The Effect of Enzymatic Hydrolysis and Maillard Reaction on the Flavor of Chicken Osteopontin
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. E-Tongue Analysis
2.4. FAAs Determination
2.5. 5′-Nucleotides Determination
2.6. TAV Determination
2.7. E-Nose Determination
2.8. Determination of Volatile Compounds
2.9. Sensory Evaluation
2.10. Statistical Analysis
3. Results
3.1. Non-Volatile Compounds Analysis
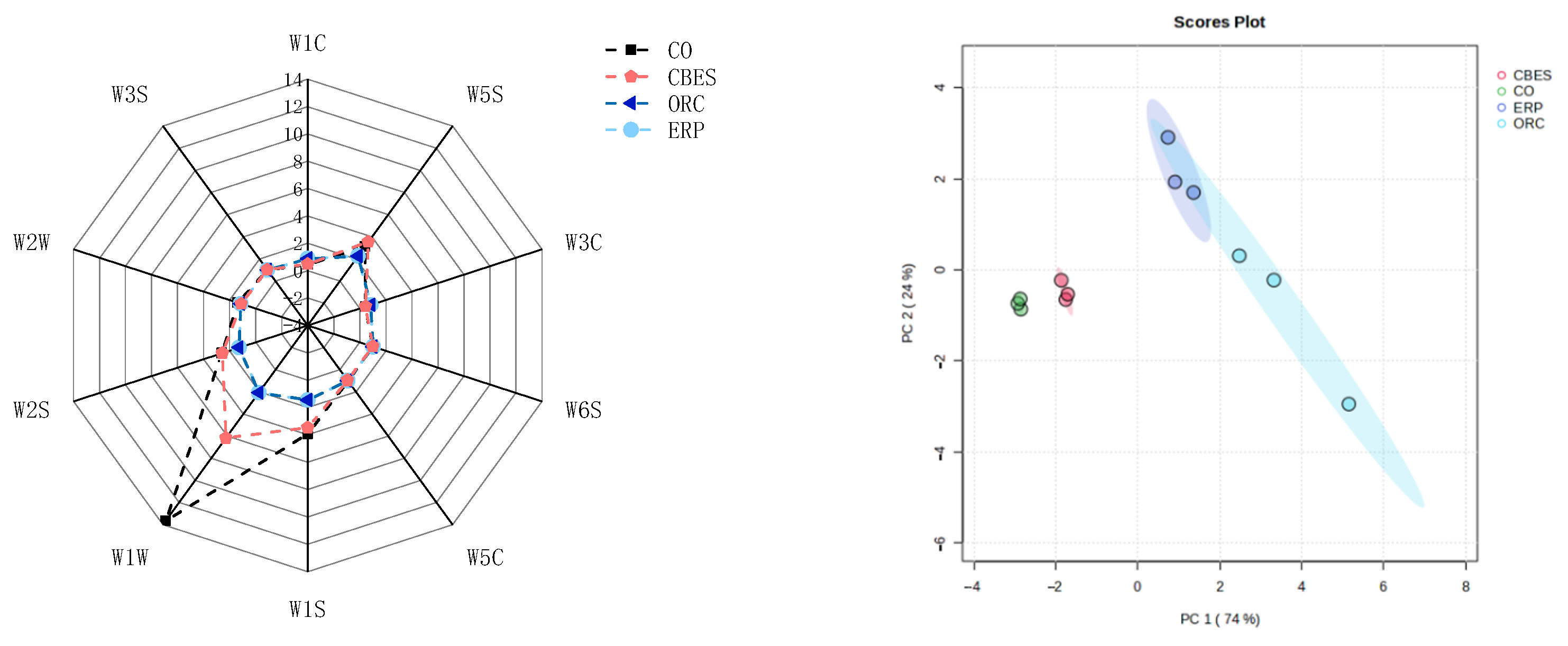
3.1.1. E-Tongue
3.1.2. FAAs Analysis
3.1.3. 5′-Nucleotide Analysis
3.2. Volatile Compounds Analysis
3.2.1. E-Nose
3.2.2. Volatile Compounds
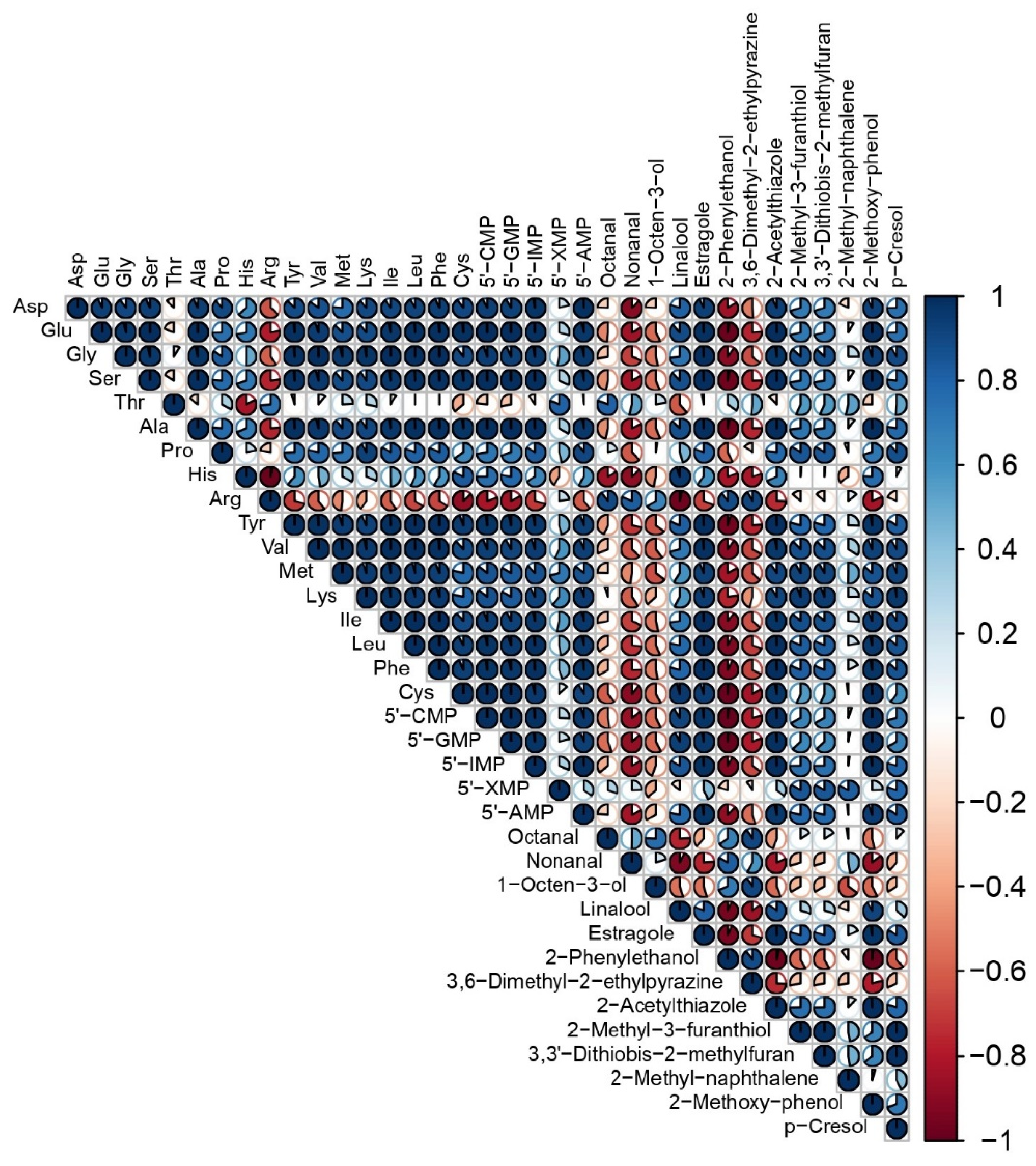
3.3. Relationships among the Variables of Taste and Aroma Traits
4. Discussion
4.1. Taste Changes in CO under Different Treatment Conditions
4.2. Aroma Changes in CO under Different Treatment Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, Q.C.; Chen, Y.W.; Zhao, L.M.; Ouyang, H.S.; Song, J. Monitoring of sausage products sold in Sichuan Province, China: A first comprehensive report on meat species authenticity determination. Sci. Rep. 2019, 9, 19074. [Google Scholar] [CrossRef]
- Cao, W.; Wang, H.L.; Zhang, J.Y.; Kan, H.; Liu, Y.; Guo, L. Effects of extraction methods on the characteristics physicochemical properties and sensory quality of collagen from spent-hens bones. Foods 2023, 12, 202. [Google Scholar] [CrossRef]
- Toldrá, F.; Milagro, L.; Reig, M. New insights into meat by-product utilization. Meat Sci. 2016, 120, 54–59. [Google Scholar] [CrossRef]
- Dong, X.B.; Li, X.; Zhang, C.H.; Wang, J.Z.; Tang, C.H.; Sun, H.M. Development of a novel method for hot-pressure extraction of protein from chicken bone and the effect of enzymatic hydrolysis on the extracts. Food Chem. 2014, 157, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, N.; Modi, V.K.; Govindaraju, K.; Radha, C.; Lalitha, R.G. Utilization of meat industry by products: Protein hydrolysate from sheep visceral mass. Bioresour. Technol. 2007, 98, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Fonkwe, L.G.; Singh, R.K. Protein recovery from mechanically deboned turkey residue by enzymic hydrolysis. Process Biochem. 1996, 31, 605–616. [Google Scholar] [CrossRef]
- Wu, Y.F.; Baek, H.H.; Gerard, P.D.; Cadwallader, K.R. Development of a meat-like process flavoring from soybean-based enzyme-hydrolyzed vegetable protein (E-HVP). J. Food Sci. 2000, 65, 1220–1227. [Google Scholar] [CrossRef]
