The Potential of Crude and Partially Purified Black Rice Bran Extracts Obtained by Ultrasound-Assisted Extraction: Anti-Glycemic, Cytotoxicity, Cytoprotective, and Antitumoral Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Ultrasound-Assisted Extraction (UAE)
2.3. Total Monomeric Anthocyanin (TMA)
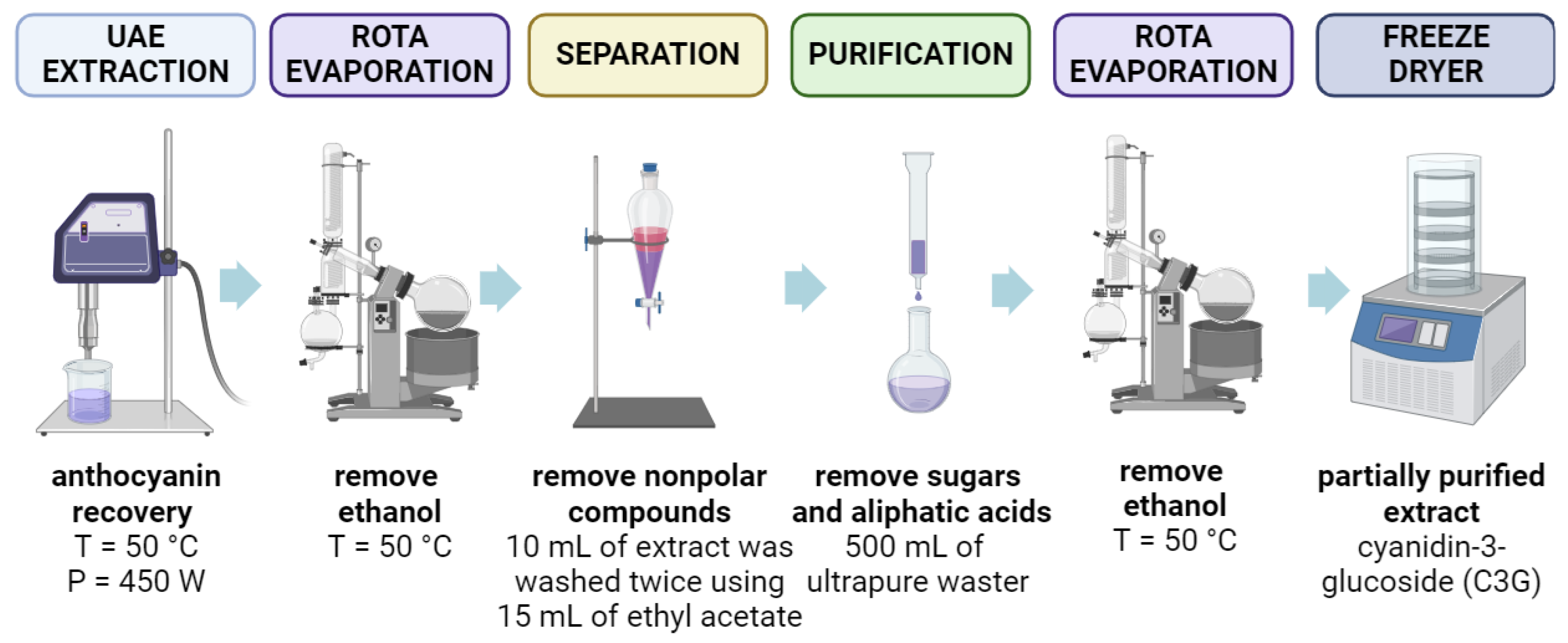
2.3.1. Partial Purification of Anthocyanin-Rich Extracts
2.3.2. Determining the Individual Anthocyanins by HPLC-MS
2.4. Antioxidant Activity
2.5. Effects of Crude and Partially Purified Anthocyanin Extracts on α-Amylase and α-Glucosidase Inhibition
2.6. Cell Culture
2.6.1. Culture Conditioning
2.6.2. Cytotoxicity Assay
2.6.3. Hydrogen Peroxide-Induced Oxidative Stress in L929 Fibroblast Cells
2.6.4. Antitumoral Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Laboratory Scale-up
3.2. Partial Purification of Anthocyanins Extracted from Black Rice Bran
3.3. Comparison of Crude and Partially Purified Anthocyanin Extracts
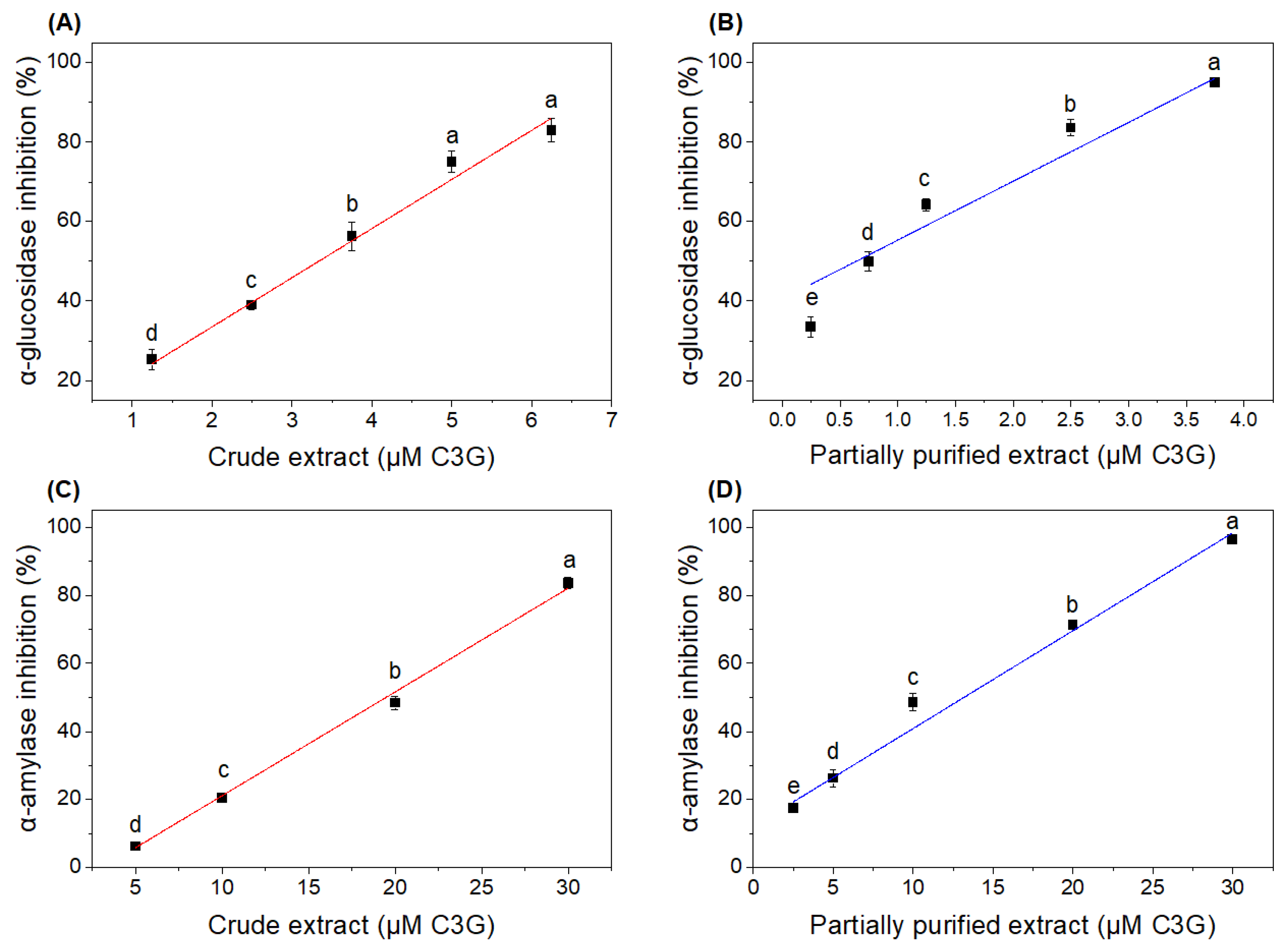
3.4. Effects of Crude and Partially Purified Extracts on In Vitro α-Amylase and α-Glucosidase Inhibition
3.5. Cell Culture
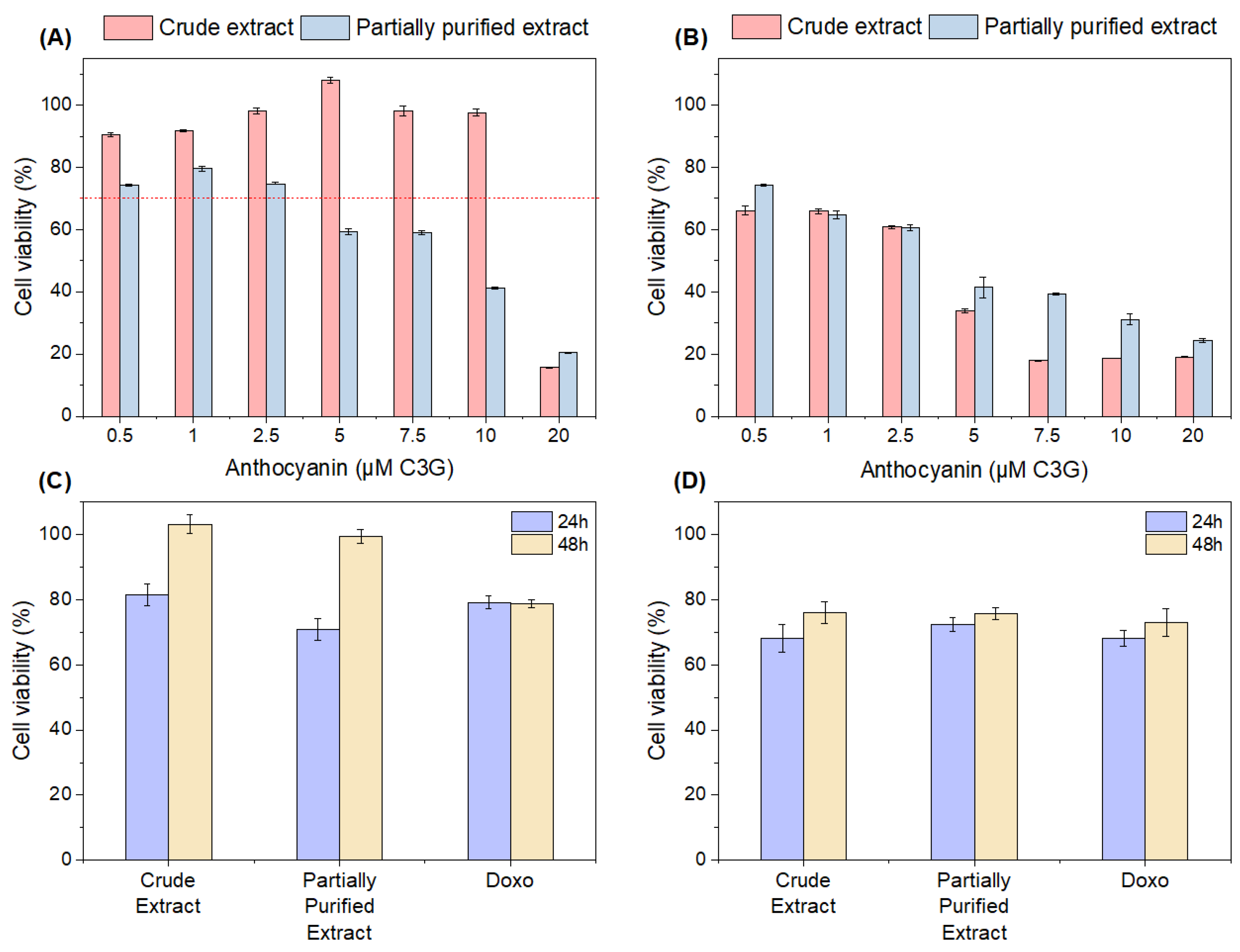
3.5.1. Cytotoxicity
3.5.2. Effects of Crude and Partially Purified Extracts on Cell Viability in H2O2-Induced L929 Cells
3.5.3. Antitumoral Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moraes, C.A.M.; Fernandes, I.J.; Calheiro, D.; Kieling, A.G.; Brehm, F.A.; Rigon, M.R.; Berwanger Filho, J.A.; Schneider, I.A.H.; Osorio, E. Review of the Rice Production Cycle: By-Products and the Main Applications Focusing on Rice Husk Combustion and Ash Recycling. Waste Manag. Res. 2014, 32, 1034–1048. [Google Scholar] [CrossRef]
- Nagrale, S.D.; Hajare, H.; Modak, P.R. Utilization of Rice Husk Ash. Int. J. Eng. Res. Appl. 2012, 2, 1–5. [Google Scholar]
- Van Hoed, V.; Depaemelaere, G.; Ayala, J.V.; Santiwattana, P.; Verhé, R.; De Greyt, W. Influence of Chemical Refining on the Major and Minor Components of Rice Bran Oil. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 315–321. [Google Scholar] [CrossRef]
