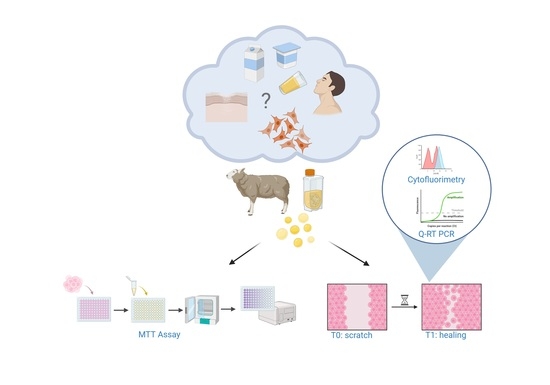
Food Safety Assessment and Nutraceutical Outcomes of Dairy By-Products: Ovine Milk Whey as Wound Repair Enhancer on Injured Human Primary Gingival Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ovine Milk Collection and Whey Preparation
2.2. Cells
2.3. 3-[4,5-Dimethylthiaoly]−2,5-diphenyltetrazolium Bromide (MTT) Assay
2.4. Wound Healing Assay
2.5. Cytofluorimetric Analysis
2.6. Quantitative Real-Time Polymerase Chain Reaction
2.7. Immunofluorescence Analysis
2.8. Statistical Analysis
3. Results
3.1. Effect of Ovine Whey on HGF-1 Viability
3.2. Ovine Whey Promotes Wound Repair in the In Vitro HGF-1 Gingiva Injury Model
3.3. Ovine Whey Enhances CD44 Antigen Density and Promotes Collagen I Up-Regulation in the In Vitro HGF-1 Gingiva Injury Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-industrial by-products: Valuable sources of bioactive compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef]
- Galali, Y.; Omar, Z.A.; Sajadi, S.M. Biologically active components in by-products of food processing. Food Sci. Nutr. 2020, 8, 3004–3022. [Google Scholar] [CrossRef]
- European Parliament. Regulation (EU) No 1169/2011 of The European Parliament and of the Council of 25 October 2011. Off. J. Eur. Union 2011. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:304:0018:0063:en:PDF (accessed on 20 December 2023).
- Ryan, M.P.; Walsh, G. The biotechnological potential of whey. Rev. Environ. Sci. Biotechnol. 2016, 15, 479–498. [Google Scholar] [CrossRef]
- Monti, L.; Donati, E.; Zambrini, A.V.; Contarini, G. Application of membrane technologies to bovine Ricotta cheese exhausted whey (scotta). Int. Dairy J. 2018, 85, 121–128. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- de Souza, R.R.; Bergamasco, R.; da Costa, S.C.; Feng, X.; Faria, S.H.B.; Gimenes, M.L. Recovery and purification of lactose from whey. Chem. Eng. Process. Process Intensif. 2010, 49, 1137–1143. [Google Scholar] [CrossRef]
- Kasapidou, E.; Sossidou, E.; Mitlianga, P. Fruit and Vegetable Co-Products as Functional Feed Ingredients in Farm Animal Nutrition for Improved Product Quality. Agriculture 2015, 5, 1020–1034. [Google Scholar] [CrossRef]
- Rațu, R.N.; Veleșcu, I.D.; Stoica, F.; Usturoi, A.; Arsenoaia, V.N.; Crivei, I.C.; Postolache, A.N.; Lipșa, F.D.; Filipov, F.; Florea, A.M.; et al. Application of Agri-Food By-Products in the Food Industry. Agriculture 2023, 13, 1559. [Google Scholar] [CrossRef]
- Berenguer, C.V.; Andrade, C.; Pereira, J.A.M.; Perestrelo, R.; Câmara, J.S. Current Challenges in the Sustainable Valorisation of Agri-Food Wastes: A Review. Processes 2023, 11, 20. [Google Scholar] [CrossRef]
- Ha, E.; Zemel, M.B. Functional properties of whey, whey components, and essential amino acids: Mechanisms underlying health benefits for active people (review). J. Nutr. Biochem. 2003, 14, 251–258. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Gaffey, J.; Sharma, M.; Dewhurst, R.J.; Moreau, B.; Newbold, J.; Clark, W.; Thakur, V.K.; Gupta, V.K. Valorization of dairy waste and by-products through microbial bioprocesses. Bioresour. Technol. 2022, 346, 126444. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef]
- Verardo, V.; Gómez-Caravaca, A.M.; Arráez-Román, D.; Hettinga, K. Recent advances in phospholipids from colostrum, milk and dairy by-products. Int. J. Mol. Sci. 2017, 18, 173. [Google Scholar] [CrossRef]
- Meisel, H. Overview on milk protein-derived peptides. Int. Dairy J. 1998, 8, 363–373. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, Y.; Wang, X.; Huang, A. Characterization of a novel antimicrobial peptide from buffalo casein hydrolysate based on live bacteria adsorption. J. Dairy Sci. 2020, 103, 11116–11128. [Google Scholar] [CrossRef]