- Begum, N.; Raza, A.; Song, H.L.; Zhang, Y.; Zhang, L.; Liu, P. Effect of thermal treatment on aroma generation from bovine bone marrow extract during enzymatic hydrolysis. J. Food Process. Preserv. 2019, 43, e14105. [Google Scholar] [CrossRef]
- Sun, H.M.; Wang, J.Z.; Zhang, C.H.; Li, X.; Xu, X.; Dong, X.B. Changes of flavor compounds of hydrolyzed chicken bone extracts during Maillard reaction. J. Food Sci. 2014, 79, C2415–C2426. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; You, M.C.; Song, H.L.; Gong, L.; Pan, W.Q. Investigation of umami and kokumi taste-active components in bovine bone marrow extract produced during enzymatic hydrolysis and Maillard reaction. Int. J. Food Sci. Technol. 2018, 53, 2465–2481. [Google Scholar] [CrossRef]
- Xu, X.R.; Zheng, Y.Y.; Song, H.L.; Gong, L.; Pan, W.Q. The effects of enzymatic hydrolysis degree of bovine bone marrow extract on flavor generation via the Maillard reaction. J. Food Meas. Charact. 2018, 13, 521–535. [Google Scholar] [CrossRef]
- Abd El-Aleem, F.S.; Taher, M.S.; Lotfy, S.N.; El-Massrey, K.F.; Fadel, H.H.M. Influence of extracted 5-nucleotides on aroma compounds and flavour acceptability of real beef soup. Int. J. Food Prop. 2017, 20, S1182–S1194. [Google Scholar] [CrossRef]
- Li, X.R.; Wang, Z.; Liu, G.S.; Zhao, J.Z.; Yu, J.Y.; Zhang, X.J.; Fan, N.Y. Effects of combined enzymes on flavoring substances of compound osteolysin hydrolysate. Sci. Technol. Food Ind. 2020, 41, 50–56. [Google Scholar] [CrossRef]
- Gu, W.G.; Zhang, J.J.; Xin, M.; Yao, Y.J.; Ji, R.; Lü, B.B.; Chen, J.C. Determination of free amino acids in the pork and its broth cooked by different methods using reversed-phase high performance liquid chromatography coupled with pre-column derivatization. Chin. J. Chromatogr. 2011, 29, 1041–1045. [Google Scholar]
- Qi, J.; Wang, H.H.; Zhou, G.H.; Xu, X.L.; Li, X.; Bai, Y. Evaluation of the taste-active and volatile compounds in stewed meat from the Chinese yellow-feather chicken breed. Int. J. Food Prop. 2017, 20, S2579–S2595. [Google Scholar] [CrossRef]
- Qi, J.; Xu, Y.; Xie, X.F.; Zhang, W.W.; Wang, H.H.; Xu, X.L. Gelatin enhances the flavor of chicken broth: A perspective on the ability of emulsions to bind volatile compounds. Food Chem. 2020, 333, 127463. [Google Scholar] [CrossRef]