- Das, A.B.; Goud, V.V.; Das, C. Extraction of Phenolic Compounds and Anthocyanin from Black and Purple Rice Bran (Oryza sativa L.) Using Ultrasound: A Comparative Analysis and Phytochemical Profiling. Ind. Crops Prod. 2017, 95, 332–341. [Google Scholar] [CrossRef]
- Leonarski, E.; Kuasnei, M.; Moraes, P.A.D.; Cesca, K.; de Oliveira, D.; Zielinski, A.A.F. Pressurized Liquid Extraction as an Eco-Friendly Approach to Recover Anthocyanin from Black Rice Bran. Innov. Food Sci. Emerg. Technol. 2023, 86, 103372. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, D.; Jin, Y.; Zhao, J.; Zhao, J.; Li, H.; Li, L.; Zhang, H.; Wang, H. In Vitro and in Vivo Inhibitory Effect of Anthocyanin-Rich Bilberry Extract on α-Glucosidase and α-Amylase. LWT 2021, 145, 111484. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, Y.; Zhou, W. Bread Fortified with Anthocyanin-Rich Extract from Black Rice as Nutraceutical Sources: Its Quality Attributes and in Vitro Digestibility. Food Chem. 2016, 196, 910–916. [Google Scholar] [CrossRef]
- Leonarski, E.; Kuasnei, M.; Cesca, K.; de Oliveira, D.; Zielinski, A.A.F. Black Rice and Its By-Products: Anthocyanin-Rich Extracts and Their Biological Potential. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Moreira, S.A.; Alexandre, E.M.C.; Pintado, M.; Saraiva, J.A. Effect of Emergent Non-Thermal Extraction Technologies on Bioactive Individual Compounds Profile from Different Plant Materials. Food Res. Int. 2019, 115, 177–190. [Google Scholar] [CrossRef]
- Belwal, T.; Devkota, H.P.; Hassan, H.A.; Ahluwalia, S.; Ramadan, M.F.; Mocan, A.; Atanasov, A.G. Phytopharmacology of Acerola (Malpighia spp.) and Its Potential as Functional Food. Trends Food Sci. Technol. 2018, 74, 99–106. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.; et al. Adsorption Properties of Macroporous Adsorbent Resins for Separation of Anthocyanins from Mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef]
