Comparative Analysis of Characteristic Volatile Compounds in Five Types of Infant Nutrition Powders by HS-GC-IMS and GC-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Color Measurements
2.3. Headspace (HS)-GC-IMS Analysis
2.4. Analysis of Fatty Acids in YYB Samples from Different Manufacturers before and after Storage Using GC-MS
2.5. Statistical Analysis
3. Results and Discussion
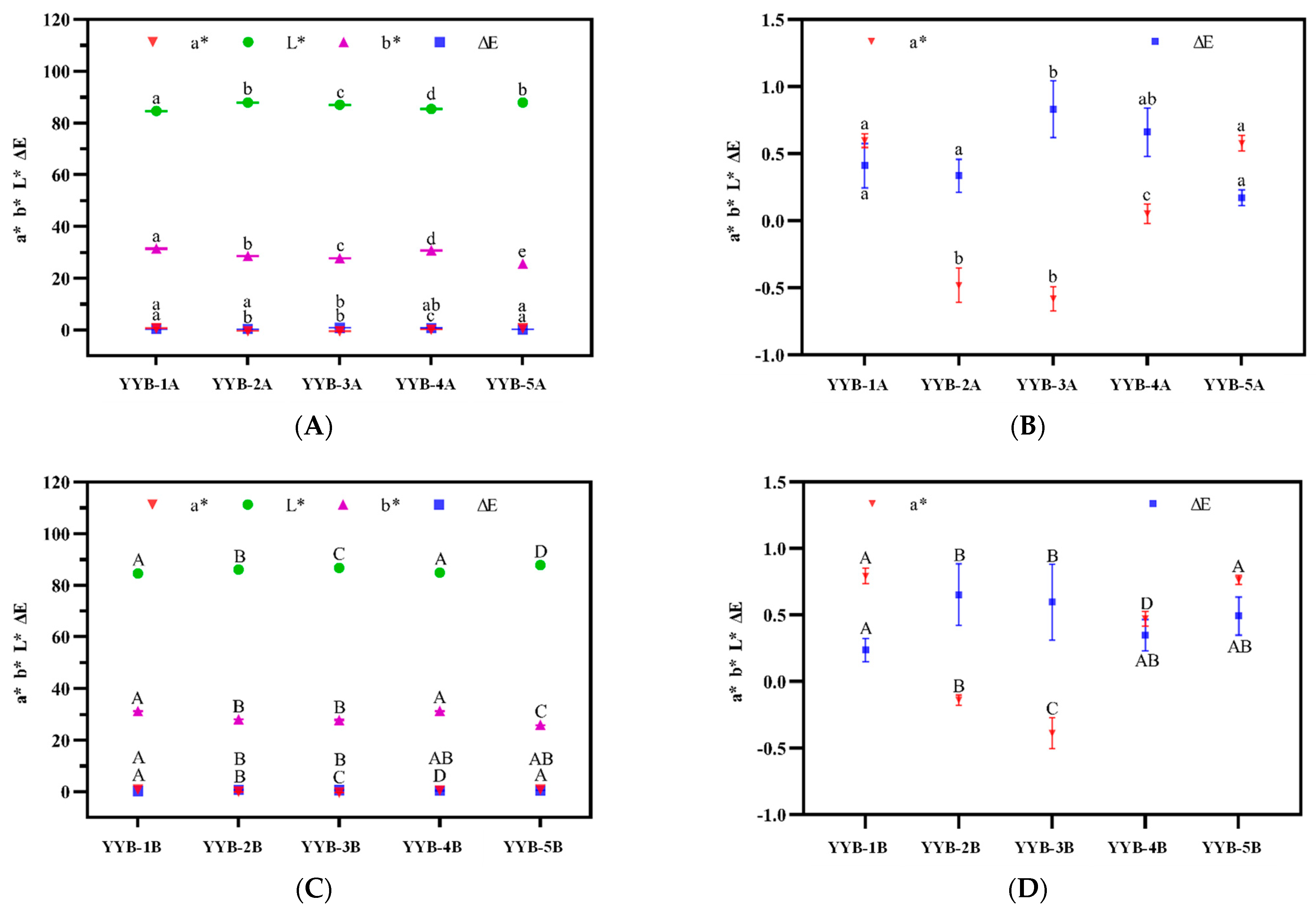
3.1. The Color Changes of Five Types of Infant Nutrition Powder after Accelerated Storage
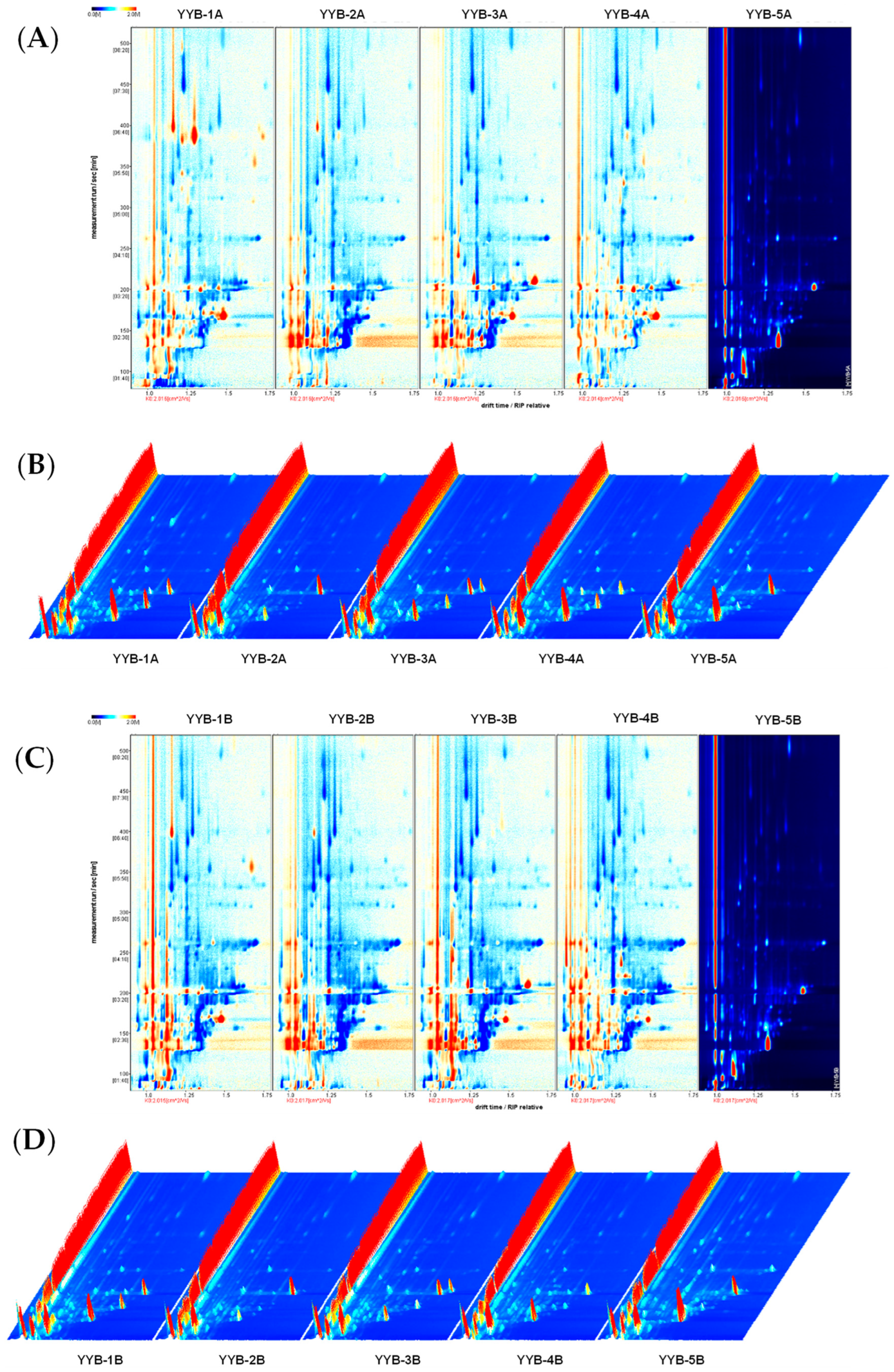
3.2. Changes in Volatile Compounds in Infant Nutrition Powder from Different Manufacturers
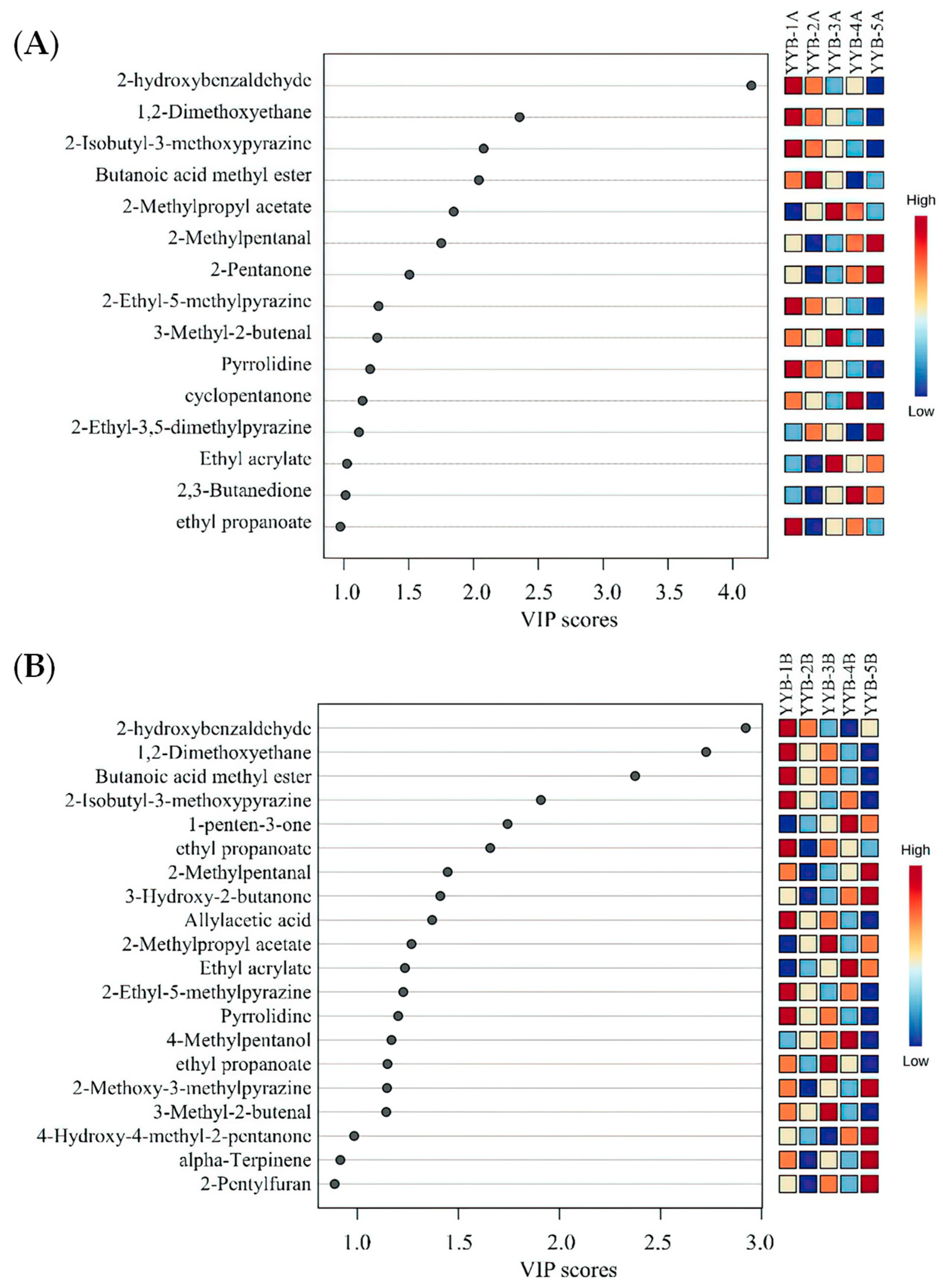
3.3. Principal Component Analysis (PCA)-Based Fingerprint Similarity Analysis
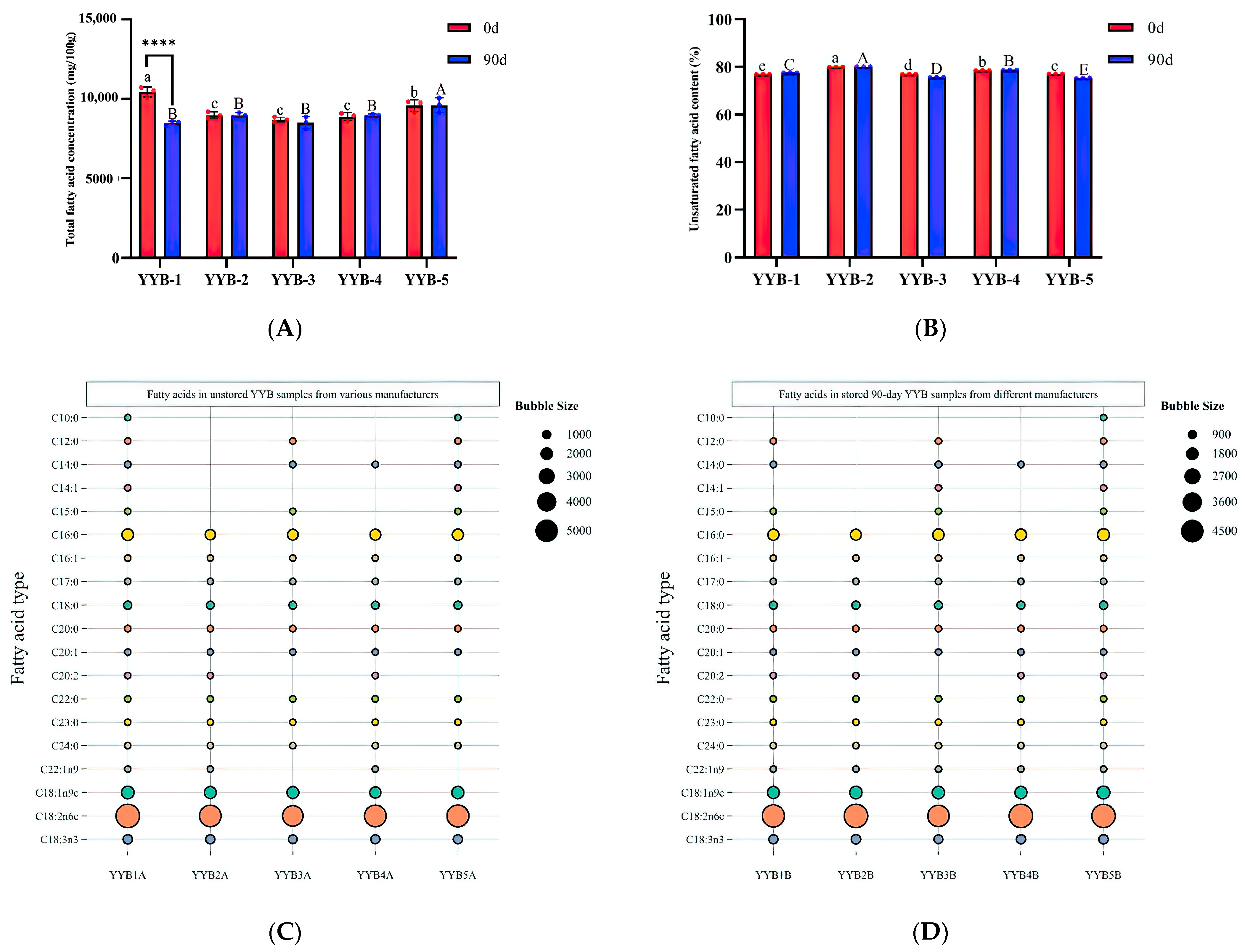
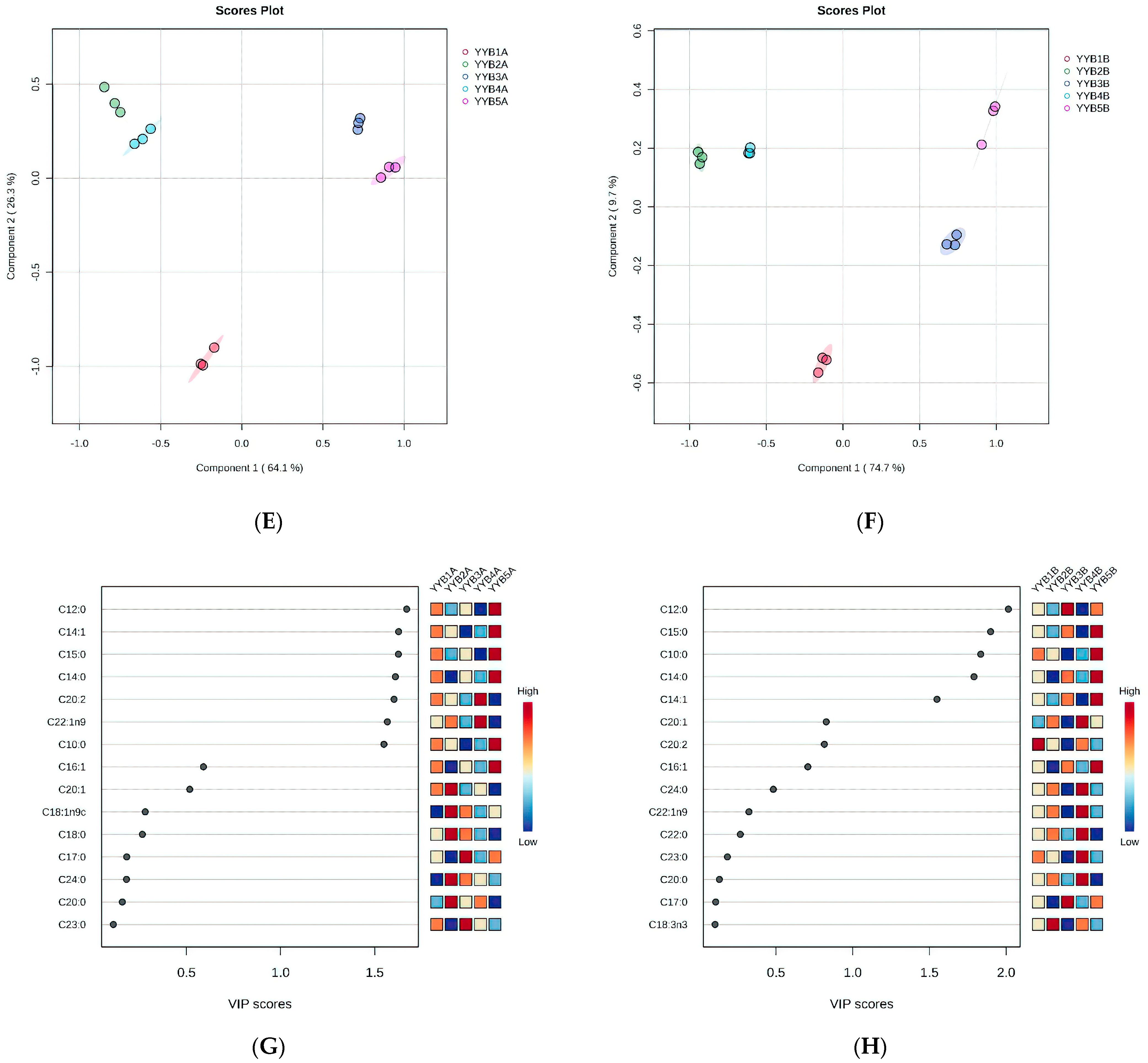
3.4. The Difference in Fatty Acid after Accelerated Storage of YYB from Different Manufacturers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Zhu, N.; Zeng, L.; Dang, S.; Zhou, J.; Yan, H. Effect of Prenatal and Postnatal Malnutrition on Intellectual Functioning in Early School-Aged Children in Rural Western China. Medicine 2016, 95, e4161. [Google Scholar] [CrossRef]
- Santos, M.; Matias, F.; Vaz, R.; Castanheira, I.; Rito, A.; Loureiro, I.; Assunção, R. A Study of Commercially Available Complementary Foods for Infants and Young Children under 36 Months. Eur. J. Public Health 2021, 31, ckab165.152. [Google Scholar] [CrossRef]
- Bartleman, J. Infant and Child Nutrition. Medicine 2019, 47, 195–198. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Huang, J.; Huo, J. Prevalence of Malnutrition and Associated Factors of Stunting among 6–23-Month-Old Infants in Central Rural China in 2019. Int. J. Environ. Res. Public Health 2021, 18, 8165. [Google Scholar] [CrossRef]
- Ju, H.; Wu, C.; Jiang, P.; Qi, L.; Lin, S. Inhibition Effect of Nitrogen-filled Technology on Flavor Degradation of Infant Nutrition Powder. Food Process. Preserv. 2022, 46, e17045. [Google Scholar] [CrossRef]
- Zhao, M.; Gou, J.; Zhang, K.; Ruan, J. Principal Components and Cluster Analysis of Trace Elements in Buckwheat Flour. Foods 2023, 12, 225. [Google Scholar] [CrossRef]