- Han, D.; Zhang, C.H.; Fauconnier, M.L.; Mi, S. Characterization and differentiation of boiled pork from Tibetan, Sanmenxia and Duroc × (Landrac × Yorkshire) pigs by volatiles profiling and chemometrics analysis. Food Res. Int. 2020, 130, 108910. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Song, H.; Wang, L.; Jing, H. Characterization of key aroma-active compounds in black garlic by sensory-directed flavor analysis. J. Agric. Food Chem. 2019, 67, 7926. [Google Scholar] [CrossRef] [PubMed]
- Spanier, A.M.; Flores, M.; Toldrá, F.; Aristoy, M.C.; Bett, K.L.; Bystricky, P.; Bland, J.M.; Shahidi, F.; Spanier, A.M.; Ho, C.T.; et al. Meat flavor: Contribution of proteins and peptides to the flavor of beef. Qual. Fresh Process. Foods 2004, 542, 33–49. [Google Scholar]
- Zhou, C.Y.; Wang, C.; Tang, C.B.; Dai, C.; Bai, Y.; Yu, X.B.; Li, C.B.; Xu, X.L.; Zhou, G.H.; Cao, J.X. Label-free proteomics reveals the mechanism of bitterness and adhesiveness in Jinhua ham. Food Chem. 2019, 297, 125012. [Google Scholar] [CrossRef]
- Harimana, Y.; Tang, X.; Le, G.W.; Xing, X.G.; Zhang, K.; Sun, Y.J. Quality parameters of black carp (Mylopharyngodon piceus) raised in lotic and lentic freshwater systems. LWT-Food Sci. Technol. 2018, 90, 45–52. [Google Scholar] [CrossRef]
- Kong, Y.; Yang, X.; Ding, Q.; Zhang, Y.Y.; Sun, B.G.; Chen, H.T. Comparison of non-volatile umami components in chicken soup and chicken enzymatic hydrolysate. Food Res. Int. 2017, 102, 559–566. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Tao NP Gong, J.; Gu, S.Q.; Xu, C.H. Comparison of non-volatile taste-active compounds between the cooked meats of pre-and post-spawning Yangtze Coilia ectenes. Fish. Sci. 2015, 81, 559–568. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Ninomiya, K. Umami and food palatability. J. Nutr. 2000, 130, 921S–926S. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Zhang, Y.F.; Jin, Y.F.; Deng, Y.; Zhao, Y.Y. Impact of high hydrostatic pressure on non-volatile and volatile compounds of squid muscles. Food Chem. 2016, 194, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Fuke, S.; Ueda, Y. Interactions between umami and other flavor characteristics. Trends Food Sci. Technol. 1996, 7, 407–411. [Google Scholar] [CrossRef]
- Van Gemert, L. Compilations of Odour Threshold Values in Air Water and Other Media (Second Enlarged and Revised Edition). 2011. Available online: https://www.academia.edu/3443692/Compilations_of_odour_threshold_values_in_air_water_and_other_media_second_enlarged_and_revised_edition_ (accessed on 16 January 2024).