- Das, A.B.; Goud, V.V.; Das, C. Adsorption/Desorption, Diffusion, and Thermodynamic Properties of Anthocyanin from Purple Rice Bran Extract on Various Adsorbents. J. Food Process Eng. 2018, 41, e12834. [Google Scholar] [CrossRef]
- Hernández, D.F.; Mojica, L.; Berhow, M.A.; Brownstein, K.; Lugo Cervantes, E.; Gonzalez de Mejia, E. Black and Pinto Beans (Phaseolus vulgaris L.) Unique Mexican Varieties Exhibit Antioxidant and Anti-Inflammatory Potential. Food Res. Int. 2023, 169, 112816. [Google Scholar] [CrossRef]
- Carail, M.; Fabiano-Tixier, A.-S.; Meullemiestre, A.; Chemat, F.; Caris-Veyrat, C. Effects of High Power Ultrasound on All-E-β-Carotene, Newly Formed Compounds Analysis by Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Ultrason. Sonochem. 2015, 26, 200–209. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1–13. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Barik, S.K.; Russell, W.R.; Moar, K.M.; Cruickshank, M.; Scobbie, L.; Duncan, G.; Hoggard, N. The Anthocyanins in Black Currants Regulate Postprandial Hyperglycaemia Primarily by Inhibiting α-Glucosidase while Other Phenolics Modulate Salivary α-Amylase, Glucose Uptake and Sugar Transporters. J. Nutr. Biochem. 2020, 78, 108325. [Google Scholar] [CrossRef]
- Ali, H.; Houghton, P.J.; Soumyanath, A. α-Amylase Inhibitory Activity of Some Malaysian Plants Used to Treat Diabetes; with Particular Reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Aguilar, A.E.M.; Fagundes, A.P.; Macuvele, D.L.P.; Cesca, K.; Porto, L.; Padoin, N.; Soares, C.; Gracher Riella, H. Green Synthesis of Nano Hydroxyapatite: Morphology Variation and Its Effect on Cytotoxicity against Fibroblast. Mater. Lett. 2021, 284, 129013. [Google Scholar] [CrossRef]
- Pitz, H.D.S.; Pereira, A.; Blasius, M.B.; Voytena, A.P.L.; Affonso, R.C.L.; Fanan, S.; Trevisan, A.C.D.; Ribeiro-Do-Valle, R.M.; Maraschin, M. In Vitro Evaluation of the Antioxidant Activity and Wound Healing Properties of Jaboticaba (Plinia peruviana) Fruit Peel Hydroalcoholic Extract. Oxid. Med. Cell. Longev. 2016, 2016, 3403586. [Google Scholar] [CrossRef]
