Assessment of Sourdough Fermentation Impact on the Antioxidant and Anti-Inflammatory Potential of Pearl Millet from Burkina Faso
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Extraction
2.3. Total Polyphenols and Flavonoids Content
2.4. Quantification of Polyphenols by HPLC-DAD
2.5. In Vitro Antioxidant Activity
2.5.1. DPPH Radical Scavenging Activity
2.5.2. ABTS Radical Scavenging Activity
2.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.4. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.6. Oxidative Hemolysis of Erythrocytes
2.7. Human Intestinal Cell Culture
2.8. RT-PCR and Quantitative Real-Time RT-PCR
2.9. Statistical Analysis
3. Results
3.1. Content of Bioactive Compounds and Antioxidant Activities In Vitro
3.2. HPLC-DAD Quantification of Phenolic Compounds
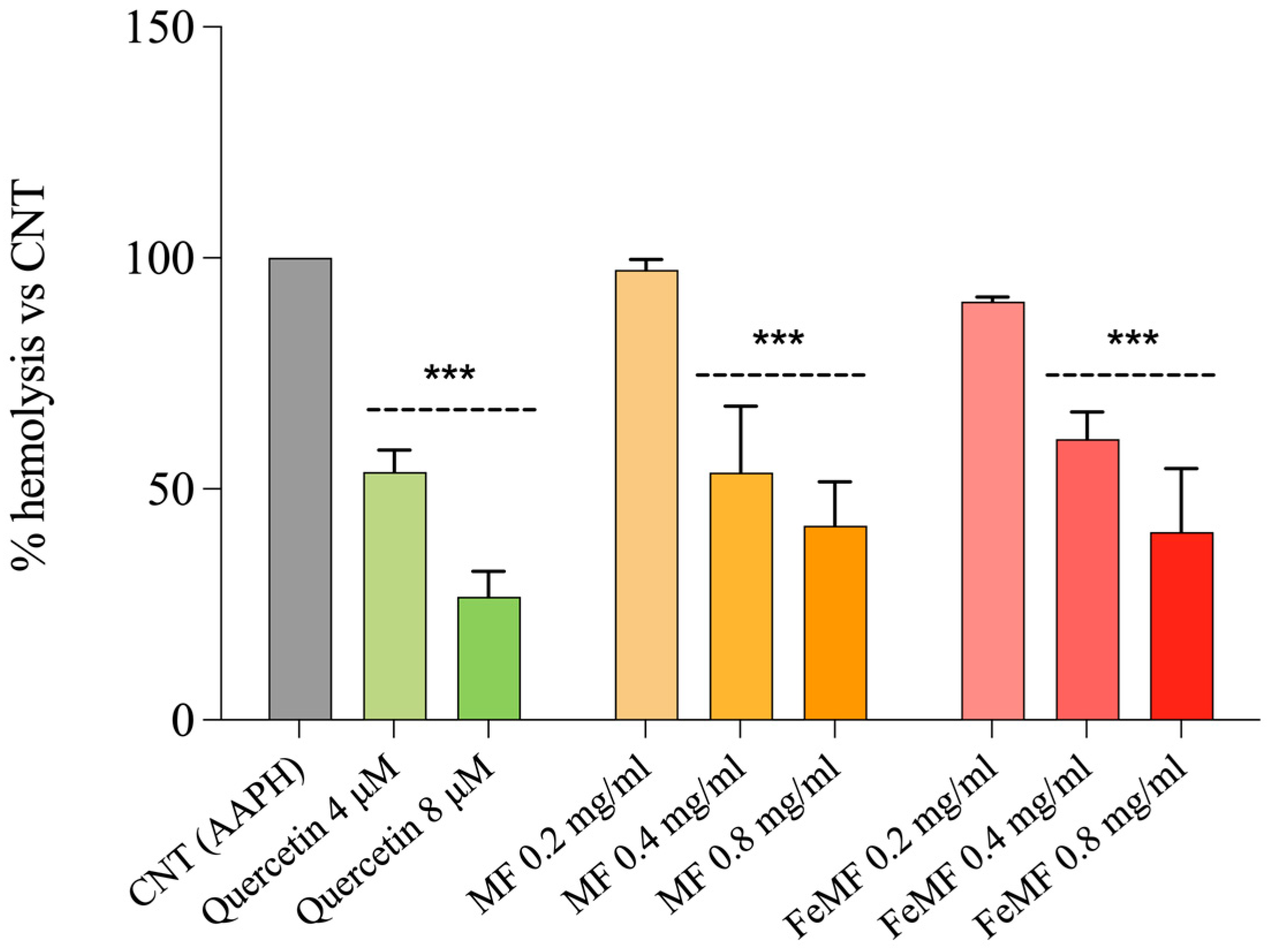
3.3. Hemolysis Test
3.4. Protective Effect against TNFα-Induced Intestinal Alterations of FeMF and MF Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Saleh, A.S.M.; Zhang, Q.; Chen, J.; Shen, Q. Millet Grains: Nutritional Quality, Processing, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2013, 12, 281–295. [Google Scholar] [CrossRef]
- International Year of Millets: Unleashing the Potential of Millets for the Well-Being of People and the Environment. Available online: https://www.fao.org/newsroom/detail/international-year-of-millets-unleashing-the-potential-of-millets-for-the-well-being-of-people-and-the-environment/en (accessed on 7 February 2024).
- Taylor, J.R.N. Chapter 1—Sorghum and millets: Taxonomy, history, distribution, and production. In Sorghum and Millets, 2nd ed.; Taylor, J.R.N., Duodu, K.G., Eds.; AACC International Press: Concord, CA, USA, 2019; pp. 1–21. ISBN 978-0-12-811527-5. [Google Scholar]
- Mahajan, P.; Bera, M.B.; Panesar, P.S.; Chauhan, A. Millet Starch: A Review. Int. J. Biol. Macromol. 2021, 180, 61–79. [Google Scholar] [CrossRef]
- Serna-Saldivar, S.O.; Espinosa-Ramírez, J. Chapter 5—Grain structure and grain chemical composition. In Sorghum and Millet, 2nd ed.; Taylor, J.R.N., Duodu, K.G., Eds.; AACC International Press: Concord, CA, USA, 2019; pp. 85–129. ISBN 978-0-12-811527-5. [Google Scholar]
- Abah, C.R.; Ishiwu, C.N.; Obiegbuna, J.E.; Oladejo, A.A. Nutritional Composition, Functional Properties and Food Applications of Millet Grains. Asian Food Sci. J. 2020, 14, 9–19. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, S.; Dar, B.N.; Singh, B. Millets as Potential Nutri-Cereals: A Review of Nutrient Composition, Phytochemical Profile and Techno-Functionality. Int. J. Food Sci. Technol. 2021, 56, 3703–3718. [Google Scholar] [CrossRef]