- Guo, Z.B.; Lin, D.; Guo, J.; Zhang, Y.; Zheng, B.D. In vitro antioxidant activity and in vivo anti-fatigue ffect of sea horse (Hippocampus) peptides. Molecules 2017, 22, 482. [Google Scholar] [CrossRef]
- Fatoumata, T.; Mohanad, B.; Guo, W.L.; Shi, Y.H. Antioxidant activities of roselle (Hibiscus sabdariffa L.) seed protein hydrolysate and its derived peptide fractions. Int. J. Food Prop. 2014, 17, 1998–2011. [Google Scholar] [CrossRef]
- Zhao, C.J.; Schieber, A.; Gnzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations—A review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef]
- Li, H.; Qin, D.; Wu, Z.Y.; Sun, B.G.; Sun, X.Y.; Huang, M.Q. Characterization of key aroma compounds in Chinese Guojing sesame-flavor baijiu by means of molecular sensory science. Food Chem. 2019, 284, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, M.; Yamada, Y.; Egi, M. Taste enhancer from the long-term ripening of miso (soybean paste). Food Chem. 2006, 99, 736–741. [Google Scholar] [CrossRef]
- Madruga, M.S.; Elmore, J.S.; Oruna-Concha, M.J.; Balagiannis, D.; Mottram, D.S. Determination of some water-soluble aroma precursors in goat meat and their enrolment on flavour profile of goat meat. Food Chem. 2010, 123, 513–520. [Google Scholar] [CrossRef]
- Xu, X.R.; Yu, M.G.; Raza, J.; Song, H.L.; Gong, L.; Pan, W.Q. Study of the mechanism of flavor compounds formed via taste-active peptides in bovine bone protein extract. LWT-Food Sci. Technol. 2021, 137, 110371. [Google Scholar] [CrossRef]
- Lynch, M.P.; Faustman, C. Effect of aldehyde lipid oxidation products on myoglobin. J. Agric. Food Chem. 2000, 48, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Tarnowicz-Ligus, S.; Trzeciak, A.M. Heck transformations of biological compounds catalyzed by phosphine-free palladium. Molecules 2018, 23, 2227. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, A.; Zhang, C.P.; Xie, J.C.; Yang, X.L. Maillard reaction chemistry in formation of critical intermediates and flavour compounds and their antioxidant properties. Food Chem. 2022, 393, 133416. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T.; Guimbertau, G.; Dubourdieu, D. Contribution of benzenemethanethiol to smoky aroma of Certainvitis vinifera L. wines. J. Agric. Food Chem. 2003, 51, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, M.; Zielinski, H. How maillard reaction influences sensorial properties (color, flavor and texture) of food products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Liu, S.Y.; Sun, H.J.; Ma, G.; Zhang, T.; Wang, L.; Pei, H.; Li, X.; Gao, L.Y. Insights into flavor and key influencing factors of Maillard reaction products: A recent update. Front. Nutr. 2022, 9, 973677. [Google Scholar] [CrossRef]
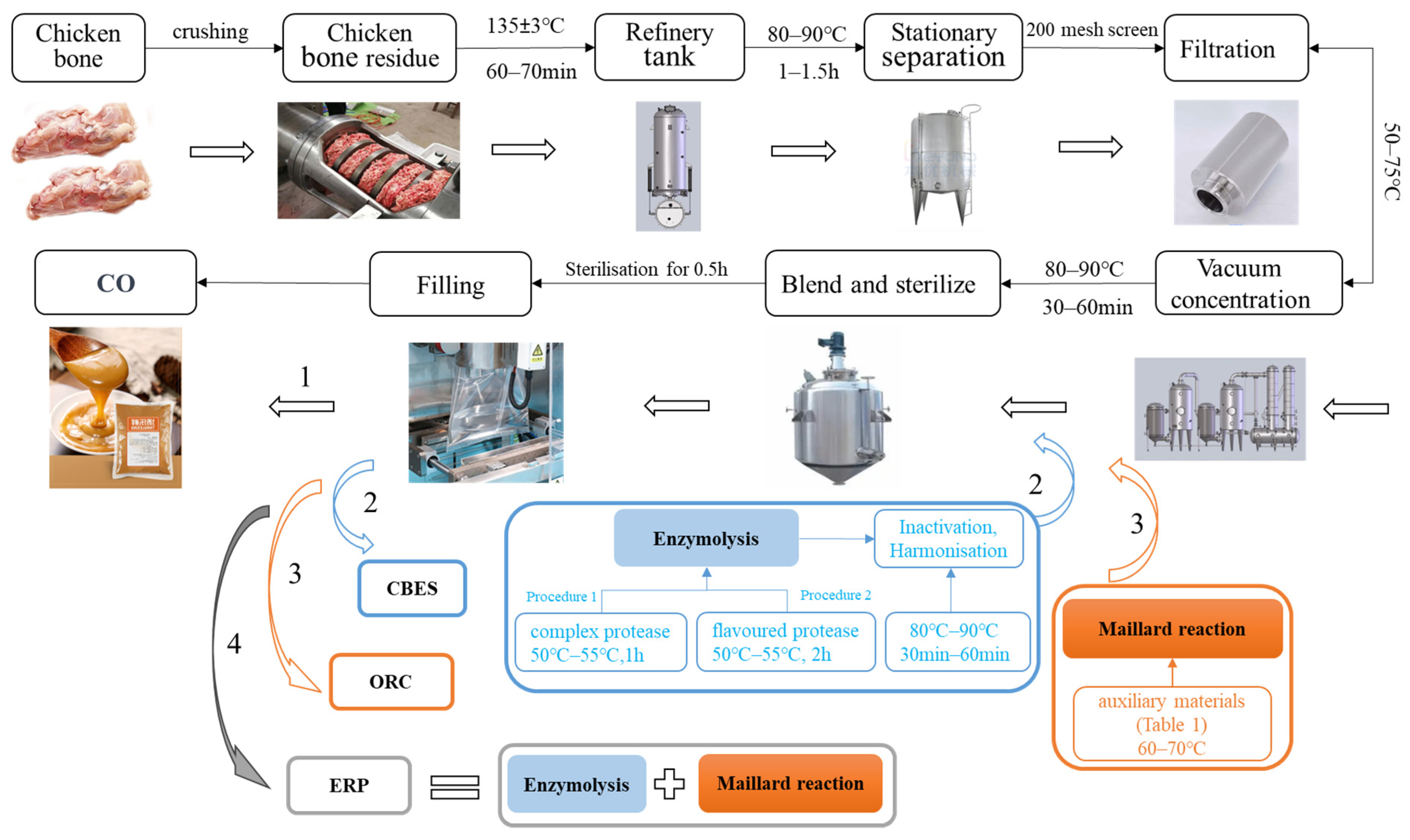


| Auxiliary Materials | Weights (kg/t) | |||
|---|---|---|---|---|
| CO | CBES | ORC | ERP | |
