Lipid-Induced Oxidative Modifications Decrease the Bioactivities of Collagen Hydrolysates from Fish Skin: The Underlying Mechanism Based on the Proteomic Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of CPs and Molecular Weight (MW) Distribution
2.3. Induction Methods of Oxidation
2.4. Measurement of Oxidation Levels of Lipid and Peptides
2.4.1. Lipid Oxidation
2.4.2. Total Sulfhydryl Content in CPs
2.4.3. Carbonyl Content in CPs
2.4.4. Schiff Base Content in CPs
2.4.5. Dityrosine in CPs
2.5. Determination of Biological Activity
2.5.1. Antioxidant Activity
2.5.2. Antihypertensive Activity
2.6. Determination of Total Amino Acids and Free Amino Acids
2.7. Identification of the Modified Peptides
2.8. Statistical Analysis
3. Results and Discussion
3.1. MW Distribution of CPs
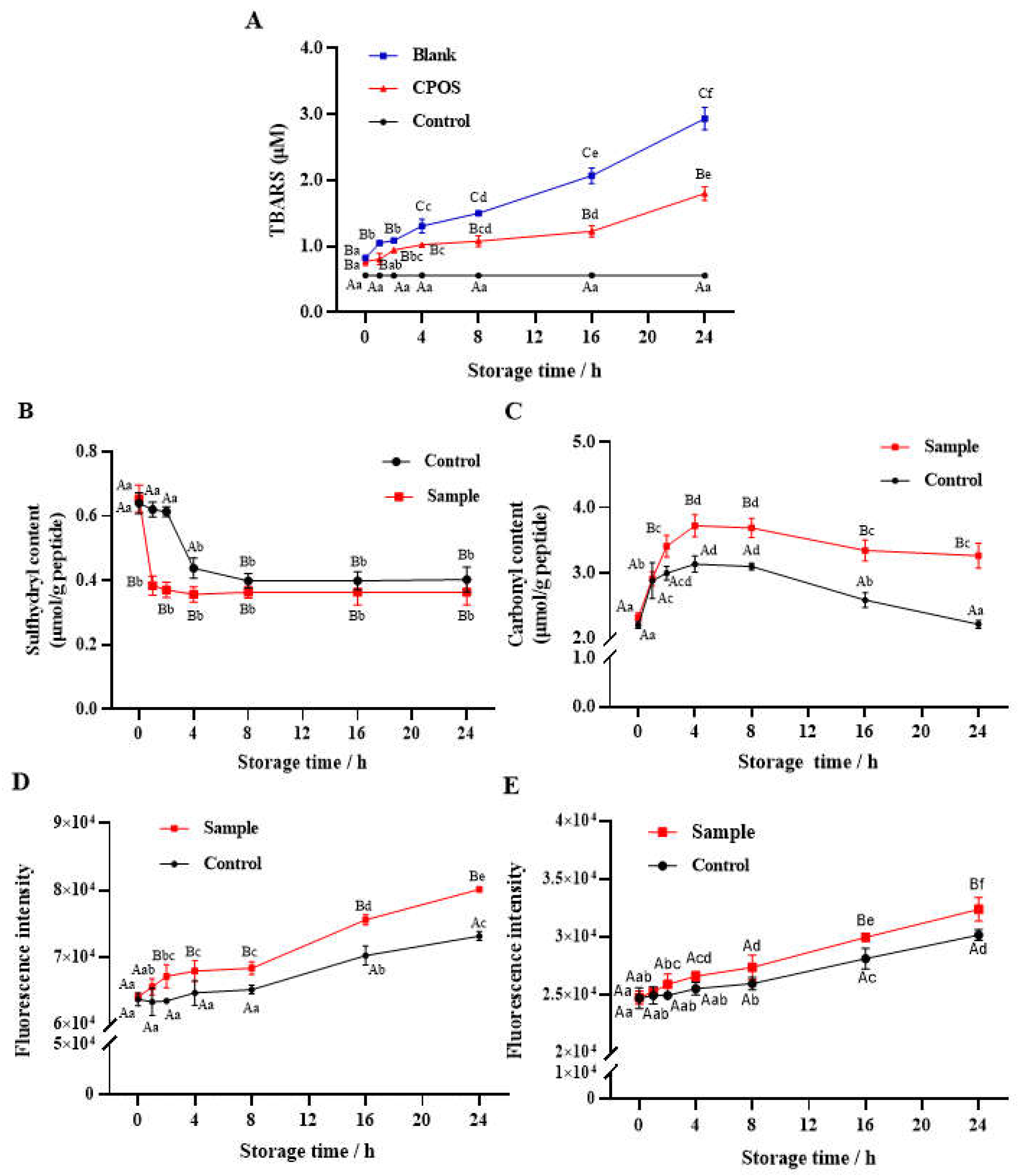
3.2. Oxidation Dynamics of Lipid and CPs
3.2.1. Lipid Oxidation
3.2.2. Sulfhydryl Content
3.2.3. Carbonyl Content
3.2.4. Schiff Base
3.2.5. Dityrosine
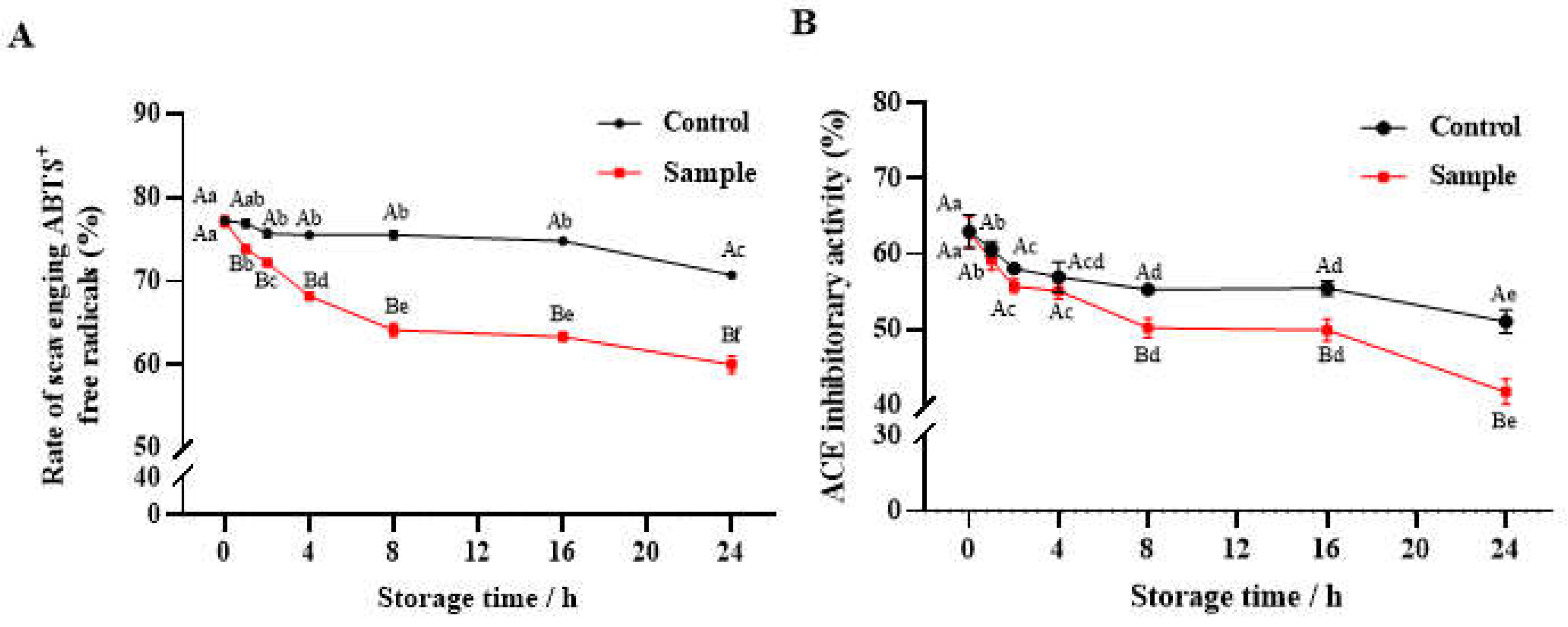
3.3. Changes in Biological Activity
3.3.1. Antioxidant Activity
3.3.2. Antihypertensive Activity
3.4. Correlation Analysis between Oxidative Behavior and Biological Activity
3.5. Changes in Total Amino Acids and Free Amino Acids
3.6. Identification of Modified Peptides
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Chen, J.; Jiang, X.; Yin, L.; Zhang, X. Antioxidant and Hypoglycaemic Effects of Tilapia Skin Collagen Peptide in Mice. Int. J. Food Sci. Technol. 2016, 51, 2157–2163. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Liu, Y.; Cui, L.; Fu, L.; Li, B. Various Bioactive Peptides in Collagen Hydrolysate from Salmo Salar Skin and the Combined Inhibitory Effects on Atherosclerosis in Vitro and in Vivo. Food Res. Int. 2022, 157, 111281. [Google Scholar] [CrossRef] [PubMed]
- Alolod, G.A.L.; Nuñal, S.N.; Nillos, M.G.G.; Peralta, J.P. Bioactivity and Functionality of Gelatin Hydrolysates from the Skin of Oneknife Unicornfish (Naso thynnoides). J. Aquat. Food Prod. Technol. 2019, 28, 1013–1026. [Google Scholar] [CrossRef]
- Liu, H.; Li, B. Separation and Identification of Collagen Peptides Derived from Enzymatic Hydrolysate of Salmo salar Skin and Their Anti-inflammatory Activity in Lipopolysaccharide (LPS)-induced RAW264.7 Inflammatory Model. J. Food Biochem. 2022, 46, e14122. [Google Scholar] [CrossRef]