- Sousa, M.H.O.; Morgan, J.M.S.; Cesca, K.; Flach, A.; de Moura, N.F. Cytotoxic Activity of Cunila angustifolia Essential Oil. Chem. Biodivers. 2020, 17, 3–7. [Google Scholar] [CrossRef]
- Gille, A.; Trautmann, A.; Posten, C.; Briviba, K. Bioaccessibility of Carotenoids from Chlorella Vulgaris and Chlamydomonas Reinhardtii. Int. J. Food Sci. Nutr. 2016, 67, 507–513. [Google Scholar] [CrossRef]
- Chen, L.; Yang, M.; Mou, H.; Kong, Q. Ultrasound-Assisted Extraction and Characterization of Anthocyanins from Purple Corn Bran. J. Food Process. Preserv. 2018, 42, e13377. [Google Scholar] [CrossRef]
- Heinonen, J.; Farahmandazad, H.; Vuorinen, A.; Kallio, H.; Yang, B.; Sainio, T. Extraction and Purification of Anthocyanins from Purple-Fleshed Potato. Food Bioprod. Process. 2016, 99, 136–146. [Google Scholar] [CrossRef]
- Kang, Y.J.; Jung, S.W.; Lee, S.J. An Optimal Extraction Solvent and Purification Adsorbent to Produce Anthocyanins from Black Rice (Oryza sativa Cv. Heugjinjubyeo). Food Sci. Biotechnol. 2014, 23, 97–106. [Google Scholar] [CrossRef]
- Chang, X.L.; Wang, D.; Chen, B.Y.; Feng, Y.M.; Wen, S.H.; Zhan, P.Y. Adsorption and Desorption Properties of Macroporous Resins for Anthocyanins from the Calyx Extract of Roselle (Hibiscus sabdariffa L.). J. Agric. Food Chem. 2012, 60, 2368–2376. [Google Scholar] [CrossRef]
- Wathon, M.H.; Beaumont, N.; Benohoud, M.; Blackburn, R.S.; Rayner, C.M. Extraction of Anthocyanins from Aronia Melanocarpa Skin Waste as a Sustainable Source of Natural Colorants. Color Technol. 2019, 135, 5–16. [Google Scholar] [CrossRef]