| CO | — | 1000 | 700 | 700 |
| Complex protease | — | 0.23 | — | 0.23 |
| Flavor protease | — | 0.38 | — | 0.38 |
| Chicken oil | — | — | 150 | 150 |
| Yeast extract | — | — | 80 | 80 |
| White Sugar | — | — | 10 | 10 |
| Glucose | — | — | 10 | 10 |
| Monosodium glutamate | — | — | 10 | 10 |
| Cysteine | — | — | 5 | 5 |
| VB1 | — | — | 5 | 5 |
| Alanine | — | — | 5 | 5 |
| Starch | — | — | 15 | 15 |
| Salt | — | — | 10 | 10 |
| Taste Compounds | Concentrations (mg/mL) | |||
|---|---|---|---|---|
| CO | CBES | ORC | ERP | |
| Amino acid | ||||
| Umami amino acids | ||||
| Asp | 67.67 ± 0.68 b | 95.45 ± 42.73 ab | 124.45 ± 19.13 a | 140.69 ± 14.77 a |
| Glu | 139.05 ± 5.94 c | 152.35 ± 29.72 c | 1317.57 ± 241.9 b | 1632.07 ± 132.86 a |
| Subtotal | 206.72 ± 6.62 d | 247.8 ± 72.45 c | 1442.02 ± 261.03 b | 1772.76 ± 147.63 a |
| Sweet amino acids | ||||
| Gly | 255.7 ± 44.63 b | 253.17 ± 47.27 b | 354.39 ± 99.69 ab | 454.67 ± 68.16 a |
| Ser | 141.84 ± 8.92 b | 152.15 ± 31.11 b | 642.69 ± 117.13 a | 797.56 ± 121.14 a |
| Thr | 333.94 ± 64.39 | 337.12 ± 57.43 | 261.6 ± 74.81 | 358.87 ± 48.57 |
| Ala | 191.9 ± 16.68 c | 203.58 ± 43.28 c | 1879.95 ± 421.88 b | 2447.27 ± 364.01 a |
| Subtotal | 994.8 ± 144.73 d | 1022.66 ± 193.31 c | 3214.58 ± 735.47 b | 4140.46 ± 618.19 a |
| Bitter amino acids | ||||
| Pro | 71.42 ± 10.11 | 76.64 ± 14.22 | 75.95 ± 21.96 | 82.09 ± 16.31 |
| His | 31.46 ± 4.97 c | 32.57 ± 6.65 c | 142.88 ± 27.7 b | 70.15 ± 12.85 a |
| Arg | 414.78 ± 88.7 | 439.51 ± 70.75 | 314.57 ± 88.84 | 375.36 ± 39.00 |
| Tyr | 54.16 ± 15.49 | 44.9 ± 5.18 | 92.85 ± 23.61 | 117.99 ± 16.97 |
| Val | 56.91 ± 8.42 b | 35.62 ± 19.51 b | 125.66 ± 32.29 a | 212.71 ± 33.11 a |
| Met | 19.54 ± 3.26 bc | 9.01 ± 1.00 c | 27.56 ± 13.21 b | 53.47 ± 11.54 a |
| Lys | 55.31 ± 3.45 b | 61.68 ± 13.5 b | 73.32 ± 13.41 b | 106.18 ± 15.22 a |
| Ile | 35.98 ± 3.63 c | 33.44 ± 4.86 c | 93.84 ± 21.33 b | 154.27 ± 24.28 a |
| Leu | 70.9 ± 9 c | 60.37 ± 10.83 c | 279.64 ± 71.08 b | 433.46 ± 72.75 a |
| Phe | 35.63 ± 8.13 c | 38.65 ± 4.67 c | 111.7 ± 29.35 b | 162.38 ± 24.6 a |
| Subtotal | 774.67 ± 145.05 c | 755.75 ± 136.95 d | 1262.02 ± 320.82 b | 1685.97 ± 250.32 a |
| Tasteless amino acids | ||||
| Cys | 12.39 ± 2.24 b | 12.77 ± 2.34 b | 775.49 ± 328.24 a | 756.96 ± 112.41 a |
| Total | 1988.58 ± 298.64 d | 2038.98 ± 405.05 c | 6694.11 ± 1626.43 b | 8356.15 ± 1128.55 a |
| Nucleotide | ||||
| 5′-CMP | 0.22 ± 0.01 c | 0.28 ± 0.04 c | 20.83 ± 1.07 b | 24.53 ± 1.17 a |
| 5′-GMP | — | — | 0.91 ± 0.04 b | 1.00 ± 0.04 a |
| 5′-IMP | 19.56 ± 1.08 d | 22.66 ± 1.07 c | 33.78 ± 1.67 b | 39.11 ± 2.21 a |
| 5′-XMP | 62.43 ± 3.70 ab | 57.86 ± 0.62 bc | 55.54 ± 3.27 c | 67.53 ± 3.68 a |
| 5′-AMP | 49.42 ± 2.47 d | 57.22 ± 1.08 c | 69.56 ± 3.56 b | 79.84 ± 3.81 a |
| Total | 131.63 ± 7.26 d | 138.02 ± 2.8 c | 180.62 ± 9.61 b | 212.01 ± 10.91 a |
| Taste Compounds | Taste Threshold in Water (mg/mL) | TAV | |||