- Kam, J.; Puranik, S.; Yadav, R.; Manwaring, H.R.; Pierre, S.; Srivastava, R.K.; Yadav, R.S. Dietary Interventions for Type 2 Diabetes: How Millet Comes to Help. Front. Plant Sci. 2016, 7, 1454. [Google Scholar] [CrossRef]
- Pei, J.; Umapathy, V.R.; Vengadassalapathy, S.; Hussain, S.F.J.; Rajagopal, P.; Jayaraman, S.; Veeraraghavan, V.P.; Palanisamy, C.P.; Gopinath, K. A Review of the Potential Consequences of Pearl Millet (Pennisetum glaucum) for Diabetes Mellitus and Other Biomedical Applications. Nutrients 2022, 14, 2932. [Google Scholar] [CrossRef]
- Belton, P.S.; Taylor, J.R.N. Sorghum and Millets: Protein Sources for Africa. Trends Food Sci. Technol. 2004, 15, 94–98. [Google Scholar] [CrossRef]
- Gabriele, M.; Pucci, L. Chapter 23—Fermentation and germination as a way to improve cereals antioxidant and antiinflammatory properties. In Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress; Hernández-Ledesma, B., Martínez-Villaluenga, C., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 477–497. ISBN 978-0-12-823482-2. [Google Scholar]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty Years of Knowledge on Sourdough Fermentation: A Systematic Review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- Gabriele, M.; Arouna, N.; Árvay, J.; Longo, V.; Pucci, L. Sourdough Fermentation Improves the Antioxidant, Antihypertensive, and Anti-Inflammatory Properties of Triticum dicoccum. Int. J. Mol. Sci. 2023, 24, 6283. [Google Scholar] [CrossRef]
- Colosimo, R.; Gabriele, M.; Cifelli, M.; Longo, V.; Domenici, V.; Pucci, L. The Effect of Sourdough Fermentation on Triticum dicoccum from Garfagnana: 1H NMR Characterization and Analysis of the Antioxidant Activity. Food Chem. 2020, 305, 125510. [Google Scholar] [CrossRef]
- Yang, T.; Ma, S.; Liu, J.; Sun, B.; Wang, X. Influences of Four Processing Methods on Main Nutritional Components of Foxtail Millet: A Review. Grain Oil Sci. Technol. 2022, 5, 156–165. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of Fermentation on the Antioxidant Activity in Plant-Based Foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Shiferaw Terefe, N.; Augustin, M.A. Fermentation for Tailoring the Technological and Health Related Functionality of Food Products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2887–2913. [Google Scholar] [CrossRef]
- Yousaf, L.; Hou, D.; Liaqat, H.; Shen, Q. Millet: A Review of Its Nutritional and Functional Changes during Processing. Food Res. Int. 2021, 142, 110197. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Peñas, E.; Frias, J. Chapter 2—Bioactive peptides in fermented foods: Production and evidence for health effects. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 23–47. ISBN 978-0-12-802309-9. [Google Scholar]