- Contreras, J.; Alcázar-Valle, M.; Lugo-Cervantes, E.; Luna-Vital, D.A.; Mojica, L. Mexican Native Black Bean Anthocyanin-Rich Extracts Modulate Biological Markers Associated with Inflammation. Pharmaceuticals 2023, 16, 874. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, M.; Zhan, Y.; Zhan, K.; Chang, X.; Yang, H.; Li, Z. Characterization and Purification of Anthocyanins from Black Peanut (Arachis hypogaea L.) Skin by Combined Column Chromatography. J. Chromatogr. A 2017, 1519, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, E.J.; Lim, Y.Y.; Choo, W.S. Antioxidant, Cytotoxic, and Antibacterial Activities of Clitoria ternatea Flower Extracts and Anthocyanin-Rich Fraction. Sci. Rep. 2022, 12, 14890. [Google Scholar] [CrossRef] [PubMed]
- Chumchoochart, W.; Sutthanut, K. Anti-Obesity Potential of Glutinous Black Rice Bran Extract: Anti-Adipogenesis and Lipolysis Induction in 3T3-L1 Adipocyte Model. Songklanakarin J. Sci. Technol. 2020, 42, 284–291. [Google Scholar] [CrossRef]
- Marathe, S.J.; Shah, N.N.; Bajaj, S.R.; Singhal, R.S. Esterification of Anthocyanins Isolated from Floral Waste: Characterization of the Esters and Their Application in Various Food Systems. Food Biosci. 2021, 40, 100852. [Google Scholar] [CrossRef]
- Sansenya, S.; Nanok, K. A-glucosidase, A-amylase Inhibitory Potential and Antioxidant Activity of Fragrant Black Rice (Thai Coloured Rice). Flavour Fragr. J. 2020, 35, 376–386. [Google Scholar] [CrossRef]
- Shimoda, H.; Aitani, M.; Tanaka, J.; Hitoe, S. Purple Rice Extract Exhibits Preventive Activities on Experimental Diabetes Models and Human Subjects. Rice Res. Open Access 2015, 3, 137. [Google Scholar] [CrossRef]
- Choi, K.; Choi, S.-I.; Park, M.H.; Han, J.-S. Cyanidin-3-O-Glucoside Ameliorates Postprandial Hyperglycemia in Diabetic Mice. J. Life Sci. 2017, 27, 32–37. [Google Scholar] [CrossRef]
- Aalim, H.; Wang, D.; Luo, Z. Black Rice (Oryza sativa L.) Processing: Evaluation of Physicochemical Properties, in Vitro Starch Digestibility, and Phenolic Functions Linked to Type 2 Diabetes. Food Res. Int. 2021, 141, 109898. [Google Scholar] [CrossRef]