|---|---|---|---|---|---|
| CO | CBES | ORC | ERP | ||
| Amino acid | |||||
| Asp | 1 | 0.07 | 0.1 | 0.12 | 0.14 |
| Ser | 1.5 | 0.09 | 0.1 | 0.43 | 0.53 |
| Glu | 0.3 | 0.46 | 0.51 | 4.39 | 5.44 |
| Gly | 1.3 | 0.20 | 0.19 | 0.27 | 0.35 |
| His | 0.2 | 0.16 | 0.16 | 0.71 | 0.35 |
| Arg | 0.5 | 0.83 | 0.88 | 0.63 | 0.75 |
| Thr | 2.6 | 0.13 | 0.13 | 0.10 | 0.14 |
| Ala | 0.6 | 0.32 | 0.34 | 3.13 | 4.08 |
| Pro | 3.0 | 0.02 | 0.03 | 0.03 | 0.03 |
| Cys | — | — | — | — | — |
| Tyr | — | — | — | — | — |
| Val | 0.4 | 0.14 | 0.09 | 0.31 | 0.53 |
| Met | 0.3 | 0.07 | 0.03 | 0.09 | 0.18 |
| Lys | 0.5 | 0.11 | 0.12 | 0.15 | 0.21 |
| Ile | 0.9 | 0.04 | 0.04 | 0.10 | 0.17 |
| Leu | 1.9 | 0.04 | 0.03 | 0.15 | 0.23 |
| Phe | 0.9 | 0.04 | 0.04 | 0.12 | 0.18 |
| Nucleotide | |||||
| 5′-AMP | 50 | 0.99 | 1.14 | 1.39 | 1.6 |
| 5′-GMP | 12.5 | — | — | 0.07 | 0.08 |
| 5′-IMP | 25 | 0.78 | 0.91 | 1.35 | 1.56 |
| No. | Compounds | LRI | Identification | Concentration (ng/g) | |||
|---|---|---|---|---|---|---|---|
| CO | CBE | ORC | ERP | ||||
| Aldehyde | |||||||
| 1 | Octanal | 1287 | MS + RI | 1.19 ± 0.12 b | 2.44 ± 0.04 a | — | 1.54 ± 0.01 b |
| 2 | Nonanal | 1390 | MS + RI | 19.86 ± 0.29 a | 13.37 ± 0.63 b | 3.70 ± 0.32 d | 8.16 ± 0.70 c |
| 3 | Furfural | 1455 | MS + RI | 2.90 ± 0.20 b | 4.10 ± 0.44 a | — | — |
| 4 | Benzaldehyde | 1508 | MS + RI | — | 26.79 ± 0.34 c | 37.68 ± 1.24 b | 55.40 ± 2.40 a |
| 5 | 5-Methyl-thiophene-2-carboxaldehyde | 1755 | MS + RI | — | — | 15.22 ± 0.67 b | 36.76 ± 1.30 a |
| 6 | 2-Carboxaldehyde pyrrole- | 2059 | MS + RI | — | — | 1.05 ± 0.04 | 0.66 ± 0.03 |
| Subtotal | 23.95 ± 0.61 d | 46.70 ± 1.45 c | 57.65 ± 2.27 b | 102.52 ± 4.44 a | |||
| Alcohols | |||||||
| 1 | 1-Octen-3-ol | 1442 | MS + RI | — | 1.15 ± 0.02 | — | — |
| 2 | 2-Ethyl-hexanol | 1480 | MS + RI | 6.75 ± 1.05 a | — | — | 1.77 ± 0.33 b |
| 3 | Linalool | 1552 | MS + RI | — | — | 5.54 ± 0.20 a | 3.50 ± 0.30 b |
| 4 | 1-Octanol | 1561 | MS + RI | 6.78 ± 1.03 a | 7.46 ± 1.24 a | 1.89 ± 0.08 c | 2.36 ± 0.26 c |
| 5 | Diethylene Glycol Mono-ethyl-Ether | 1619 | MS + RI | — | — | 4.92 ± 0.60 b | 7.47 ± 0.45 a |
| 6 | 2-Furanmethanol | 1669 | MS + RI | 11.00 ± 1.35 b | 10.61 ± 1.00 b | 8.73 ± 0.87 c | 22.23 ± 2.03 a |
| 7 | Estragole | 1685 | MS + RI | — | — | 9.73 ± 1.02 b | 15.61 ± 1.25 a |
| 8 | Alpha-Terpineol | 1690 | MS + RI | — | — | 6.72 ± 0.60 | 5.92 ± 0.52 |
| 9 | Benzyl alcohol | 1878 | MS + RI | 1.36 ± 0.06 d | 2.30 ± 0.35 c | 16.01 ± 1.50 b | 19.59 ± 1.08 a |
| 10 | Phenylethyl alcohol | 1919 | MS + RI | 0.91 ± 0.05 | 1.14 ± 0.04 | — | — |
| Subtotal | 26.79 ± 3.54 c | 22.65 ± 2.65 d | 53.54 ± 4.87 b | 78.45 ± 6.22 a | |||
| Ketones | |||||||
| 1 | Acetophenone | 1645 | MS + RI | 7.00 ± 1.06 a | 1.72 ± 0.13 b | — | — |
| 2 | N-Methyl-2-pyrrolidone | 1662 | MS + RI | — | 3.17 ± 0.46 a | 1.95 ± 0.22 b | 3.18 ± 0.30 a |