- Wu, H.; Rui, X.; Li, W.; Xiao, Y.; Zhou, J.; Dong, M. Whole-Grain Oats (Avena sativa L.) as a Carrier of Lactic Acid Bacteria and a Supplement Rich in Angiotensin I-Converting Enzyme Inhibitory Peptides through Solid-State Fermentation. Food Funct. 2018, 9, 2270–2281. [Google Scholar] [CrossRef]
- Algonaiman, R.; Alharbi, H.F.; Barakat, H. Antidiabetic and Hypolipidemic Efficiency of Lactobacillus plantarum Fermented Oat (Avena sativa) Extract in Streptozotocin-Induced Diabetes in Rats. Fermentation 2022, 8, 267. [Google Scholar] [CrossRef]
- Xiao, X.; Bai, J.; Zhang, J.; Wu, J.; Dong, Y. Inhibitory Effect of Fermented Selected Barley Extracts with Lactobacillus plantarum Dy-1 on the Proliferation of Human HT-29 Cells. J. Food Biochem. 2019, 43, e12989. [Google Scholar] [CrossRef]
- Verni, M.; Verardo, V.; Rizzello, C.G. How Fermentation Affects the Antioxidant Properties of Cereals and Legumes. Foods 2019, 8, 362. [Google Scholar] [CrossRef]
- Balli, D.; Bellumori, M.; Pucci, L.; Gabriele, M.; Longo, V.; Paoli, P.; Melani, F.; Mulinacci, N.; Innocenti, M. Does Fermentation Really Increase the Phenolic Content in Cereals? A Study on Millet. Foods 2020, 9, 303. [Google Scholar] [CrossRef]
- Shahbazi, R.; Sharifzad, F.; Bagheri, R.; Alsadi, N.; Yasavoli-Sharahi, H.; Matar, C. Anti-Inflammatory and Immunomodulatory Properties of Fermented Plant Foods. Nutrients 2021, 13, 1516. [Google Scholar] [CrossRef]
- Gabriele, M.; Pucci, L.; Árvay, J.; Longo, V. Anti-Inflammatory and Antioxidant Effect of Fermented Whole Wheat on TNFα-Stimulated HT-29 and NF-κB Signaling Pathway Activation. J. Funct. Foods 2018, 45, 392–400. [Google Scholar] [CrossRef]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J Agric Food Chem 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Barreira, J.C.M.; Sousa, M.J.; Carvalho, A.M.; Ferreira, I.C.F.R. Targeting Excessive Free Radicals with Peels and Juices of Citrus Fruits: Grapefruit, Lemon, Lime and Orange. Food Chem. Toxicol. 2010, 48, 99–106. [Google Scholar] [CrossRef]
- Chelucci, E.; Chiellini, C.; Cavallero, A.; Gabriele, M. Bio-Functional Activities of Tuscan Bee Pollen. Antioxidants 2023, 12, 115. [Google Scholar] [CrossRef]
- Mikstacka, R.; Rimando, A.M.; Ignatowicz, E. Antioxidant Effect of Trans-Resveratrol, Pterostilbene, Quercetin and Their Combinations in Human Erythrocytes In Vitro. Plant Foods Hum. Nutr. 2010, 65, 57–63. [Google Scholar] [CrossRef]
- Hassan, Z.M.; Sebola, N.A.; Mabelebele, M. The Nutritional Use of Millet Grain for Food and Feed: A Review. Agric. Food Secur. 2021, 10, 16. [Google Scholar] [CrossRef]
