472. [Google Scholar] [CrossRef]
- Yu, L.; Xu, Y.; Feng, H.; Li, S.F. Separation and determination of toxic pyrrolizidine alkaloids in traditional Chinese herbal medicines by micellar electrokinetic chromatography with organic modifier. Electrophoresis 2005, 26, 3397–3404. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Schoch, T.K.; Stegelmeier, B.L.; Gardner, D.R.; Than, K.A.; Molyneux, R.J. Development of enzyme-linked immunosorbent assays for the hepatotoxic alkaloids riddelliine and riddelliine N-oxide. J. Agric. Food Chem. 2001, 49, 4144–4151. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, S.F. Dynamic pH junction-sweeping capillary electrophoresis for online preconcentration of toxic pyrrolizidine alkaloids in Chinese herbal medicine. Electrophoresis 2005, 26, 4360–4367. [Google Scholar] [CrossRef]
- Kowalczyk, E.; Sieradzki, Z.; Kwiatek, K. Determination of Pyrrolizidine Alkaloids in Honey with Sensitive Gas Chromatography-Mass Spectrometry Method. Food Anal. Methods 2018, 11, 1345–1355. [Google Scholar] [CrossRef]
- Guo, Q.; Yang, Y.; Li, J.; Shao, B.; Zhang, J. Screening for Plant Toxins in Honey and Herbal Beverage by Ultrahigh-Performance Liquid Chromatography-Ion Mobility-Quadrupole Time of Flight Mass Spectrometry. Am. J. Anal. Chem. 2022, 13, 108–134. [Google Scholar] [CrossRef]
- Rizzo, S.; Celano, R.; Piccinelli, A.L.; Russo, M.; Rastrelli, L. Target screening method for the quantitative determination of 118 pyrrolizidine alkaloids in food supplements, herbal infusions, honey and teas by liquid chromatography coupled to quadrupole orbitrap mass spectrometry. Food Chem. 2023, 423, 136306. [Google Scholar] [CrossRef]
- Sixto, A.; Perez-Parada, A.; Niell, S.; Heinzen, H. GC-MS and LC-MS/MS workflows for the identification and quantitation of pyrrolizidine alkaloids in plant extracts, a case study: Echium plantagineum. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2019, 29, 500–503. [Google Scholar] [CrossRef]
- Ma, C.; Liu, Y.; Zhu, L.; Ji, H.; Song, X.; Guo, H.; Yi, T. Determination and regulation of hepatotoxic pyrrolizidine alkaloids in food: A critical review of recent research. Food Chem. Toxicol. 2018, 119, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Crews, C.; Berthiller, F.; Krska, R. Update on analytical methods for toxic pyrrolizidine alkaloids. Anal. Bioanal. Chem. 2010, 396, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Izcara, S.; Casado, N.; Morante-Zarcero, S.; Sierra, I. A Miniaturized QuEChERS Method Combined with Ultrahigh Liquid Chromatography Coupled to Tandem Mass Spectrometry for the Analysis of Pyrrolizidine Alkaloids in Oregano Samples. Foods 2020, 9, 1319. [Google Scholar] [CrossRef] [PubMed]
- Kaczynski, P.; Lozowicka, B. A novel approach for fast and simple determination pyrrolizidine alkaloids in herbs by ultrasound-assisted dispersive solid phase extraction method coupled to liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2020, 187, 113351. [Google Scholar] [CrossRef] [PubMed]
- Dzuman, Z.; Jonatova, P.; Stranska-Zachariasova, M.; Prusova, N.; Brabenec, O.; Novakova, A.; Fenclova, M.; Hajslova, J. Development of a new LC-MS method for accurate and sensitive determination of 33 pyrrolizidine and 21 tropane alkaloids in plant-based food matrices. Anal. Bioanal. Chem. 2020, 412, 7155–7167. [Google Scholar] [CrossRef]
- De Jesus Inacio, L.; Lanza, I.; Merlanti, R.; Contiero, B.; Lucatello, L.; Serva, L.; Bisutti, V.; Mirisola, M.; Tenti, S.; Segato, S.; et al. Discriminant analysis of pyrrolizidine alkaloid contamination in bee pollen based on near-infrared data from lab-stationary and portable spectrometers. Eur. Food Res. Technol. 2020, 246, 2471–2483. [Google Scholar] [CrossRef]