- Pintado, M.E.; Malcata, F.X.; Lopes da Silva, J.A. Comparative characterization of whey protein concentrates from ovine, caprine and bovine breeds. LWT 1999, 32, 231–237. [Google Scholar] [CrossRef]
- Casper, J.; Wendorff, W.; Thomas, D. Functional properties of whey protein concentrates from caprine and ovine specialty cheese wheys. J. Dairy Sci. 1999, 82, 265–271. [Google Scholar] [CrossRef]
- Grellier, J.; Rushton, L.; Briggs, D.J.; Nieuwenhuijsen, M.J. Assessing the human health impacts of exposure to disinfection by-products—A critical review of concepts and methods. Environ. Int. 2015, 78, 61–81. [Google Scholar] [CrossRef]
- Behne, S.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk Assessment of Chlorogenic and Isochlorogenic Acids in Coffee By-Products. Molecules 2023, 28, 5540. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food by-products and food wastes: Are they safe enough for their valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Rao, M.; Bast, A.; de Boer, A. Valorized food processing by-products in the EU: Finding the balance between safety, nutrition, and sustainability. Sustainability 2021, 13, 4428. [Google Scholar] [CrossRef]
- Lopreiato, V.; Ceniti, C.; Trimboli, F.; Fratto, E.; Marotta, M.; Britti, D.; Morittu, V. Evaluation of the capillary electrophoresis method for measurement of immunoglobulin concentration in ewe colostrum. J. Dairy Sci. 2017, 100, 6465–6469. [Google Scholar] [CrossRef]
- Trimboli, F.; Costanzo, N.; Lopreiato, V.; Ceniti, C.; Morittu, V.M.; Spina, A.; Britti, D. Detection of buffalo milk adulteration with cow milk by capillary electrophoresis analysis. J. Dairy Sci. 2019, 102, 5962–5970. [Google Scholar] [CrossRef]
- Ceniti, C.; Ambrosio, R.L.; Bria, J.; Di Vito, A.; Tilocca, B.; Anastasio, A.; Britti, D.; Morittu, V.M.; Chiarella, E. Utilization of Dairy By-Products as a Source of Functional and Health Compounds—The Role of Ovine Colostrum and Milk Whey on Chronic Myeloid Leukemia Cells. Foods 2023, 12, 1752. [Google Scholar] [CrossRef]
- Chiarella, E.; Lombardo, N.; Lobello, N.; Piazzetta, G.L.; Morrone, H.L.; Mesuraca, M.; Bond, H.M. Deficit in adipose differentiation in mesenchymal stem cells derived from chronic rhinosinusitis nasal polyps compared to nasal mucosal tissue. Int. J. Mol. Sci. 2020, 21, 9214. [Google Scholar] [CrossRef]
- Mesuraca, M.; Nisticò, C.; Lombardo, N.; Piazzetta, G.L.; Lobello, N.; Chiarella, E. Cellular and Biochemical Characterization of Mesenchymal Stem Cells from Killian Nasal Polyp. Int. J. Mol. Sci. 2022, 23, 13214. [Google Scholar] [CrossRef]
- Chiarella, E.; Carrà, G.; Scicchitano, S.; Codispoti, B.; Mega, T.; Lupia, M.; Pelaggi, D.; Marafioti, M.G.; Aloisio, A.; Giordano, M.; et al. Umg lenti: Novel lentiviral vectors for efficient transgene- And reporter gene expression in human early hematopoietic progenitors. PLoS ONE 2014, 9, e114795. [Google Scholar] [CrossRef]
- Di Vito, A.; Giudice, A.; Chiarella, E.; Malara, N.; Bennardo, F.; Fortunato, L. In Vitro Long-Term Expansion and High Osteogenic Potential of Periodontal Ligament Stem Cells: More Than a Mirage. Cell Transplant. 2019, 28, 129–139. [Google Scholar] [CrossRef]
- Di Vito, A.; Chiarella, E.; Sovereto, J.; Bria, J.; Perrotta, I.D.; Salatino, A.; Baudi, F.; Sacco, A.; Antonelli, A.; Biamonte, F.; et al. Novel insights into the pharmacological modulation of human periodontal ligament stem cells by the amino-bisphosphonate Alendronate. Eur. J. Cell Biol. 2023, 102, 151354. [Google Scholar] [CrossRef]
- O’Donoghue, L.T.; Murphy, E.G. Nondairy food applications of whey and milk permeates: Direct and indirect uses. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2652–2677. [Google Scholar] [CrossRef]