- Kitabayashi, K.; Tanimoto, S.; Kikutani, H.; Ohkita, T.; Mabuchi, R.; Shimoda, M. Effect of Nitrogen Gas Packaging on Odor Development in Yellowtail Seriola Quinqueradiata Muscle during Ice Storage. Fish. Sci. 2019, 85, 247–257. [Google Scholar] [CrossRef]
- Clarke, H.J.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Correlating Volatile Lipid Oxidation Compounds with Consumer Sensory Data in Dairy Based Powders during Storage. Antioxidants 2020, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, K.; Yang, R.; Dong, Y.; Lin, S. Mechanism of Aroma Compounds Changes from Sea Cucumber Peptide Powders (SCPPs) under Different Storage Conditions. Food Res. Int. 2020, 128, 108757. [Google Scholar] [CrossRef]
- Manzi, P.; Di Costanzo, M.; Mattera, M. Updating Nutritional Data and Evaluation of Technological Parameters of Italian Milk. Foods 2013, 2, 254–273. [Google Scholar] [CrossRef]
- Walczak, J.; Bocian, S.; Kowalkowski, T.; Trziszka, T.; Buszewski, B. Determination of Omega Fatty Acid Profiles in Egg Yolk by HILIC-LC-MS and GC-MS. Food Anal. Methods 2017, 10, 1264–1272. [Google Scholar] [CrossRef]
- Rao, Q.; Rocca-Smith, J.R.; Schoenfuss, T.C.; Labuza, T.P. Accelerated Shelf-Life Testing of Quality Loss for a Commercial Hydrolysed Hen Egg White Powder. Food Chem. 2012, 135, 464–472. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Charoenrein, S.; Roos, Y.H. Porosity and Water Activity Effects on Stability of Crystalline β-Carotene in Freeze-Dried Solids. J. Food Sci. 2012, 77, E313–E320. [Google Scholar] [CrossRef]
- Damjanovic Desic, S.; Birlouez-Aragon, I. The FAST Index—A Highly Sensitive Indicator of the Heat Impact on Infant Formula Model. Food Chem. 2011, 124, 1043–1049. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y. Characteristics and Antioxidant Activity of Maillard Reaction Products from Fructose-Glycine Oligomer. Food Sci. Biotechnol. 2010, 19, 929–940. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, Y.; Chi, Y. Changes in the Gel Characteristics of Two Hen Egg White Powders Modified by Dry Heating and the Maillard Reaction during Long-Term Storage. LWT 2019, 109, 123–129. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary Fibre and Fibre-Rich by-Products of Food Processing: Characterisation, Technological Functionality and Commercial Applications: A Review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, L.; Zhang, Y.; Shi, J.; Tan, C.P.; Zheng, Z.; Liu, Y. Lipid Profiles of Human Milk and Infant Formulas: A Comparative Lipidomics Study. Foods 2023, 12, 600. [Google Scholar] [CrossRef] [PubMed]