- Cui, L.; Li, B. Enrichment of Antiplatelet Peptides and Removal of Fishy Odor from Silver Carp Skin Collagen Hydrolysates by Macroporous Resins: pH Value of Loading Sample Affects the Peptides Separation. Food Chem. 2023, 411, 135481. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, S.; Liu, H.; Li, B. Osteogenic Peptides in Collagen Hydrolysates: Stimulate Differentiation of MC3T3-E1 Cells via Β1 Integrin-FAK-ERK1/2 Signaling Pathway and Smad1 Protein. Food Biosci. 2022, 47, 101775. [Google Scholar] [CrossRef]
- Kamdem, J.P.; Tsopmo, A. Reactivity of Peptides within the Food Matrix. J. Food Biochem. 2019, 43, e12489. [Google Scholar] [CrossRef]
- Evangelho, J.A.D.; Vanier, N.L.; Pinto, V.Z.; Berrios, J.J.D.; Dias, A.R.G.; Zavareze, E.D.R. Black Bean (Phaseolus vulgaris L.) Protein Hydrolysates: Physicochemical and Functional Properties. Food Chem. 2017, 214, 460–467. [Google Scholar] [CrossRef]
- Rao, Q.; Klaassen Kamdar, A.; Labuza, T.P. Storage Stability of Food Protein Hydrolysates—A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1169–1192. [Google Scholar] [CrossRef]
- Yu, Y.; Guan, S.; Li, X.; Sun, B.; Lin, S.; Gao, F. Physicochemical and Functional Properties of Egg White Peptide Powders under Different Storage Conditions. J. Food Sci. Technol. 2023, 60, 732–741. [Google Scholar] [CrossRef]
- Kerkaert, B.; Mestdagh, F.; Cucu, T.; Shrestha, K.; Van Camp, J.; De Meulenaer, B. The Impact of Photo-Induced Molecular Changes of Dairy Proteins on Their ACE-Inhibitory Peptides and Activity. Amino Acids 2012, 43, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M. The Chemistry of Protein Oxidation in Food. Angew. Chem. Int. Ed. 2019, 58, 16742–16763. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Evaluation of the Bioactive Potential of Foods Fortified with Fish Protein Hydrolysates. Food Res. Int. 2020, 137, 109572. [Google Scholar] [CrossRef] [PubMed]
- Hematyar, N.; Rustad, T.; Sampels, S.; Kastrup Dalsgaard, T. Relationship between Lipid and Protein Oxidation in Fish. Aquac. Res. 2019, 50, 1393–1403. [Google Scholar] [CrossRef]
- Feng, R.; Liang, W.; Liu, Y.; Luo, Y.; Tan, Y.; Hong, H. Protein Oxidation Affected the Digestibility and Modification Sites of Myofibrillar Proteins from Bighead Carp Fillets Treated with Hydroxyl Radicals and Endogenous Oxidizing System. Food Chem. 2023, 409, 135279. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhang, C.; Lin, H.; Yang, P.; Hong, P.; Jiang, Z. Isolation and Characterisation of Acid-Solubilised Collagen from the Skin of Nile Tilapia (Oreochromis niloticus). Food Chem. 2009, 116, 879–883. [Google Scholar] [CrossRef]