- Sharma, R. Whey Proteins in Functional Foods. In Whey Proteins from Milk to Medicine; Academic Press: Cambridge, MA, USA, 2019; pp. 637–663. [Google Scholar] [CrossRef]
- Babenyshev, S.P.; Zhidkov, V.E.; Mamay, D.S.; Utkin, V.P.; Shapakov, N.A. Ultrafiltration of modified milk whey. Foods Raw Mater. 2016, 4, 101–110. [Google Scholar] [CrossRef]
- León-López, A.; Pérez-Marroquín, X.A.; Estrada-Fernández, A.G.; Campos-Lozada, G.; Morales-Peñaloza, A.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Milk Whey Hydrolysates as High Value-Added Natural Polymers: Functional Properties and Applications. Polymers 2022, 14, 1258. [Google Scholar] [CrossRef]
- Agostoni, C.; Bresson, J.-L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific Opinion on the substantiation of health claims related to whey protein and increase in satiety leading to a reduction in energy intake (ID 425), contribution to the maintenance or achievement of a normal body weight (ID 1683), growth or mainten. EFSA J. 2010, 8, 1818. [Google Scholar] [CrossRef]
- The International Dairy Federation (IDF) Collaborated with Codex Alimentarius in the. 2017. 18/07/2017. Available online: https://fil-idf.org/news_insights/new-standard-dairy-permeate-powders-adopted-codex/ (accessed on 20 December 2023).
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Whey basic protein isolates as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2018, 16, e05360. [Google Scholar] [CrossRef]
- Sattin, E.; Andreani, N.A.; Carraro, L.; Lucchini, R.; Fasolato, L.; Telatin, A.; Balzan, S.; Novelli, E.; Simionati, B.; Cardazzo, B. A multi-omics approach to evaluate the quality of milk whey used in ricotta cheese production. Front. Microbiol. 2016, 7, 1272. [Google Scholar] [CrossRef]
- Lam, F.-C.; Bukhsh, A.; Rehman, H.; Waqas, M.K.; Shahid, N.; Khaliel, A.M.; Elhanish, A.; Karoud, M.; Telb, A.; Khan, T.M. Efficacy and safety of whey protein supplements on vital sign and physical performance among athletes: A network meta-analysis. Front. Pharmacol. 2019, 10, 317. [Google Scholar] [CrossRef]
- Yamauchi, K.; Toida, T.; Nishimura, S.; Nagano, E.; Kusuoka, O.; Teraguchi, S.; Hayasawa, H.; Shimamura, S.; Tomita, M. 13-Week oral repeated administration toxicity study of bovine lactoferrin in rats. Food Chem. Toxicol. 2000, 38, 503–512. [Google Scholar] [CrossRef]
- Luz, C.; Izzo, L.; Ritieni, A.; Mañes, J.; Meca, G. Antifungal and antimycotoxigenic activity of hydrolyzed goat whey on Penicillium spp: An application as biopreservation agent in pita bread. LWT 2020, 118, 108717. [Google Scholar] [CrossRef]
- Slozhenkina, M.I.; Gorlov, I.F.; Kryuchkova, V.V.; I Mosolova, N.; Bochkareva, A.; Serova, O.P. Functional fermented milk whey product: Assessment of quality and safety. Proc. IOP Conf. Ser. Earth Environ. Sci. 2020, 548, 082030. [Google Scholar] [CrossRef]
- Boga, B.; Akbulut, M.; Maytalman, E.; Kozanoglu, I. Effect of milk and whey on proliferation and differentiation of placental stromal cells. Cytotechnology 2023, 75, 391–401. [Google Scholar] [CrossRef]
- Nassrallah, A.; Abdel-Mobdy, A.E. Biochemical investigation of goat milk casein and whey protein crude methanol extract. Egypt. J. Chem. 2021, 64, 6213–6219. [Google Scholar] [CrossRef]
- Wielento, A.; Lagosz-Cwik, K.; Potempa, J.; Grabiec, A. The Role of Gingival Fibroblasts in the Pathogenesis of Periodontitis. J. Dent. Res. 2023, 102, 489–496. [Google Scholar] [CrossRef]
- Di Vito, A.; Bria, J.; Antonelli, A.; Mesuraca, M.; Barni, T.; Giudice, A.; Chiarella, E. A Review of Novel Strategies for Human Periodontal Ligament Stem Cell Ex Vivo Expansion: Are They an Evidence-Based Promise for Regenerative Periodontal Therapy? Int. J. Mol. Sci. 2023, 24, 7798. [Google Scholar] [CrossRef]