- Corral, S.; Salvador, A.; Flores, M. Salt Reduction in Slow Fermented Sausages Affects the Generation of Aroma Active Compounds. Meat Sci. 2013, 93, 776–785. [Google Scholar] [CrossRef]
- Pan, X.; Bi, S.; Lao, F.; Wu, J. Factors Affecting Aroma Compounds in Orange Juice and Their Sensory Perception: A Review. Food Res. Int. 2023, 169, 112835. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, M.; Umano, K.; Shibamoto, T. Analysis of Volatile Compounds Formed from Fish Oil Heated with Cysteine and Trimethylamine Oxide. J. Agric. Food Chem. 1998, 46, 5232–5237. [Google Scholar] [CrossRef]
- Fraatz, M.A.; Goldmann, M.; Geissler, T.; Gross, E.; Backes, M.; Hilmer, J.-M.; Ley, J.; Rost, J.; Francke, A.; Zorn, H. Biotechnological Production of Methyl-Branched Aldehydes. J. Agric. Food Chem. 2018, 66, 2387–2392. [Google Scholar] [CrossRef]
- Jeleń, H.; Gracka, A. Characterization of Aroma Compounds: Structure, Physico-chemical and Sensory Properties. In Flavour; Guichard, E., Salles, C., Morzel, M., Le Bon, A., Eds.; Wiley: New York, NY, USA, 2016; pp. 126–153. ISBN 978-1-118-92941-4. [Google Scholar]
- Tong, L.; Yi, H.; Wang, J.; Pan, M.; Chi, X.; Hao, H.; Ai, N. Effect of Preheating Treatment before Defatting on the Flavor Quality of Skim Milk. Molecules 2019, 24, 2824. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhu, X.; Sun, R.; Jiang, W.; Zhang, D.; Liu, H.; Sun, B. Sensory Attributes and Characterization of Aroma Profiles of Fermented Sausages Based on Fibrous-like Meat Substitute from Soybean Protein and Coprinus Comatus. Food Chem. 2022, 373, 131537. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.E.; Chambers, D.H. Descriptive Analysis and Consumer Acceptance of Black Walnut Cultivars in a Sugar Cookie Base. LWT-Food Sci. Technol. 2013, 53, 139–145. [Google Scholar] [CrossRef]
- Xie, J.; Gänzle, M. Microbiology of Fermented Soy Foods in Asia: Can We Learn Lessons for Production of Plant Cheese Analogues? Int. J. Food Microbiol. 2023, 407, 110399. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.; Jansson, T.; Eggers, N.; Clausen, M.R.; Larsen, L.B.; Jensen, H.B.; Ray, C.; Sundgren, A.; Andersen, H.J.; Bertram, H.C. Storage-Induced Changes in the Sensory Characteristics and Volatiles of Conventional and Lactose-Hydrolyzed UHT Processed Milk. Eur. Food Res. Technol. 2015, 240, 1247–1257. [Google Scholar] [CrossRef]