- Han, H.; Jiang, C.; Wang, C.; Lu, Y.; Wang, Z.; Chai, Y.; Zhang, X.; Liu, X.; Lu, C.; Chen, H. Dissipation pattern and conversion of pyrrolizidine alkaloids (PAs) and pyrrolizidine alkaloid N-oxides (PANOs) during tea manufacturing and brewing. Food Chem. 2022, 390, 133183. [Google Scholar] [CrossRef]
- Mulder, P.P.; de Witte, S.L.; Stoopen, G.M.; van der Meulen, J.; van Wikselaar, P.G.; Gruys, E.; Groot, M.J.; Hoogenboom, R.L. Transfer of pyrrolizidine alkaloids from various herbs to eggs and meat in laying hens. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 1826–1839. [Google Scholar] [CrossRef]
- Jeong, S.H.; Choi, E.Y.; Kim, J.; Lee, C.; Kang, J.; Cho, S.; Ko, K.Y. LC-ESI-MS/MS Simultaneous Analysis Method Coupled with Cation-Exchange Solid-Phase Extraction for Determination of Pyrrolizidine Alkaloids on Five Kinds of Herbal Medicines. J. AOAC Int. 2021, 104, 1514–1525. [Google Scholar] [CrossRef]
- Stegelmeier, B.L.; James, L.F.; Panter, K.E.; Ralphs, M.H.; Gardner, D.R.; Molyneux, R.J.; Pfister, J.A. The pathogenesis and toxicokinetics of locoweed (Astragalus and Oxytropis spp.) poisoning in livestock. J. Nat. Toxins 1999, 8, 35–45. [Google Scholar]
- Woolford, L.; Fletcher, M.T.; Boardman, W.S. Suspected pyrrolizidine alkaloid hepatotoxicosis in wild southern hairy-nosed wombats (Lasiorhinus latifrons). J. Agric. Food Chem. 2014, 62, 7413–7418. [Google Scholar] [CrossRef]
- Stillman, A.E.; Huxtable, R.; Consroe, P.; Kohnen, P.; Smith, S. Hepatic Veno-Occlusive Disease Due to Pyrrolizidine (Senecio) Poisoning in Arizona. Gastroenterology 1977, 73, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Bull, L.B.; Dick, A.T.; Mc, K.J. The acute toxic effects of heliotrine and lasiocarpine, and their N-oxides, on the rat. J. Pathol. Bacteriol. 1958, 75, 17–25. [Google Scholar] [CrossRef]
- Litvinchuk, M.D.; Gaiduk, R.I.; Kit, V.I. Spasmolytic properties of pyrrolizidine alkaloids. Farmakol. Toksikol. 1979, 42, 509–511. [Google Scholar] [PubMed]
- Schoental, R. Hepatotoxic activity of retrorsine, senkirkine and hydroxysenkirkine in newborn rats, and the role of epoxides in carcinogenesis by pyrrolizidine alkaloids and aflatoxins. Nature 1970, 227, 401–402. [Google Scholar] [CrossRef]
- Culvenor, C.C.J.; Downing, D.T.; Edgar, J.A.; Jago, M.V. Pyrrolizidine Alkaloids as Alkylating and Antimitotic Agents. Ann. N. Y. Acad. Sci. 1969, 163, 837–847. [Google Scholar] [CrossRef]
- Li, Y.H.; Kan, W.L.T.; Li, N.; Lin, G. Assessment of pyrrolizidine alkaloid-induced toxicity in an in vitro screening model. J. Ethnopharmacol. 2013, 150, 560–567. [Google Scholar] [CrossRef]
- Field, R.A.; Stegelmeier, B.L.; Colegate, S.M.; Brown, A.W.; Green, B.T. An in vitro comparison of the cytotoxic potential of selected dehydropyrrolizidine alkaloids and some N-oxides. Toxicon 2015, 97, 36–45. [Google Scholar] [CrossRef]
- Ji, L.; Liu, T.; Wang, Z. Pyrrolizidine alkaloid clivorine induced oxidative injury on primary cultured rat hepatocytes. Hum. Exp. Toxicol. 2010, 29, 303–309. [Google Scholar] [CrossRef]
- Nuringtyas, T.R.; Verpoorte, R.; Klinkhamer, P.G.; van Oers, M.M.; Leiss, K.A. Toxicity of pyrrolizidine alkaloids to Spodoptera exigua using insect cell lines and injection bioassays. J. Chem. Ecol. 2014, 40, 609–616. [Google Scholar] [CrossRef]
- Gluck, J.; Waizenegger, J.; Braeuning, A.; Hessel-Pras, S. Pyrrolizidine Alkaloids Induce Cell Death in Human HepaRG Cells in a Structure-Dependent Manner. Int. J. Mol. Sci. 2020, 22, 202. [Google Scholar] [CrossRef]