- Gao, S.; Fu, Z.; Zhang, L.; Li, B.; Tan, Y.; Hong, H.; Luo, Y. Oxidation and Side-Chain Modifications Decrease Gastrointestinal Digestibility and Transport of Proteins from Salted Bighead Carp Fillets after Frozen Storage. Food Chem. 2023, 428, 136747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, Y.; Cheng, X.; Meng, M.; Luo, Y.; Li, B. The Anti-Photoaging Effect of Antioxidant Collagen Peptides from Silver Carp (Hypophthalmichthys molitrix) Skin Is Preferable to Tea Polyphenols and Casein Peptides. Food Funct. 2017, 8, 1698–1707. [Google Scholar] [CrossRef]
- Xu, M.; Lian, Z.; Chen, X.; Yao, X.; Lu, C.; Niu, X.; Xu, M.; Zhu, Q. Effects of Resveratrol on Lipid and Protein Co-Oxidation in Fish Oil-Enriched Whey Protein Isolate Emulsions. Food Chem. 2021, 365, 130525. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, D.-Y.; Liu, Z.-Y.; Yin, F.-W.; Liu, Z.-Q.; Li, D.-Y.; Shahidi, F. Hydrolysis and Oxidation of Lipids in Mussel Mytilus edulis during Cold Storage. Food Chem. 2019, 272, 109–116. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.; Garland, D.; Oliver, C.; Amici, A.; Climent, I.; Lenz, A.; Ahn, B.; Shaltiel, S.; Stadtman, E. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Kylli, P.; Puolanne, E.; Kivikari, R.; Heinonen, M. Fluorescence Spectroscopy as a Novel Approach for the Assessment of Myofibrillar Protein Oxidation in Oil-in-Water Emulsions. Meat Sci. 2008, 80, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.; Delsignore, M.; Lin, S. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J. Biol. Chem. 1987, 262, 9902–9907. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Changes in the Antioxidant Activity of Loach (Misgurnus anguillicaudatus) Protein Hydrolysates during a Simulated Gastrointestinal Digestion. Food Chem. 2010, 120, 810–816. [Google Scholar] [CrossRef]
- Yang, Y.-J.; He, H.-Y.; Wang, F.-Z.; Ju, X.-R.; Yuan, J.; Wang, L.-F.; Aluko, R.E.; He, R. Transport of Angiotensin Converting Enzyme and Renin Dual Inhibitory Peptides LY, RALP and TF across Caco-2 Cell Monolayers. J. Funct. Foods 2017, 35, 303–314. [Google Scholar] [CrossRef]