| Subtotal | 7.00 ± 1.06 a | 4.89 ± 0.59 b | 1.95 ± 0.22 d | 3.18 ± 0.30 c | |||
| Pyrazines | |||||||
| 1 | 2-Methylpyrazine | 1276 | MS + RI | 1.73 ± 0.07 b | 1.86 ± 0.03 b | 1.56 ± 0.04 c | 2.85 ± 0.12 a |
| 2 | 2,6-Dimethylpyrazine | 1319 | MS + RI | 26.69 ± 1.26 a | 24.03 ± 1.22 a | 5.17 ± 0.78 c | 9.71 ± 1.20 b |
| 3 | 2,5-Dimethylyrazine | 1323 | MS + RI | — | — | 5.43 ± 0.20 | 6.05 ± 0.32 |
| 4 | 2,3-Dimethylpyrazine | 1346 | MS + RI | — | — | 1.28 ± 0.05 | 1.91 ± 0.03 |
| 5 | 6-Methyl-2-ethylpyrazine | 1390 | MS + RI | 1.62 ± 0.05 c | 1.75 ± 0.07 c | 2.50 ± 0.20 b | 4.76 ± 0.43 a |
| 6 | 5-Methyl-2-ethylpyrazine | 1399 | MS + RI | 2.89 ± 0.25 b | 3.87 ± 0.44 a | 1.6 ± 0.19 c | 2.86 ± 0.25 b |
| 7 | Trimethylpyrazine | 1413 | MS + RI | 6.86 ± 0.74 d | 7.48 ± 0.75 c | 11.43 ± 1.10 b | 15.08 ± 1.55 a |
| 8 | 2,5-Dimethyl-3-ethylpyrazine | 1449 | MS + RI | 4.22 ± 0.42 b | 6.48 ± 0.65 a | 2.37 ± 0.20 c | 3.37 ± 0.34 b |
| Subtotal | 44.01 ± 2.79 a | 45.47 ± 3.16 a | 31.41 ± 2.76 b | 46.6 ± 4.24 a | |||
| Furans | |||||||
| 1 | 2-Pentylfuran | 1249 | MS + RI | — | 1.60 ± 0.08 b | 1.02 ± 0.12 b | 5.60 ± 0.49 a |
| 2 | 2-Acetylfuran | 1479 | MS + RI | — | — | 37.12 ± 2.33 b | 49.51 ± 1.5 a |
| Subtotal | — | 1.60 ± 0.08 c | 38.14 ± 2.45 b | 55.11 ± 1.99 a | |||
| Pyrrole, thiazole | |||||||
| 1 | 2-Acetylthiazole | 1643 | MS + RI | — | — | 5.05 ± 0.39 b | 7.02 ± 0.89 a |
| 2 | Benzothiazole | 1961 | MS + RI | 3.45 ± 0.40 a | 1.62 ± 0.12 b | 0.98 ± 0.03 c | 0.80 ± 0.09 c |
| 3 | 2-Acetylpyrrole | 1971 | MS + RI | 1.57 ± 0.12 b | 1.77 ± 0.20 b | 26.26 ± 2.04 a | 23.15 ± 1.98 a |
| Subtotal | 5.02 ± 0.52 b | 3.39 ± 0.32 b | 27.24 ± 2.07 a | 23.95 ± 2.07 a | |||
| Sulfurs | |||||||
| 1 | Ethanethioic acid S-ethyl ester | 1109 | MS + RI | — | — | — | 2.32 ± 0.28 |
| 2 | 2-Methyl-3-furanthiol | 1315 | MS + RI | — | — | — | 2.00 ± 0.09 |
| 3 | Bis(2-Methyl-3-furyl) disulfide | 2100 | MS + RI | — | — | — | 4.30 ± 0.38 |
| Subtotal | — | — | — | 8.62 ± 0.75 | |||
| Acids | |||||||
| 1 | Acetic acid | 1460 | MS + RI | 1.96 ± 0.89 c | 1.85 ± 0.85 c | 18.58 ± 1.56 b | 49.72 ± 4.25 a |
| 2 | Isovaleric acid | 1680 | MS + RI | — | — | 5.98 ± 0.34 b | 8.3 ± 0.55 a |
| 3 | Heptanoic acid | 1934 | MS + RI | — | — | 2.09 ± 0.10 | 2.22 ± 0.13 |
| 4 | Hexanoic acid | 1954 | MS + RI | — | — | 6.45 ± 0.67 b | 8.40 ± 0.85 a |
| Subtotal | 1.96 ± 0.89 c | 1.85 ± 0.85 c | 33.10 ± 2.67 b | 68.64 ± 5.78 a | |||
| Esters | |||||||
| 1 | Butyrolactone | 1626 | MS + RI | 2.05 ± 0.31 c | 1.82 ± 0.23 c | 3.04 ± 0.35 b | 5.02 ± 0.67 a |
| 2 | 5-Decanolide | 2220 | MS + RI | 0.53 ± 0.05 b | 0.70 ± 0.05 b | 1.26 ± 0.16 a | 1.90 ± 0.20 a |
| Subtotal | 2.58 ± 0.36 c | 2.52 ± 0.28 c | 4.30 ± 0.51 b | 6.92 ± 0.87 a | |||
| Others | |||||||
| 1 | D-Limonene | 1105 | MS + RI | — | — | 3.89 ± 0.42 b | 9.16 ± 0.92 a |
| 2 | Styrene | 1254 | MS + RI | 1.80 ± 0.20 | 1.79 ± 0.18 | — | — |