- Govindaraju, P.; Todd, L.; Shetye, S.; Monslow, J.; Puré, E. CD44-dependent inflammation, fibrogenesis, and collagenolysis regulates extracellular matrix remodeling and tensile strength during cutaneous wound healing. Matrix Biol. 2019, 75–76, 314–330. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Miyake, T.; Ehsanipour, E.A.; Kelly, P.J.; Becker, L.M.; McGrail, D.J.; Sugimoto, H.; LeBleu, V.S.; Ge, Y.; Kalluri, R. Dermal αSMA+ myofibroblasts orchestrate skin wound repair via β1 integrin and independent of type I collagen production. EMBO J. 2022, 41, e109470. [Google Scholar] [CrossRef]
- Rujirachotiwat, A.; Suttamanatwong, S. Curcumin Promotes Collagen Type I, Keratinocyte Growth Factor-1, and Epidermal Growth Factor Receptor Expressions in the In Vitro Wound Healing Model of Human Gingival Fibroblasts. Eur. J. Dent. 2021, 15, 63–70. [Google Scholar] [CrossRef]
- Lifshits, L.A.; Rabin, M.; Tohar, R.; Netti, F.; Gabay, M.; Sova, M.; Bar, D.Z.; Weinberg, E.; Adler-Abramovich, L.; Gal, M. Enhancement of Collagen-I Levels in Human Gingival Fibroblasts by Small Molecule Activation of HIF-1α. J. Agric. Food Chem. 2023, 71, 7829–7835. [Google Scholar] [CrossRef]
- Eslami, H.; Motahari, P.; Safari, E.; Seyyedi, M. Evaluation effect of low level helium-neon laser and Iranian propolis extract on collagen type I gene expression by human gingival fibroblasts: An in vitro study. Laser Ther. 2017, 26, 105–112. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D.; Garg, A.P.; Gupta, S. Whey proteins: A novel source of bioceuticals. Middle East J. Sci. Res. 2012, 12, 365–375. [Google Scholar] [CrossRef]
- Takada, Y.; Aoe, S.; Kumegawa, M. Whey protein stimulates the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biochem. Biophys. Res. Commun. 1996, 223, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Xu, R. Effect of whey protein on the proliferation and differentiation of osteoblasts. J. Dairy Sci. 2009, 92, 3014–3018. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, A.A.; Larki-Harchegani, A.; Shabib, S.; Jalali, A.; Rezaei, A.; Housmand, G. Wound healing property of milk in full thickness wound model of rabbit. Int. J. Surg. 2018, 54, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Purup, S.; Nielsen, S.D.; Le, T.T.; Bertelsen, H.; Sørensen, J.; Larsen, L.B. Wound Healing Properties of Commercial Milk Hydrolysates in Intestinal Cells. Int. J. Pept. Res. Ther. 2019, 25, 483–491. [Google Scholar] [CrossRef]
- Panahipour, L.; Nasserzare, S.; Amer, Z.; Brücke, F.; Stähli, A.; Kreissl, A.; Haiden, N.; Gruber, R. The anti-inflammatory effect of milk and dairy products on periodontal cells: An in vitro approach. Clin. Oral Investig. 2019, 23, 1959–1966. [Google Scholar] [CrossRef]
- Zhao, C.; Ashaolu, T.J. Bioactivity and safety of whey peptides. LWT 2020, 134, 109935. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceniti, C.; Di Vito, A.; Ambrosio, R.L.; Anastasio, A.; Bria, J.; Britti, D.; Chiarella, E. Food Safety Assessment and Nutraceutical Outcomes of Dairy By-Products: Ovine Milk Whey as Wound Repair Enhancer on Injured Human Primary Gingival Fibroblasts. Foods 2024, 13, 683. https://doi.org/10.3390/foods13050683
Ceniti C, Di Vito A, Ambrosio RL, Anastasio A, Bria J, Britti D, Chiarella E. Food Safety Assessment and Nutraceutical Outcomes of Dairy By-Products: Ovine Milk Whey as Wound Repair Enhancer on Injured Human Primary Gingival Fibroblasts. Foods. 2024; 13(5):683. https://doi.org/10.3390/foods13050683
Chicago/Turabian StyleCeniti, Carlotta, Anna Di Vito, Rosa Luisa Ambrosio, Aniello Anastasio, Jessica Bria, Domenico Britti, and Emanuela Chiarella. 2024. "Food Safety Assessment and Nutraceutical Outcomes of Dairy By-Products: Ovine Milk Whey as Wound Repair Enhancer on Injured Human Primary Gingival Fibroblasts" Foods 13, no. 5: 683. https://doi.org/10.3390/foods13050683