- Trabelsi, I.; Ben Slima, S.; Ktari, N.; Triki, M.; Abdehedi, R.; Abaza, W.; Moussa, H.; Abdeslam, A.; Ben Salah, R. Incorporation of Probiotic Strain in Raw Minced Beef Meat: Study of Textural Modification, Lipid and Protein Oxidation and Color Parameters during Refrigerated Storage. Meat Sci. 2019, 154, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Padilla, P.; Carvalho, L.; Martín, L.; Carrapiso, A.; Delgado, J. Malondialdehyde Interferes with the Formation and Detection of Primary Carbonyls in Oxidized Proteins. Redox Biol. 2019, 26, 101277. [Google Scholar] [CrossRef]
- Estévez, M. Protein Carbonyls in Meat Systems: A Review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Estévez, M.; Díaz-Velasco, S.; Martínez, R. Protein Carbonylation in Food and Nutrition: A Concise Update. Amino Acids 2022, 54, 559–573. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Zhu, H.; Xiong, Y.L. Mass Spectrometric Evidence of Malonaldehyde and 4-Hydroxynonenal Adductions to Radical-Scavenging Soy Peptides. J. Agric. Food Chem. 2012, 60, 9727–9736. [Google Scholar] [CrossRef] [PubMed]
- Morzel, M.; Gatellier, P.; Sayd, T.; Renerre, M.; Laville, E. Chemical Oxidation Decreases Proteolytic Susceptibility of Skeletal Muscle Myofibrillar Proteins. Meat Sci. 2006, 73, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yang, X.; Wang, T.; Li, L.; Wu, Y.; Zhou, Y.; You, L. Purification and Identification of Antioxidant Peptides from Round Scad (Decapterus maruadsi) Hydrolysates by Consecutive Chromatography and Electrospray Ionization-Mass Spectrometry. Food Chem. Toxicol. 2020, 135, 110882. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, C.; Tsai, E.; Chen, G. Preparation and Identification of Novel Antihypertensive Peptides from the In Vitro Gastrointestinal Digestion of Marine Cobia Skin Hydrolysates. Nutrients 2019, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Milic, I.; Melo, T.; Domingues, M.R.; Domingues, P.; Fedorova, M. Heterogeneity of Peptide Adducts with Carbonylated Lipid Peroxidation Products: MS/MS Characterization of Peptide-Lipid Adducts. J. Mass Spectrom. 2015, 50, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhuang, S.; Zhang, L.; Lametsch, R.; Tan, Y.; Li, B.; Hong, H.; Luo, Y. Proteomic Evidence of Protein Degradation and Oxidation in Brined Bighead Carp Fillets during Long-Term Frozen Storage. Food Chem. 2024, 433, 137312. [Google Scholar] [CrossRef]