| 3 | Naphthalene | 1707 | MS + RI | — | — | 5.75 ± 0.34 b | 8.52 ± 0.59 a |
| 4 | Anethole | 1817 | MS + RI | — | — | 8.74 ± 0.67 | 7.46 ± 0.83 |
| 5 | Guaiacol | 1860 | MS + RI | — | — | 6.96 ± 0.44 b | 8.02 ± 0.36 a |
| 6 | 2-Methylnaphthalene | 1877 | MS + RI | 3.11 ± 0.31 a | 1.87 ± 0.22 b | 2.02 ± 0.18 b | 2.93 ± 0.21 a |
| 7 | Dimethyl sulfone | 1912 | MS + RI | 0.77 ± 0.09 | — | — | 1.35 ± 0.14 |
| 8 | Maltol | 1978 | MS + RI | — | — | 8.51 ± 0.85 | 8.63 ± 0.67 |
| 9 | Ethyl maltol | 1980 | MS + RI | — | — | 446.70 ± 45.89 b | 453.71 ± 56.77 a |
| 10 | Phenol | 1992 | MS + RI | 2.11 ± 0.18 a | 1.73 ± 0.16 a | 1.59 ± 0.12 b | 1.82 ± 0.15 a |
| 11 | p-Cresol | 2089 | MS + RI | 3.15 ± 0.32 c | 3.65 ± 0.40 b | 4.10 ± 0.37 b | 14.04 ± 1.55 a |
| 12 | 2,4-Di-tert-butylphenol | 2321 | MS + RI | 1.60 ± 0.20 a | 1.03 ± 0.15 b | — | — |
| 13 | Indole | 2435 | MS + RI | — | — | 3.47 ± 0.58 b | 4.38 ± 0.42 a |
| Subtotal | 12.54 ± 1.3 c | 10.05 ± 1.11 c | 491.72 ± 49.86 b | 520.02 ± 62.61 a | |||
| Total | 123.85 ± 11.07 d | 139.12 ± 10.49 c | 739.07 ± 67.68 b | 914.01 ± 89.27 a | |||
| Compounds | Threshold in Water (ng/g) | OAVs | |||
|---|---|---|---|---|---|
| CO | CBES | ORC | ERP | ||
| Aldehyde | |||||
| Octanal | 0.41 | 3 | 6 | — | 4 |
| Nonanal | 1 | 20 | 13 | 4 | 8 |
| Alcohols | |||||
| 1-Octen-3-ol | 1 | — | 1 | — | — |
| Linalool | 0.01 | — | — | 554 | 350 |
| Estragole | 6 | — | — | 2 | 3 |
| 2-Phenylethanol | 0.015 | 61 | 76 | — | — |
| Pyrazines | |||||
| 3,6-Dimethyl-2-ethylpyrazine | 0.4 | 11 | 16 | 6 | 8 |
| Pyrrole, thiazole | |||||
| 2-Acetylthiazole | 3 | — | — | 2 | 2 |
| Sulfurs | |||||
| 2-Methyl-3-furanthiol | 0.0004 | — | — | — | 5004 |
| 3,3′-Dithiobis-2-methylfuran | 0.00002 | — | — | — | 214,942 |
| Others | |||||
| 2-Methyl-naphthalene | 3 | 1 | <1 | <1 | <1 |
| 2-Methoxy-phenol | 0.48 | — | — | 15 | 17 |
| p-Cresol | 2.7 | 1 | 4 | 2 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Bi, K.; Wu, G.; Yang, P.; Li, H.; Jia, W.; Zhang, C. The Effect of Enzymatic Hydrolysis and Maillard Reaction on the Flavor of Chicken Osteopontin. Foods 2024, 13, 702. https://doi.org/10.3390/foods13050702
Xu X, Bi K, Wu G, Yang P, Li H, Jia W, Zhang C. The Effect of Enzymatic Hydrolysis and Maillard Reaction on the Flavor of Chicken Osteopontin. Foods. 2024; 13(5):702. https://doi.org/10.3390/foods13050702
Chicago/Turabian StyleXu, Xiong, Ke Bi, Guangyu Wu, Ping Yang, Hongjun Li, Wei Jia, and Chunhui Zhang. 2024. "The Effect of Enzymatic Hydrolysis and Maillard Reaction on the Flavor of Chicken Osteopontin" Foods 13, no. 5: 702. https://doi.org/10.3390/foods13050702
APA StyleXu, X., Bi, K., Wu, G., Yang, P., Li, H., Jia, W., & Zhang, C. (2024). The Effect of Enzymatic Hydrolysis and Maillard Reaction on the Flavor of Chicken Osteopontin. Foods, 13(5), 702. https://doi.org/10.3390/foods13050702