- Hu, H.; Shi, A.; Liu, H.; Liu, L.; Fauconnier, M.L.; Wang, Q. Study on Key Aroma Compounds and Its Precursors of Peanut Oil Prepared with Normal- and High-Oleic Peanuts. Foods 2021, 10, 3036. [Google Scholar] [CrossRef]
- Friedman, M. Food Browning and Its Prevention: An Overview. J. Agric. Food Chem. 1996, 44, 631–653. [Google Scholar] [CrossRef]
- Amores-Arrocha, A.; Sancho-Galán, P.; Jiménez-Cantizano, A.; Palacios, V. A Comparative Study on Volatile Compounds and Sensory Profile of White and Red Wines Elaborated Using Bee Pollen versus Commercial Activators. Foods 2021, 10, 1082. [Google Scholar] [CrossRef]
- Cheng, H.; Erichsen, H.; Soerensen, J.; Petersen, M.A.; Skibsted, L.H. Optimising Water Activity for Storage of High Lipid and High Protein Infant Formula Milk Powder Using Multivariate Analysis. Int. Dairy J. 2019, 93, 92–98. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Ye, Z.; Zhao, J.; Xue, X.; Heyden, Y.V.; Sun, Q. Classification of Chinese Honeys According to Their Floral Origin by near Infrared Spectroscopy. Food Chem. 2012, 135, 338–342. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.; Caramujo, M. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Roos, Y.H.; Miao, S. Effect of Maltodextrins on the Stability and Release of Volatile Compounds of Oil-in-Water Emulsions Subjected to Freeze–Thaw Treatment. Food Hydrocoll. 2015, 50, 219–227. [Google Scholar] [CrossRef]
- Liu, Y.; He, C.; Song, H. Comparison of SPME Versus SAFE Processes for the Analysis of Flavor Compounds in Watermelon Juice. Food Anal. Methods 2018, 11, 1677–1689. [Google Scholar] [CrossRef]
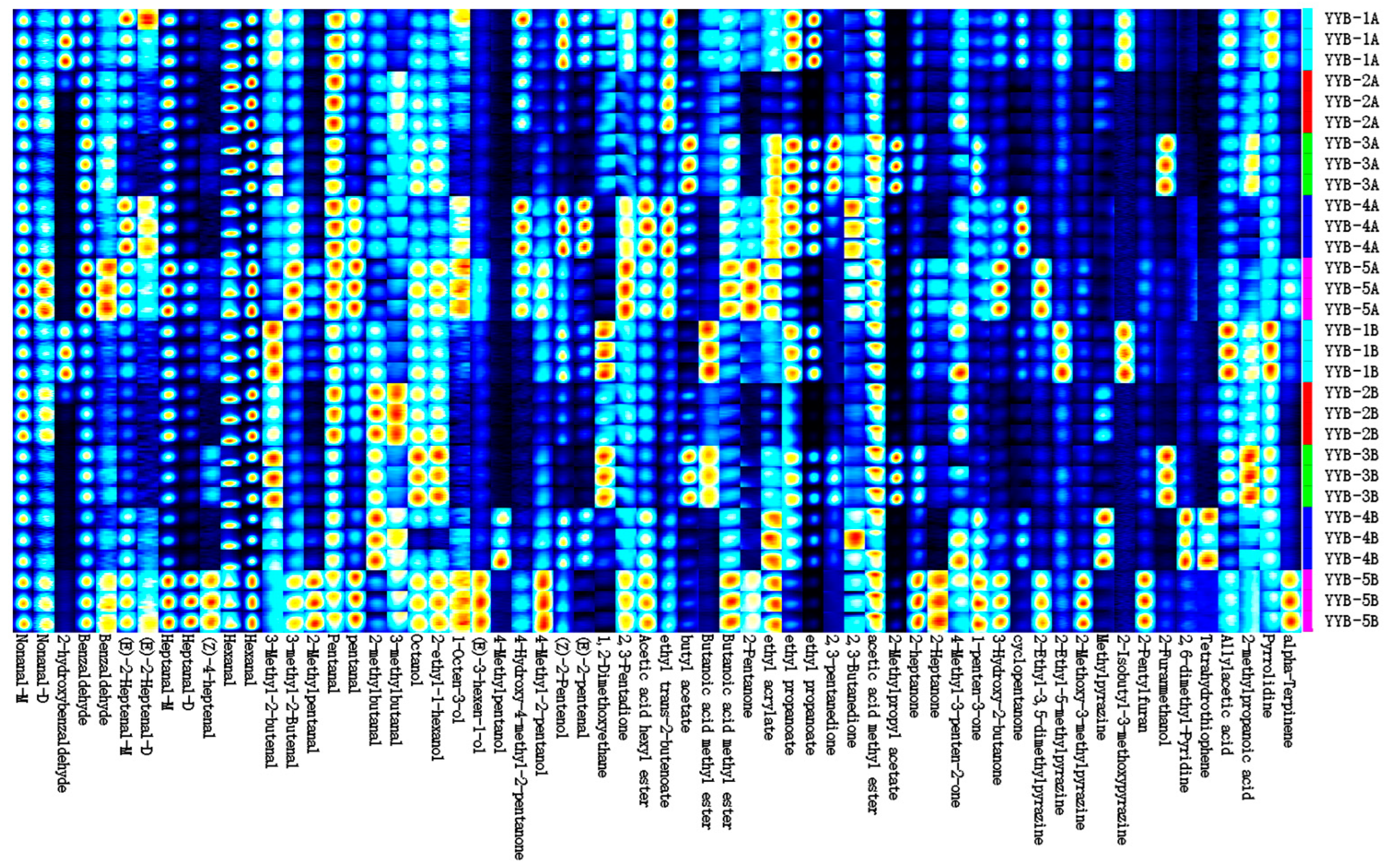

| No. | Compound | CAS | Formula | MW | RI 1 | Rt 2 | Dt 3 |
|---|---|---|---|---|---|---|---|
| 1 | nonanal (M) | 124-19-6 | C9H18O | 142.2 | 1107.9 | 506.703 | 1.94623 |
| 2 | nonanal (D) | 124-19-6 | C9H18O | 142.2 | 1108.2 | 507.28 | 1.47979 |
| 3 | 2-hydroxybenzaldehyde | 90-02-8 | C7H6O2 | 122.1 | 1041.5 | 400.178 | 1.16322 |
| 4 | benzaldehyde (M) | 100-52-7 | C7H6O | 106.1 | 962.9 | 311.291 | 1.14982 |