- Sante-Lhoutellier, V.; Aubry, L.; Gatellier, P. Effect of Oxidation on in Vitro Digestibility of Skeletal Muscle Myofibrillar Proteins. J. Agric. Food Chem. 2007, 55, 5343–5348. [Google Scholar] [CrossRef] [PubMed]
- Yathisha, U.G.; Bhat, I.; Karunasagar, I.; Mamatha, B.S. Antihypertensive Activity of Fish Protein Hydrolysates and Its Peptides. Crit. Rev. Food Sci. Nutr. 2019, 59, 2363–2374. [Google Scholar] [CrossRef]
- Soladoye, O.P.; Juárez, M.L.; Aalhus, J.L.; Shand, P.; Estévez, M. Protein Oxidation in Processed Meat: Mechanisms and Potential Implications on Human Health. Compr. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, C.; Ji, H. Two Novel Bioactive Peptides from Antarctic Krill with Dual Angiotensin Converting Enzyme and Dipeptidyl Peptidase IV Inhibitory Activities. J. Food Sci. 2017, 82, 1742–1749. [Google Scholar] [CrossRef]
| Component | Relative Content (%) | ||
|---|---|---|---|
| >1000 Da | 500–1000 Da | <500 Da | |
| Collagen hydrolysates | 16.75 | 25.04 | 58.22 |
| CPs | 24.42 | 30.12 | 45.47 |
| Time | Variables | Sulfhydryl | Carbonyl | Schiff Base | Dityrosine | Antioxidant | Antihypertensive |
|---|---|---|---|---|---|---|---|
| 0–4 h | Sulfhydryl | \ | −0.830 ** | −0.646 * | −0.677 * | 0.762 ** | 0.832 ** |
| Carbonyl | \ | 0.721 ** | 0.739 ** | −0.947 ** | −0.911 ** | ||
| Schiff base | \ | 0.842 ** | −0.754 ** | −0.717 ** | |||
| Dityrosine | \ | −0.777 ** | −0.800 ** | ||||
| Antioxidant | \ | 0.858 ** | |||||
| Antihypertensive | \ | ||||||
| 4–8 h | Sulfhydryl | \ | 0.674 | 0.472 | −0.036 | 0.134 | 0.028 |
| Carbonyl | \ | −0.217 | −0.480 | 0.304 | 0.150 | ||
| Schiff base | \ | 0.279 | −0.036 | 0.04 | |||
| Dityrosine | \ | −0.549 | −0.630 | ||||
| Antioxidant | \ | 0.964 ** | |||||
| Antihypertensive | \ | ||||||
| 8–24 h | Sulfhydryl | \ | 0.104 | 0.096 | 0.059 | 0.242 | −0.107 |
| Carbonyl | \ | −0.707 * | −0.790 * | 0.542 | 0.618 | ||
| Schiff base | \ | 0.939 ** | −0.820 ** | −0.779 * | |||
| Dityrosine | \ | −0.801 ** | −0.865 ** | ||||
| Antioxidant | \ | 0.860 ** | |||||
| Antihypertensive | \ | ||||||
| 0–24 h | Sulfhydryl | \ | −0.767 ** | −0.405 | −0.422 | 0.613 ** | 0.565 ** |
| Carbonyl | \ | 0.302 | 0.303 | −0.623 ** | −0.516 * | ||
| Schiff base | \ | 0.969 ** | −0.863 ** | −0.895 ** | |||
| Dityrosine | \ | −0.882 ** | −0.933 ** | ||||