- Ji, L.; Chen, Y.; Liu, T.; Wang, Z. Involvement of Bcl-xL degradation and mitochondrial-mediated apoptotic pathway in pyrrolizidine alkaloids-induced apoptosis in hepatocytes. Toxicol. Appl. Pharmacol. 2008, 231, 393–400. [Google Scholar] [CrossRef]
- Ji, L.L.; Zhang, M.; Sheng, Y.C.; Wang, Z.T. Pyrrolizidine alkaloid clivorine induces apoptosis in human normal liver L-02 cells and reduces the expression of p53 protein. Toxicol. In Vitro 2005, 19, 41–46. [Google Scholar] [CrossRef]
- Ebmeyer, J.; Franz, L.; Lim, R.; Niemann, B.; Glatt, H.; Braeuning, A.; Lampen, A.; Hessel-Pras, S. Sensitization of Human Liver Cells Toward Fas-Mediated Apoptosis by the Metabolically Activated Pyrrolizidine Alkaloid Lasiocarpine. Mol. Nutr. Food Res. 2019, 63, e1801206. [Google Scholar] [CrossRef]
- Xiong, A.Z.; Yang, L.; Ji, L.L.; Wang, Z.Y.; Yang, X.J.; Chen, Y.; Wang, X.L.; Wang, C.H.; Wang, Z.T. UPLC-MS based metabolomics study on Senecio scandens and S-vulgaris: An approach for the differentiation of two Senecio herbs with similar morphology but different toxicity. Metabolomics 2012, 8, 614–623. [Google Scholar] [CrossRef]
- Chan, P.C.; Haseman, J.K.; Prejean, J.D.; Nyska, A. Toxicity and carcinogenicity of riddelliine in rats and mice. Toxicol. Lett. 2003, 144, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Reddy, J.K. Malignant neoplasms in rats fed lasiocarpine. Br. J. Cancer 1978, 37, 289–293. [Google Scholar] [CrossRef]
- Chen, T.; Mei, N.; Fu, P.P. Genotoxicity of pyrrolizidine alkaloids. J. Appl. Toxicol. 2010, 30, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.M.; Mori, H.; Hirono, I.; Nagao, M. Genotoxicity of pyrrolizidine alkaloids in the hepatocyte primary culture/DNA-repair test. Mutat. Res. 1980, 79, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.L.; Schoofs, G.M.; Schwass, D.E.; Molyneux, R.J. Genotoxic activity of a series of pyrrolizidine alkaloids in primary hepatocyte-mediated V79 cell mutagenesis and DNA repair assay. J. Nat. Toxins 1996, 5, 7–24. [Google Scholar]
- Xia, Q.S.; Zhao, Y.W.; Von Tungeln, L.S.; Doerge, D.R.; Lin, G.; Cai, L.N.; Fu, P.P. Pyrrolizidine Alkaloid-Derived DNA Adducts as a Common Biological Biomarker of Pyrrolizidine Alkaloid-Induced Tumorigenicity. Chem. Res. Toxicol. 2013, 26, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- Louisse, J.; Rijkers, D.; Stoopen, G.; Holleboom, W.J.; Delagrange, M.; Molthof, E.; Mulder, P.P.J.; Hoogenboom, R.; Audebert, M.; Peijnenburg, A. Determination of genotoxic potencies of pyrrolizidine alkaloids in HepaRG cells using the gammaH2AX assay. Food Chem. Toxicol. 2019, 131, 110532. [Google Scholar] [CrossRef] [PubMed]
- Fielden, M.R.; Kolaja, K.L. The role of early in vivo toxicity testing in drug discovery toxicology. Expert Opin. Drug Saf. 2008, 7, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Barlow, S.; Chesson, A.; Collins, J.; Fernandes, T.; Flynn, A.; Hardy, T.; Jansson, B.; Knaap, A.; Kuiper, H.; Le Neindre, P.; et al. Opinion of the Scientific Committee on a request from EFSA related to A Harmonised Approach for Risk Assessment of Substances Which are both Genotoxic and Carcinogenic. EFSA J. 2005, 3, 282. [Google Scholar] [CrossRef]
- Barlow, S.; Renwick, A.G.; Kleiner, J.; Bridges, J.W.; Busk, L.; Dybing, E.; Edler, L.; Eisenbrand, G.; Fink-Gremmels, J.; Knaap, A.; et al. Risk assessment of substances that are both genotoxic and carcinogenic—Report of an International Conference organized by EFSA and WHO with support of ILSI Europe. Food Chem. Toxicol. 2006, 44, 1636–1650. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. The Use of the Benchmark Dose Approach in Health Risk Assessmentt; U.S. Environmental Protection Agency: Washington, DC, USA, 1995.