| 5 | benzaldehyde (D) | 100-52-7 | C7H6O | 106.1 | 963.3 | 311.572 | 1.46808 |
| 6 | (E)-2-heptenal (M) | 18829-55-5 | C7H12O | 112.2 | 958.6 | 307.64 | 1.25807 |
| 7 | (E)-2-heptenal (D) | 188255-5 | C7H12O | 112.2 | 959.9 | 308.764 | 1.67159 |
| 8 | heptanal (M) | 111-71-7 | C7H14O | 114.2 | 901.7 | 263.425 | 1.333 |
| 9 | heptanal (D) | 111-71-7 | C7H14O | 114.2 | 900.4 | 262.499 | 1.69684 |
| 10 | (Z)-4-heptenal | 6728-31-0 | C7H12O | 112.2 | 888.5 | 254.841 | 1.63226 |
| 11 | hexanal (M) | 66-25-1 | C6H12O | 100.2 | 783 | 206.799 | 1.26096 |
| 12 | hexanal (D) | 66-25-1 | C6H12O | 100.2 | 776.9 | 203.497 | 1.56295 |
| 13 | 3-methyl-2-butenal | 107-86-8 | C5H8O | 84.1 | 781.1 | 205.79 | 1.35933 |
| 14 | 2-methylpentanal | 123-15-9 | C6H12O | 100.2 | 748.4 | 188.78 | 1.51291 |
| 15 | pentanal (M) | 110-62-3 | C5H10O | 86.1 | 691.7 | 162.582 | 1.18891 |
| 16 | pentanal (D) | 110-62-3 | C5H10O | 86.1 | 690.6 | 162.115 | 1.42669 |
| 17 | 2-methylbutanal | 96-17-3 | C5H10O | 86.1 | 664.8 | 153.085 | 1.16143 |
| 18 | 3-methylbutanal | 590-86-3 | C5H10O | 86.1 | 650.9 | 148.517 | 1.17545 |
| 19 | 3-methyl-2-Butenal | 107-86-8 | C5H8O | 84.1 | 744.4 | 186.822 | 1.11091 |
| 20 | octanol | 111-87-5 | C8H18O | 130.2 | 1046.7 | 407.636 | 1.45537 |
| 21 | 2-ethyl-1-hexanol | 104-76-7 | C8H18O | 130.2 | 1038.2 | 395.604 | 1.39794 |
| 22 | 1-octen-3-ol | 3391-86-4 | C8H16O | 128.2 | 961.9 | 310.449 | 1.56875 |
| 23 | (E)-3-hexen-1-ol | 928-97-2 | C6H12O | 100.2 | 851.8 | 237.042 | 1.26041 |
| 24 | 4-methylpentanol | 626-89-1 | C6H14O | 102.2 | 848.2 | 235.399 | 1.30886 |
| 25 | 4-hydroxy-4-methyl-2-pentanone | 123-42-2 | C6H12O2 | 116.2 | 838.4 | 230.893 | 1.51444 |
| 26 | 4-methyl-2-pentanol | 108-11-2 | C6H14O | 102.2 | 754.4 | 191.766 | 1.25491 |
| 27 | (Z)-2-pentenol | 1576-95-0 | C5H10O | 86.1 | 773.7 | 201.776 | 1.45079 |
| 28 | (E)-2-pentenal | 1576-87-0 | C5H8O | 84.1 | 767.9 | 198.744 | 1.10396 |
| 29 | 1,2-dimethoxyethane | 110-71-4 | C4H10O2 | 90.1 | 649.6 | 148.092 | 1.13222 |
| 30 | 2,3-pentadione | 600-14-6 | C5H8O2 | 100.1 | 654.6 | 149.7 | 1.27829 |
| 31 | 2-heptanone (M) | 110-43-0 | C7H14O | 114.2 | 889.8 | 255.489 | 1.26345 |
| 32 | 2-heptanone (D) | 110-43-0 | C7H14O | 114.2 | 898.8 | 261.399 | 1.66038 |