| Antioxidant | \ | 0.943 ** | |||||
| Antihypertensive | \ |
| Type C | Amino Acid B | Sample A | Control A | |||
|---|---|---|---|---|---|---|
| 0 h | 1 h | 4 h | 24 h | Con-24 h | ||
| Total | Asp | 43.24 ± 1.45 ab | 45.20 ± 0.63 a | 42.79 ± 0.23 ab | 43.50 ± 0.44 ab | 42.24 ± 0.36 b |
| Glu | 67.62 ± 0.67 b | 71.04 ± 0.07 a | 67.09 ± 0.59 b | 70.63 ± 0.16 a | 66.85 ± 0.15 b | |
| Hyp | 80.53 ± 0.16 a | 78.20 ± 0.65 b | 78.03 ± 0.49 b | 73.41 ± 0.65 c | 77.49 ± 0.05 b | |
| Cys-Cys | 0.28 ± 0.01 | 0.30 ± 0.03 | 0.31 ± 0.06 | 0.27 ± 0.03 | 0.27 ± 0.07 | |
| Ser | 22.63 ± 0.02 c | 24.75 ± 0.13 a | 23.61 ± 0.16 b | 24.69 ± 0.07 a | 22.76 ± 0.58 bc | |
| Gly | 162.96 ± 0.77 bc | 168.58 ± 0.82 a | 161.56 ± 1.87 c | 167.24 ± 0.15 ab | 161.16 ± 1.66 c | |
| His | 5.50 ± 0.09 ab | 5.69 ± 0.07 a | 5.47 ± 0.00 b | 5.09 ± 0.05 c | 5.53 ± 0.04 ab | |
| Arg | 58.96 ± 0.27 a | 57.12 ± 0.60 bc | 55.82 ± 0.57 c | 56.03 ± 0.51 bc | 57.57 ± 0.26 ab | |
| Thr | 15.84 ± 0.53 a | 14.56 ± 0.35 ab | 14.01 ± 0.25 b | 14.16 ± 0.01 ab | 14.44 ± 0.80 ab | |
| Ala | 59.39 ± 0.39 bc | 61.25 ± 0.18 a | 58.34 ± 0.60 c | 60.39 ± 0.25 ab | 58.78 ± 0.79 bc | |
| Pro | 138.28 ± 2.73 a | 133.62 ± 1.20 a | 121.93 ± 0.03 b | 93.86 ± 0.98 c | 136.45 ± 0.64 a | |
| Tyr | 2.40 ± 0.14 a | 2.25 ± 0.02 a | 0.98 ± 0.06 bc | 0.88 ± 0.02 c | 1.15 ± 0.01 b | |
| Val | 11.41 ± 0.09 ab | 11.82 ± 0.26 a | 10.96 ± 0.01 b | 11.42 ± 0.63 ab | 11.09 ± 0.10 b | |
| Met | 4.12 ± 0.05 b | 3.65 ± 0.09 c | 3.12 ± 0.11 e | 3.37 ± 0.04 d | 5.31 ± 0.05 a | |
| Ile | 5.46 ± 0.16 | 5.59 ± 0.07 | 5.39 ± 0.06 | 5.41 ± 0.08 | 5.40 ± 0.04 | |
| Leu | 12.47 ± 0.41 | 12.98 ± 0.39 | 12.02 ± 0.22 | 12.55 ± 0.13 | 12.42 ± 0.37 | |
| Phe | 11.67 ± 0.29 | 11.88 ± 0.09 | 11.41 ± 0.17 | 11.74 ± 0.16 | 11.39 ± 0.04 | |
| Lys | 28.88 ± 0.02 a | 27.77 ± 0.54 b | 25.91 ± 0.49 c | 26.11 ± 0.23 bc | 27.60 ± 0.61 ab | |
| BAAs | 93.33 ± 0.38 a | 90.58 ± 1.21 a | 87.21 ± 1.05 b | 87.23 ± 0.79 b | 90.71 ± 0.90 a | |
| AAAs | 110.86 ± 2.12 bc | 116.24 ± 0.70 a | 109.89 ± 0.82 c | 114.12 ± 0.28 ab | 109.09 ± 0.20 c | |
| ARAAs | 14.07 ± 0.43 a | 14.13 ± 0.12 a | 12.39 ± 0.11 b | 12.62 ± 0.18 b | 12.54 ± 0.05 b | |
| HAAs | 242.18 ± 1.51 a | 240.78 ± 1.78 a | 223.16 ± 1.09 b | 198.75 ± 0.86 c | 240.84 ± 1.73 a | |
| Total | 731.63 ± 1.86 a | 736.24 ± 3.22 a | 698.75 ± 4.14 c | 680.76 ± 0.10 d | 717.87 ± 5.88 b | |