- Akkarachiyasit, S.; Yibchok-Anun, S.; Wacharasindhu, S.; Adisakwattana, S. In Vitro Inhibitory Effects of Cyandin-3-Rutinoside on Pancreatic α-Amylase and Its Combined Effect with Acarbose. Molecules 2011, 16, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Shafique, Z.; Amjad, S.T.; Bin Asad, M.H.H. Enzymes Inhibitors from Natural Sources with Antidiabetic Activity: A Review. Phyther. Res. 2019, 33, 41–54. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products As-Amylase and-Glucosidase Inhibitors and Their Hypoglycaemic Potential in the Treatment of Diabetes: An Update; Bentham Science Publishers: Sharjah, United Arab Emirates, 2010; Volume 10. [Google Scholar]
- Hui, X.; Wu, G.; Han, D.; Stipkovits, L.; Wu, X.; Tang, S.; Brennan, M.A.; Brennan, C.S. The Effects of Bioactive Compounds from Blueberry and Blackcurrant Powders on the Inhibitory Activities of Oat Bran Pastes against α-Amylase and α-Glucosidase Linked to Type 2 Diabetes. Food Res. Int. 2020, 138, 109756. [Google Scholar] [CrossRef]
- Sangkitikomol, W.; Tencomnao, T.; Rocejanasaroj, A. Effects of Thai Black Sticky Rice Extract on Oxidative Stress and Lipid Metabolism Gene Expression in Hepg2 Cells. Genet. Mol. Res. 2010, 9, 2086–2095. [Google Scholar] [CrossRef]
- Aprodu, I.; Milea, S.A.; Anghel, R.M.; Enachi, E.; Barbu, V.; Crăciunescu, O.; Râpeanu, G.; Bahrim, G.E.; Oancea, A.; Stănciuc, N. New Functional Ingredients Based on Microencapsulation of Aqueous Anthocyanin-Rich Extracts Derived from Black Rice (Oryza sativa L.). Molecules 2019, 24, 3389. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Liu, Y.; Xu, C.; Liu, J. Antiproliferative and Proapoptotic Activities of Anthocyanin and Anthocyanidin Extracts from Blueberry Fruits on B16-F10 Melanoma Cells. Food Nutr. Res. 2017, 61, 1325308. [Google Scholar] [CrossRef] [PubMed]
- Ereminas, G.; Majiene, D.; Sidlauskas, K.; Jakstas, V.; Ivanauskas, L.; Vaitiekaitis, G.; Liobikas, J. Neuroprotective Properties of Anthocyanidin Glycosides against H2O2-Induced Glial Cell Death Are Modulated by Their Different Stability and Antioxidant Activity in Vitro. Biomed. Pharmacother. 