| 33 | 4-methyl-3-penten-2-one | 141-79-7 | C6H10O | 98.1 | 794.5 | 211.715 | 1.46891 |
| 34 | 1-penten-3-one | 1629-58-9 | C5H8O | 84.1 | 681.8 | 158.862 | 1.32791 |
| 35 | 3-hydroxy-2-butanone | 513-86-0 | C4H8O2 | 88.1 | 734.7 | 182.086 | 1.35775 |
| 36 | cyclopentanone | 120-92-3 | C5H8O | 84.1 | 770.7 | 200.21 | 1.32899 |
| 37 | 2-ethyl-3,5-dimethylpyrazine | 13925-07-0 | C8H12N2 | 136.2 | 1074.5 | 450.057 | 1.22834 |
| 38 | 2-ethyl-5-methylpyrazine | 13360-64-0 | C7H10N2 | 122.2 | 1008.8 | 356.311 | 1.68005 |
| 39 | 2-methoxy-3-methylpyrazine | 2847-30-5 | C6H8N2O | 124.1 | 986.4 | 331.832 | 1.16217 |
| 40 | methylpyrazine | 109-08-0 | C5H6N2 | 94.1 | 816.9 | 221.302 | 1.08503 |
| 41 | 2-isobutyl-3-methoxypyrazine | 24683-00-9 | C9H14N2O | 166.2 | 1176.1 | 645.781 | 1.81887 |
| 42 | acetic acid, hexyl ester | 142-92-7 | C8H16O2 | 144.2 | 1009.5 | 357.195 | 1.40756 |
| 43 | ethyl trans-2-butenoate | 623-70-1 | C6H10O2 | 114.1 | 841.1 | 232.123 | 1.18045 |
| 44 | butyl acetate | 123-86-4 | C6H12O2 | 116.2 | 792.7 | 210.973 | 1.24106 |
| 45 | butanoic acid methyl ester (M) | 623-42-7 | C5H10O2 | 102.1 | 716.9 | 173.741 | 1.13044 |
| 46 | butanoic acid methyl ester (D) | 623-42-7 | C5H10O2 | 102.1 | 723.7 | 176.881 | 1.41501 |
| 47 | 2-pentanone | 107-87-9 | C5H10O | 86.1 | 691.1 | 162.327 | 1.38112 |
| 48 | ethyl acrylate | 140-88-5 | C5H8O2 | 100.1 | 704.3 | 168.064 | 1.41033 |
| 49 | ethyl propanoate (M) | 105-37-3 | C5H10O2 | 102.1 | 703.6 | 167.745 | 1.48045 |
| 50 | ethyl propanoate (D) | 105-37-3 | C5H10O2 | 102.1 | 704.5 | 168.17 | 1.16611 |
| 51 | 2,3-pentanedione | 600-14-6 | C5H8O2 | 100.1 | 625.4 | 140.48 | 1.28563 |
| 52 | 2,3-butanedione | 431-03-8 | C4H6O2 | 86.1 | 582.1 | 127.802 | 1.1808 |
| 53 | acetic acid methyl ester | 79-20-9 | C3H6O2 | 74.1 | 522.1 | 112.125 | 1.1826 |
| 54 | 2-methylpropyl acetate | 110-19-0 | C6H12O2 | 116.2 | 791.3 | 210.377 | 1.62248 |
| 55 | 2-pentylfuran | 3777-69-3 | C9H14O | 138.2 | 997.1 | 341.859 | 1.25701 |
| 56 | 2-Furanmethanol | 98-00-0 | C5H6O2 | 98.1 | 862 | 241.901 | 1.14061 |
| 57 | allylacetic acid | 591-80-0 | C5H8O2 | 100.1 | 901.2 | 263.103 | 1.43113 |
| 58 | 2-methylpropanoic acid | 79-31-2 | C4H8O2 | 88.1 | 770.9 | 200.307 | 1.36084 |
| 59 | Tetrahydrothiophene | 110-01-0 | C4H8S | 88.2 | 816.9 | 221.302 | 1.33391 |