- European Food Safety Authority (EFSA). Opinion of the Scientific Committee on a Request from EFSA Related to a Harmonised Approach for Risk Assessment of Substances Which Are Both Genotoxic and Carcinogenic; European Food Safety Authority (EFSA): Parma, Italy, 2005; p. 282.
- Chen, L.; Zhang, Q.; Yi, Z.W.; Chen, Y.; Xiao, W.H.; Su, D.; Shi, W.B. Risk Assessment of (Herbal) Teas Containing Pyrrolizidine Alkaloids (PAs) Based on Margin of Exposure Approach and Relative Potency (REP) Factors. Foods 2022, 11, 2946. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Renwick, A.G.; Constable, A.; Dybing, E.; Muller, D.J.; Schlatter, J.; Slob, W.; Tueting, W.; van Benthem, J.; Williams, G.M.; et al. Approaches to the risk assessment of genotoxic carcinogens in food: A critical appraisal. Food Chem. Toxicol. 2006, 44, 1613–1635. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mulder, P.P.J.; Louisse, J.; Peijnenburg, A.; Wesseling, S.; Rietjens, I. Risk assessment for pyrrolizidine alkaloids detected in (herbal) teas and plant food supplements. Regul. Toxicol. Pharmacol. 2017, 86, 292–302. [Google Scholar] [CrossRef]
- Merz, K.; Schrenk, D. Relative potency factors for a preliminary toxicological risk assessment of pyrrolizidine alkaloids in food and herbal medicines. Toxicol. Lett. 2016, 2016, S217–S218. [Google Scholar] [CrossRef]
- Bandini, T.B.; Spisso, B.F. Detection, dietary exposure assessment and risk evaluation of quinolones and pyrrolizidine alkaloids in commercial honey from Brazil. Food Addit. Contam. Part B Surveill. 2022, 15, 89–97. [Google Scholar] [CrossRef]
- Committee in Toxicity of Chemicals in Food, Consumer, Products and the Environment (COT). Statement on Pyrrolizidine Alkaloids in Food; Committee in Toxicity of Chemicals in Food, Consumer, Products and the Environment: London, UK, 2008.
- The Dutch National Institute for Public Health and the Environment (RIVM). Risicobeoordeling Inzake de Aanwezigheid van Pyrrolzidine Alkaloiden in Honing; The Dutch National Institute for Public Health and the Environment: Utrecht, The Netherlands, 2007.
- European Medicines Agency (EMA). Public Statement on Contamination of Herbal Medicinal Products/Traditional Herbal Medicinal Products with Pyrrolizidine Alkaloids; European Medicines Agency: Amsterdam, The Netherlands, 2016.
- World Health Organization (WHO). Pyrrolizidine Alkaloids—Environmental Health Criteria 80; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- National Toxicology Program. A Strategic Roadmap for Establishing New Approaches to Evaluate the Safety of Chemicals and Medical Products in the United States; National Toxicology Program; National Institutes of Health: Bethesda, MD, USA, 2018.