- Muthamilarasan, M.; Dhaka, A.; Yadav, R.; Prasad, M. Exploration of Millet Models for Developing Nutrient Rich Graminaceous Crops. Plant Sci. Int. J. Exp. Plant Biol. 2016, 242, 89–97. [Google Scholar] [CrossRef]
- Gabaza, M.; Shumoy, H.; Muchuweti, M.; Vandamme, P.; Raes, K. Effect of Fermentation and Cooking on Soluble and Bound Phenolic Profiles of Finger Millet Sour Porridge. J. Agric. Food Chem. 2016, 64, 7615–7621. [Google Scholar] [CrossRef]
- Đorđević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Effect of Fermentation on Antioxidant Properties of Some Cereals and Pseudo Cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Hubert, J.; Berger, M.; Nepveu, F.; Paul, F.; Daydé, J. Effects of Fermentation on the Phytochemical Composition and Antioxidant Properties of Soy Germ. Food Chem. 2008, 109, 709–721. [Google Scholar] [CrossRef]
- Adebo, O.A.; Gabriela Medina-Meza, I. Impact of Fermentation on the Phenolic Compounds and Antioxidant Activity of Whole Cereal Grains: A Mini Review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef]
- Salar, R.K.; Purewal, S.S.; Sandhu, K.S. Fermented Pearl Millet (Pennisetum glaucum) with in Vitro DNA Damage Protection Activity, Bioactive Compounds and Antioxidant Potential. Food Res. Int. 2017, 100, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Zhao, H.; Lu, Z.; Lu, F.; Bie, X.; Zhang, C. Improved Physicochemical and Functional Properties of Dietary Fiber from Millet Bran Fermented by Bacillus natto. Food Chem. 2019, 294, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Semwal, D.K. Health Benefits and Limitations of Rutin—A Natural Flavonoid with High Nutraceutical Value. Phytochem. Lett. 2021, 46, 119–128. [Google Scholar] [CrossRef]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- Moazzen, A.; Öztinen, N.; Ak-Sakalli, E.; Koşar, M. Structure-Antiradical Activity Relationships of 25 Natural Antioxidant Phenolic Compounds from Different Classes. Heliyon 2022, 8, e10467. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Li, Z.; Wu, T.; Jensen, G.S.; Schauss, A.G.; Wu, X. Anti-Oxidant Capacities of Flavonoid Compounds Isolated from Acai Pulp (Euterpe oleracea Mart.). Food Chem. 2010, 122, 610–617. [Google Scholar] [CrossRef]
- Loganayaki, N.; Siddhuraju, P.; Manian, S. Antioxidant Activity and Free Radical Scavenging Capacity of Phenolic Extracts from Helicteres isora L. and Ceiba pentandra L. J. Food Sci. Technol. 2013, 50, 687–695. [Google Scholar] [CrossRef]
- Singh, A.-K.; Patel, P.K.; Choudhary, K.; Joshi, J.; Yadav, D.; Jin, J.-O. Quercetin and Coumarin Inhibit Dipeptidyl Peptidase-IV and Exhibits Antioxidant Properties: In Silico, In Vitro, Ex Vivo. Biomolecules 2020, 10, 207. [Google Scholar] [CrossRef]