| 60 | alpha-Terpinene | 99-86-5 | C10H16 | 136.2 | 1016.4 | 366.043 | 1.19378 |
| 61 | Pyrrolidine | 123-75-1 | C4H9N | 71.1 | 690.5 | 162.065 | 1.2915 |
| 62 | 2,6-dimethyl-pyridine | 108-48-5 | C7H9N | 107.2 | 884.1 | 252.671 | 1.08859 |
| YYB-1A | YYB-2A | YYB-3A | YYB-4A | YYB-5A | |
|---|---|---|---|---|---|
| C10:0 | 8.76 ± 0.57 | – | – | – | 8.64 ± 0.57 |
| C12:0 | 23.22 ± 1.27 a | – | 18.54 ± 0.34 b | – | 22.95 ± 2.3 a |
| C14:0 | 85.12 ± 2.06 a | – | 59.69 ± 0.8 b | 13.55 ± 0.56 c | 83.64 ± 2.72 a |
| C14:1 | 4.78 ± 0.21 | – | – | – | 5.31 ± 0.59 |
| C15:0 | 9.38 ± 0.34 a | – | 6.22 ± 0.14 b | – | 9.25 ± 0.25 a |
| C16:0 | 1577.94 ± 39.09 a | 1208.29 ± 21.36 c | 1341.29 ± 20.37 b | 1344.17 ± 30.57 b | 1443.27 ± 43.66 a |
| C16:1 | 14.18 ± 0.9 a | 3.75 ± 0.2 c | 10.72 ± 1.09 b | 4.9 ± 0.25 c | 14.32 ± 0.7 a |
| C17:0 | 14.48 ± 0.02 a | 9.6 ± 0.61 c | 12.44 ± 0.17 b | 10.18 ± 0.69 c | 13.54 ± 0.72 ab |
| C18:0 | 537.25 ± 13.39 a | 460.45 ± 7.03 bc | 451.89 ± 6.28 c | 420.22 ± 9.44 d | 482.12 ± 14.92 b |
| C18:1n9c | 1888.58 ± 47.77 a | 1669.97 ± 29.74 c | 1655.6 ± 24.31 c | 1487.73 ± 33.55 d | 1772.41 ± 58.71 b |
| C18:2n6c | 5233.62 ± 125.89 a | 4663.31 ± 81.66 b | 4314 ± 66.52 c | 4687.39 ± 104.83 b | 4756.86 ± 143.07 b |
| C18:3n3 | 902.37 ± 20.6 a | 837.98 ± 20.19 bc | 724.84 ± 10.47 d | 793.14 ± 17.31 c | 844.84 ± 25.2 b |
| C20:0 | 38.13 ± 1.29 a | 33.67 ± 1.56 b | 30.79 ± 0.57 c | 32.54 ± 0.74 bc | 32.48 ± 1.26 bc |
| C20:1 | 16.75 ± 1.38 a | 14.26 ± 3.58 a | 11.55 ± 1.54 a | 12.58 ± 0.36 a | 11.33 ± 2.16 a |
| C20:2 | 5.20 ± 0.45 a | 3.98 ± 0.62 a | – | 4.35 ± 0.16 a | – |
| C22:0 | 55.08 ± 2.89 a | 46.33 ± 1.23 bc | 43.58 ± 0.43 c | 47.67 ± 2.99 bc | 49.23 ± 1.68 b |
| C22:1n9 | 5.3 ± 0.52 a | 4.36 ± 0.45 a | – | 5.09 ± 0.85 a | – |
| C23:0 | 7.58 ± 0.85 a | 5.24 ± 0.27 c | 6.87 ± 0.21 ab | 5.92 ± 0.71 bc | 6.95 ± 0.67 ab |
| C24:0 | 17.33 ± 1.2 a | 15.72 ± 0.78 ab | 15.29 ± 0.4 ab | 14.76 ± 0.31 b | 16.24 ± 0.59 ab |
| YYB-1B | YYB-2B | YYB-3B | YYB-4B | YYB-5B | |
|---|---|---|---|---|---|
| C10:0 | – | – | – | – | 12.48 ± 0.45 |
| C12:0 | 15.08 ± 0.14 c | – | 28.78 ± 0.88 b | – | 31.63 ± 1.32 a |
| C14:0 | 58.4 ± 0.48 c | – | 83.8 ± 3.15 b | 18.72 ± 0.43 d | 110.81 ± 3.93 a |
| C14:1 | – | – | 4.98 ± 0.19 | – | 6.66 ± 1.01 |
| C15:0 | 6.32 ± 0.41 c | – | 8.92 ± 0.25 b | – | 12.78 ± 0.25 a |