2017, 94, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Kang, M.; Xie, Q.; Xu, B.; Sun, C.; Chen, K.; Wu, Y. Anthocyanins from Chinese Bayberry Extract Protect β Cells from Oxidative Stress-Mediated Injury via HO-1 Upregulation. J. Agric. Food Chem. 2011, 59, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, P.; Xue, H.; Li, Q. Cyanidin-3-Glucoside Prevents Hydrogen Peroxide (H2O2)-Induced Oxidative Damage in HepG2 Cells. Biotechnol. Lett. 2020, 42, 2453–2466. [Google Scholar] [CrossRef] [PubMed]
- Palungwachira, P.; Tancharoen, S.; Dararat, P.; Nararatwanchai, T. Anthocyanins Isolated from Oryza sativa L. Protect Dermal Fibroblasts from Hydrogen Peroxide-Induced Cell Death. J. Nat. Sci. Biol. Med. 2020, 11, 45–54. [Google Scholar] [CrossRef]
- Xu, L.; Choi, T.H.; Kim, S.; Kim, S.H.; Chang, H.W.; Choe, M.; Kwon, S.Y.; Hur, J.A.; Shin, S.C.; Chung, J., II; et al. Anthocyanins from Black Soybean Seed Coat Enhance Wound Healing. Ann. Plast. Surg. 2013, 71, 415–420. [Google Scholar] [CrossRef]
- Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Ultrasound-Assisted Enzymatic Extraction of Anthocyanins from Raspberry Wine Residues: Process Optimization, Isolation, Purification, and Bioactivity Determination. Food Anal. Methods 2021, 14, 1369–1386. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Xu, X. Cyanidin-3-Glucoside Suppresses the Progression of Lung Adenocarcinoma by Downregulating TP53I3 and Inhibiting PI3K/AKT/MTOR Pathway. World J. Surg. Oncol. 2021, 19, 232. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.N.; Panchanathan, R.; Lee, W.S.; Kim, H.J.; Kim, D.H.; Choi, Y.H.; Kim, G.S.; Shin, S.C.; Hong, S.C. Anthocyanins from the Fruit of Vitis coignetiae Pulliat Inhibit TNF-Augmented Cancer Proliferation, Migration, and Invasion in A549 Cells. Asian Pacific J. Cancer Prev. 2017, 18, 2919–2923. [Google Scholar] [CrossRef]
| Frequency Power (W) | Real Frequency Power (W) | Ultrasound Intensity (W/cm2) | ||
|---|---|---|---|---|
| 15 mL Reactor | 300 mL Reactor | 15 mL Reactor | 300 mL Reactor | |