- Shi, J.; Shan, S.; Li, H.; Song, G.; Li, Z. Anti-Inflammatory Effects of Millet Bran Derived-Bound Polyphenols in LPS-Induced HT-29 Cell via ROS/miR-149/Akt/NF-κB Signaling Pathway. Oncotarget 2017, 8, 74582–74594. [Google Scholar] [CrossRef]

| TP (mg GAE/g dw) | TF (mg CE/g dw) | DPPH EC50 (mg mL−1) | FRAP (µM Fe2+) | ABTS (μmol TE/g dw) | ORAC (μmol TE/100g dw) | |
|---|---|---|---|---|---|---|
| FeMF | 3.25 ± 0.09 *** | 2.84 ± 0.39 * | 1.83 ± 0.23 *** | 1476.3 ± 0.92 ** | 19.09 ± 2.64 ns | 640.4 ± 30.97 * |
| MF | 1.83 ± 0.02 | 1.29 ± 0.53 | 3.16 ± 0.08 | 1336.2 ± 30.33 | 15.65 ± 1.95 | 498.4 ± 78.48 |
| Phenolic Compound | Concentration | Wavelength | Class | Rt | |
|---|---|---|---|---|---|
| FeMF | MF | ||||
| Gallic acid | 106.26 ± 1.30 | <LOD | 320 | Phenolic acid | 2.669 |
| 4-OH Benzoic acid | 141.15 ± 1.24 *** | 98.80 ± 3.27 | 265 | Carboxylic acid | 4.830 |
| Vanillic acid | 39.58 ± 0.55 *** | 23.87 ± 0.74 | 265 | Phenolic acid | 5.260 |
| Rutin | 4.49 ± 0.11 | <LOD | 265 | Flavonol | 5.955 |
| Vitexin | 296.88 ± 2.50 *** | 56.88 ±1.80 | 320 | Flavone | 6.275 |
| Iso-Quercitrin | <LOD | <LOD | 372 | Flavonol | 6.987 |
| trans-p-Coumaric acid | <LOD | 6.48 ± 0.42 | 320 | Hydroxycinnamic acid | 7.444 |
| trans-Ferulic acid | <LOD | <LOD | 320 | Hydroxycinnamic acid | 8.219 |
| Quercetin | 0.96 ± 0.06 | <LOD | 372 | Flavonol | 17.363 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabriele, M.; Cavallero, A.; Tomassi, E.; Arouna, N.; Árvay, J.; Longo, V.; Pucci, L. Assessment of Sourdough Fermentation Impact on the Antioxidant and Anti-Inflammatory Potential of Pearl Millet from Burkina Faso. Foods 2024, 13, 704. https://doi.org/10.3390/foods13050704
Gabriele M, Cavallero A, Tomassi E, Arouna N, Árvay J, Longo V, Pucci L. Assessment of Sourdough Fermentation Impact on the Antioxidant and Anti-Inflammatory Potential of Pearl Millet from Burkina Faso. Foods. 2024; 13(5):704. https://doi.org/10.3390/foods13050704
Chicago/Turabian StyleGabriele, Morena, Andrea Cavallero, Elena Tomassi, Nafiou Arouna, Július Árvay, Vincenzo Longo, and Laura Pucci. 2024. "Assessment of Sourdough Fermentation Impact on the Antioxidant and Anti-Inflammatory Potential of Pearl Millet from Burkina Faso" Foods 13, no. 5: 704. https://doi.org/10.3390/foods13050704
APA StyleGabriele, M., Cavallero, A., Tomassi, E., Arouna, N., Árvay, J., Longo, V., & Pucci, L. (2024). Assessment of Sourdough Fermentation Impact on the Antioxidant and Anti-Inflammatory Potential of Pearl Millet from Burkina Faso. Foods, 13(5), 704. https://doi.org/10.3390/foods13050704