- Rietjens, I.M.C.M.; Louisse, J.; Punt, A. Tutorial on physiologically based kinetic modeling in molecular nutrition and food research. Mol. Nutr. Food Res. 2011, 55, 941–956. [Google Scholar] [CrossRef]
- Chiu, W.A.; Barton, H.A.; DeWoskin, R.S.; Schlosser, P.; Thompson, C.M.; Sonawane, B.; Lipscomb, J.C.; Krishnan, K. Evaluation of physiologically based pharmacokinetic models for use in risk assessment. J. Appl. Toxicol. 2007, 27, 218–237. [Google Scholar] [CrossRef]
- Clewell, R.A.; Clewell, H.J., 3rd. Development and specification of physiologically based pharmacokinetic models for use in risk assessment. Regul. Toxicol. Pharmacol. 2008, 50, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Paini, A.; Tan, Y.M.; Sachana, M.; Worth, A. Gaining acceptance in next generation PBK modelling approaches for regulatory assessments—An OECD international effort. Comput. Toxicol. 2021, 18, 100163. [Google Scholar] [CrossRef]
- Chen, L.; Peijnenburg, A.; de Haan, L.; Rietjens, I. Prediction of in vivo genotoxicity of lasiocarpine and riddelliine in rat liver using a combined in vitro-physiologically based kinetic modelling-facilitated reverse dosimetry approach. Arch. Toxicol. 2019, 93, 2385–2395. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Vervoort, J.; Rietjens, I.M.; van Ravenzwaay, B.; Louisse, J. Use of physiologically based kinetic modeling-facilitated reverse dosimetry of in vitro toxicity data for prediction of in vivo developmental toxicity of tebuconazole in rats. Toxicol. Lett. 2017, 266, 85–93. [Google Scholar] [CrossRef]
- Abdullah, R.; Alhusainy, W.; Woutersen, J.; Rietjens, I.M.; Punt, A. Predicting points of departure for risk assessment based on in vitro cytotoxicity data and physiologically based kinetic (PBK) modeling: The case of kidney toxicity induced by aristolochic acid I. Food Chem. Toxicol. 2016, 92, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; van Ravenzwaay, B.; Fabian, E.; Rietjens, I.; Louisse, J. Towards a generic physiologically based kinetic model to predict in vivo uterotrophic responses in rats by reverse dosimetry of in vitro estrogenicity data. Arch. Toxicol. 2018, 92, 1075–1088. [Google Scholar] [CrossRef]
- Thompson, C.M.; Sonawane, B.; Barton, H.A.; DeWoskin, R.S.; Lipscomb, J.C.; Schlosser, P.; Chiu, W.A.; Krishnan, K. Approaches for applications of physiologically based pharmacokinetic models in risk assessment. J. Toxicol. Environ. Health Part B Crit. Rev. 2008, 11, 519–547. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Zhu, L.; Li, Q.X.; Shi, T.; Zhang, Z.; Wu, X.; Yang, T.; Hua, R.; Cao, H. Pyrrolizidine Alkaloids in Tea (Camellia sinensis L.) from Weeds through Weed-Soil-Tea Transfer and Risk Assessment of Tea Intake. J. Agric. Food Chem. 2023, 71, 19045–19053. [Google Scholar] [CrossRef]
| PAs | Cell Line | Exposure Dose (μM) | Exposure Time (h) | IC50/IC20 (μM) | References |
|---|---|---|---|---|---|
| Seneciphylline | HepG2 | 62.5, 125, 250, 500, 1000 | 24 | 660 a | [70] |
| Clivorine | 130 a | ||||
| Retrorsine | 270 a | ||||
| Platyphylline | 850 a | ||||
| Senecionine | 340 a | ||||
| Lasiocarpine | CLR-2118 | 19, 38, 75, 300 | 24 | 14 b | [71] |
| Senecionine | mouse primary hepatocytes | 1, 3, 10, 30, 100 | 48 | 5.41 b | [78] |
| Adonifoline | 49.91 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-S.; Qiu, J.; Mu, X.-Y.; Qian, Y.-Z.; Chen, L. Levels, Toxic Effects, and Risk Assessment of Pyrrolizidine Alkaloids in Foods: A Review. Foods 2024, 13, 536. https://doi.org/10.3390/foods13040536
Lu Y-S, Qiu J, Mu X-Y, Qian Y-Z, Chen L. Levels, Toxic Effects, and Risk Assessment of Pyrrolizidine Alkaloids in Foods: A Review. Foods. 2024; 13(4):536. https://doi.org/10.3390/foods13040536
Chicago/Turabian StyleLu, Yu-Shun, Jing Qiu, Xi-Yan Mu, Yong-Zhong Qian, and Lu Chen. 2024. "Levels, Toxic Effects, and Risk Assessment of Pyrrolizidine Alkaloids in Foods: A Review" Foods 13, no. 4: 536. https://doi.org/10.3390/foods13040536
APA StyleLu, Y.-S., Qiu, J., Mu, X.-Y., Qian, Y.-Z., & Chen, L. (2024). Levels, Toxic Effects, and Risk Assessment of Pyrrolizidine Alkaloids in Foods: A Review. Foods, 13(4), 536. https://doi.org/10.3390/foods13040536