| C16:0 | 1275.75 ± 15.89 cd | 1208.58 ± 16.96 d | 1369.07 ± 50.41 b | 1322.08 ± 12.38 bc | 1533.36 ± 58.91 a |
| C16:1 | 9.91 ± 0.32 c | 4.15 ± 0.5 e | 13.88 ± 0.74 a | 5.16 ± 0.31 d | 18.7 ± 1.39 b |
| C17:0 | 11.33 ± 0.31 c | 10.14 ± 0.44 c | 13.14 ± 0.39 b | 10.62 ± 0.31 c | 15 ± 0.84 a |
| C18:0 | 426.06 ± 5.2 cd | 454.51 ± 6.56 bc | 459.67 ± 16.97 b | 422.56 ± 3.39 d | 518.35 ± 19.87 a |
| C18:1n9c | 1516.01 ± 17.7 c | 1661.15 ± 23.36 b | 1617.03 ± 58.37 bc | 1575.71 ± 15.22 bc | 1764.33 ± 66.27 a |
| C18:2n6c | 4317.87 ± 53.22 b | 4679.68 ± 64.51 a | 4118.66 ± 152.18 b | 4668.69 ± 43.4 a | 4641.05 ± 177.19 a |
| C18:3n3 | 743.13 ± 8.96 c | 844.06 ± 11.94 a | 692.85 ± 25.44 d | 795.23 ± 6.78 b | 824.63 ± 32.44 ab |
| C20:0 | 29.94 ± 0.79 bc | 33.33 ± 0.3 a | 28.08 ± 1.63 c | 33.85 ± 0.11 a | 31.79 ± 1.45 ab |
| C20:1 | 9.29 ± 1.68 b | 12.68 ± 1.59 ab | 8.2 ± 1.92 b | 14.28 ± 0.3 a | 12.72 ± 2.64 ab |
| C20:2 | 6.58 ± 0.97 a | 3.88 ± 0.04 b | – | 4.04 ± 0.37 b | 3.41 ± 0.1 b |
| C22:0 | 43.23 ± 0.74 c | 43.73 ± 0.95 bc | 40.2 ± 0.94 d | 52.16 ± 0.86 a | 46.12 ± 1.89 b |
| C22:1n9 | 4.22 ± 0.66 a | 4.37 ± 0.22 a | 3.95 ± 0.35 a | 5.07 ± 0.92 a | 5 ± 0.1 a |
| C23:0 | 6.37 ± 0.24 a | 5.92 ± 0.47 a | 5.22 ± 0.68 a | 6.5 ± 0.3 a | 6.62 ± 1.16 a |
| C24:0 | 13.54 ± 0.23 c | 15.98 ± 0.26 b | 13.01 ± 0.9 c | 18.66 ± 1.34 a | 16.01 ± 0.7 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Li, J.; Guo, X. Comparative Analysis of Characteristic Volatile Compounds in Five Types of Infant Nutrition Powders by HS-GC-IMS and GC-MS. Foods 2024, 13, 648. https://doi.org/10.3390/foods13050648
Yang Z, Li J, Guo X. Comparative Analysis of Characteristic Volatile Compounds in Five Types of Infant Nutrition Powders by HS-GC-IMS and GC-MS. Foods. 2024; 13(5):648. https://doi.org/10.3390/foods13050648
Chicago/Turabian StyleYang, Zhihua, Jinjin Li, and Xiaoming Guo. 2024. "Comparative Analysis of Characteristic Volatile Compounds in Five Types of Infant Nutrition Powders by HS-GC-IMS and GC-MS" Foods 13, no. 5: 648. https://doi.org/10.3390/foods13050648
APA StyleYang, Z., Li, J., & Guo, X. (2024). Comparative Analysis of Characteristic Volatile Compounds in Five Types of Infant Nutrition Powders by HS-GC-IMS and GC-MS. Foods, 13(5), 648. https://doi.org/10.3390/foods13050648