| 300 | 24.61 | 71.31 | 3.48 | 2.52 |
| 350 | 27.89 | 78.04 | 3.95 | 2.76 |
| 380 | 28.85 | 80.58 | 4.08 | 2.85 |
| 400 | 30.99 | 85.69 | 4.39 | 3.03 |
| 450 | 32.18 | 88.31 | 4.55 | 3.12 |
| Analysis | Extracts | |
|---|---|---|
| Crude | Partially Purified | |
| TMA (mg/g) | 1.06 ± 0.04 b | 4.44 ± 0.60 a |
| C3G (mg/g) | 1.03 ± 0.01 b | 3.97 ± 0.02 a |
| DPPH IC50 (mg/mL) | 1.57 ± 0.03 a | 0.67 ± 0.01 b |
| ABTS IC50 (mg/mL) | 1.26 ± 0.01 a | 0.33 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonarski, E.; Kuasnei, M.; Santos, E.H.; Moraes, P.A.D.; Cesca, K.; Oliveira, D.d.; Zielinski, A.A.F. The Potential of Crude and Partially Purified Black Rice Bran Extracts Obtained by Ultrasound-Assisted Extraction: Anti-Glycemic, Cytotoxicity, Cytoprotective, and Antitumoral Effects. Foods 2024, 13, 597. https://doi.org/10.3390/foods13040597
Leonarski E, Kuasnei M, Santos EH, Moraes PAD, Cesca K, Oliveira Dd, Zielinski AAF. The Potential of Crude and Partially Purified Black Rice Bran Extracts Obtained by Ultrasound-Assisted Extraction: Anti-Glycemic, Cytotoxicity, Cytoprotective, and Antitumoral Effects. Foods. 2024; 13(4):597. https://doi.org/10.3390/foods13040597
Chicago/Turabian StyleLeonarski, Eduardo, Mayara Kuasnei, Eloisa H. Santos, Paulo A. D. Moraes, Karina Cesca, Débora de Oliveira, and Acácio A. F. Zielinski. 2024. "The Potential of Crude and Partially Purified Black Rice Bran Extracts Obtained by Ultrasound-Assisted Extraction: Anti-Glycemic, Cytotoxicity, Cytoprotective, and Antitumoral Effects" Foods 13, no. 4: 597. https://doi.org/10.3390/foods13040597
APA StyleLeonarski, E., Kuasnei, M., Santos, E. H., Moraes, P. A. D., Cesca, K., Oliveira, D. d., & Zielinski, A. A. F. (2024). The Potential of Crude and Partially Purified Black Rice Bran Extracts Obtained by Ultrasound-Assisted Extraction: Anti-Glycemic, Cytotoxicity, Cytoprotective, and Antitumoral Effects. Foods, 13(4), 597. https://doi.org/10.3390/foods13040597