| Free | Total | 3.62 ± 0.03 e | 6.81 ± 0.05 d | 7.52 ± 0.04 c | 12.84 ± 0.04 a | 7.92 ± 0.01 b |
| Sample a | 0 h | 4 h | 24 h | |
|---|---|---|---|---|
| Modifications b | Oxidation | 9.78 | 14.30 | 12.41 |
| Di-oxidation | 10.68 | 10.36 | 8.61 | |
| GGS | 2.59 | 4.84 | 3.61 | |
| AAS | 1.35 | 1.46 | 1.11 | |
| AAA | 0.75 | 0.90 | 0.46 | |
| MDA | 0.34 | 0.85 | 0.65 | |
| Carbonylation | 0.15 | 0.68 | 0.19 | |
| HNE | 0.15 | 0.45 | 0.19 | |
| Sensitive amino acid residue | Met (M) | 7.17 | 22.89 | 14.16 |
| Lys (K) | 18.80 | 21.84 | 22.48 | |
| Arg (R) | 12.91 | 17.68 | 14.81 | |
| Phe (F) | 2.98 | 6.59 | 4.55 | |
| Pro (P) | 2.44 | 2.76 | 2.27 | |
| Asn (N) | 1.61 | 1.95 | 1.72 | |
| Ala (A) | 0 | 0.15 | 0.07 | |
| O:P | 40.17 | 41.71 | 41.36 | |
| Common peptide segment c | PGPGPM | PGPGPM | PGPGPM(+15.99) | PGPGPM(+15.99) |
| PGAR | PGAR(+15.99)-X | PGAR(+15.99)-X | PGAR(+54.01)-X | |
| GPAGPR | GPAGPR(+31.99)-X | GPAGPR(−43.05)-X | GPAGPR(−43.05)-X | |
| KAPDPFR | KAPDPFR | K(−1.03)APDPFR | K(+14.96)APDPFR | |
| KAPDPLR | K(−1.03)APDPLR-X | K(+14.96)APDPLR-X | K(+14.96)APDPLR-X | |
| GPTGR | X1- GPTGR-X2 | X1- GPTGR(+12.98)-X2 | X1- GPTGR(−43.05)-X2 | |
| KGPSG | X1- KGPSG-X2 | X1- KGPSG-X2 | X1-K(+156.12) GPSG -X2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gou, F.; Gao, S.; Li, B. Lipid-Induced Oxidative Modifications Decrease the Bioactivities of Collagen Hydrolysates from Fish Skin: The Underlying Mechanism Based on the Proteomic Strategy. Foods 2024, 13, 583. https://doi.org/10.3390/foods13040583
Gou F, Gao S, Li B. Lipid-Induced Oxidative Modifications Decrease the Bioactivities of Collagen Hydrolysates from Fish Skin: The Underlying Mechanism Based on the Proteomic Strategy. Foods. 2024; 13(4):583. https://doi.org/10.3390/foods13040583
Chicago/Turabian StyleGou, Fengjie, Song Gao, and Bo Li. 2024. "Lipid-Induced Oxidative Modifications Decrease the Bioactivities of Collagen Hydrolysates from Fish Skin: The Underlying Mechanism Based on the Proteomic Strategy" Foods 13, no. 4: 583. https://doi.org/10.3390/foods13040583
APA StyleGou, F., Gao, S., & Li, B. (2024). Lipid-Induced Oxidative Modifications Decrease the Bioactivities of Collagen Hydrolysates from Fish Skin: The Underlying Mechanism Based on the Proteomic Strategy. Foods, 13(4), 583. https://doi.org/10.3390/foods13040583






