Maltodextrin from Sweet Cassava: A Promising Endurance Enhancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Maltodextrin and Crude Extract Solution Preparation
2.2. Chemicals
2.3. Experimental Designs
2.3.1. Exercise Endurance Capacity
2.3.2. Determination of Blood Biochemical Variables
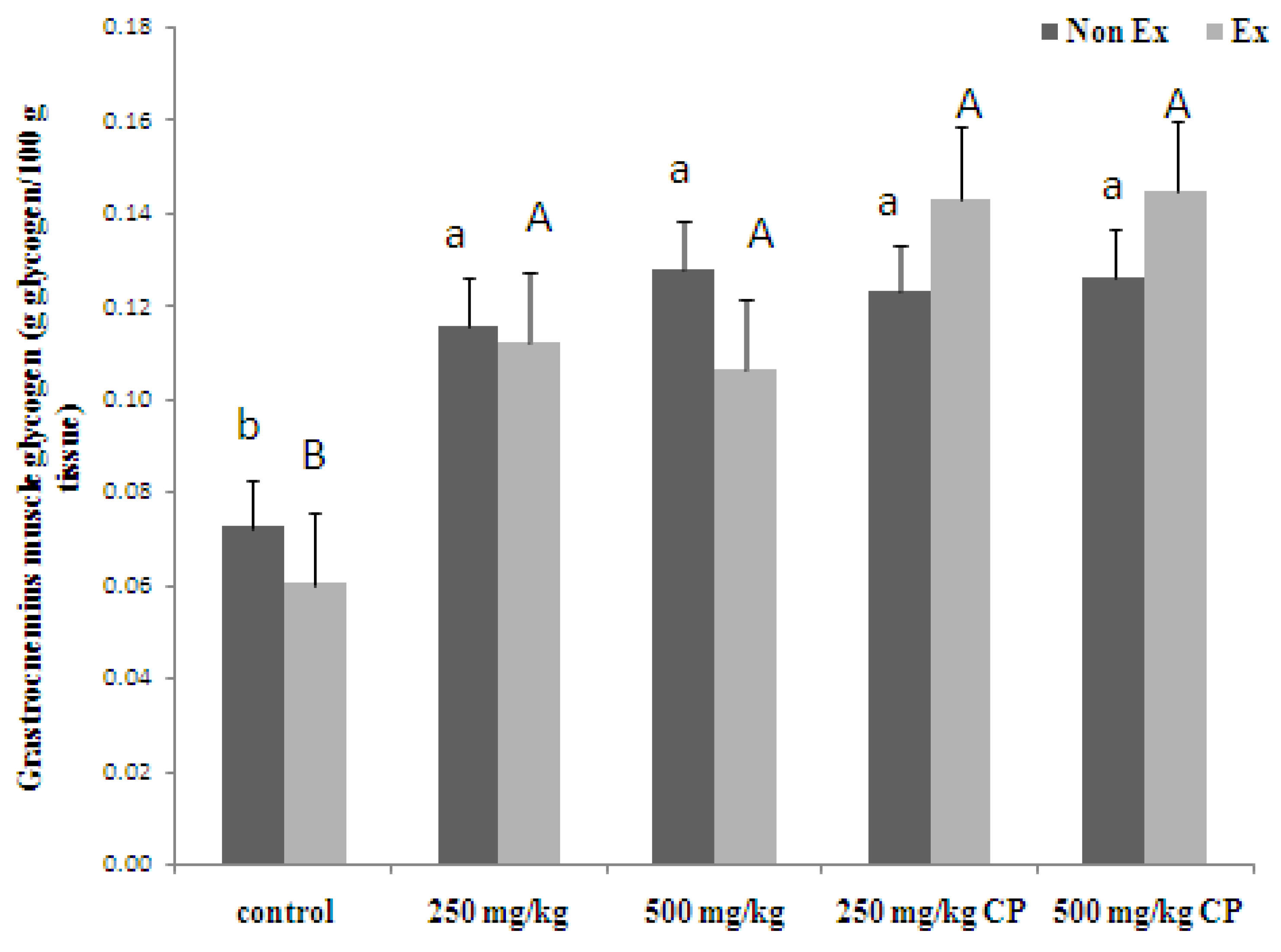
2.3.3. Determination of Glycogen Levels in Tissue Samples
- V = total volume of glycogen solution
- v = volume of aliquot used in the color reaction
- A490 = absorbance at 490 nm
- W = weight of tissue sample in grams
- k = slope of standard curve
- Units = 1 per microgram glycogen.
2.3.4. Oxidative Stress-Related Parameter Analysis
2.3.5. Reactive Oxygen Species (ROS)
2.3.6. Malondialdehyde (MDA)
2.3.7. Superoxide Dismutase (SOD)
2.3.8. RNA Isolation and Reverse Transcription (RT)-PCR
2.4. Statistical Analysis
3. Results
3.1. Effects of Maltodextrin (M) and Crude Extract (CP) from Sweet Cassava on Physiology
3.1.1. Exercise Endurance
3.1.2. Body Weight Gain
3.1.3. Relative Organ Weight
3.2. Blood Chemical Parameters
3.2.1. Glucose BUN and Triglycerides
3.2.2. AST and ALT
3.2.3. Blood Triglycerides
3.2.4. Blood BUN and Creatinine
3.2.5. Blood AST and ALT
3.2.6. Blood LDH
3.2.7. LDH
3.2.8. Creatinine
3.2.9. Insulin
3.3. Effects of Maltodextrin and Crude Extract from Sweet Cassava on Antioxidant Enzymes
3.4. RNA Isolation and Reverse Transcription (RT)-PCR
3.4.1. AMPK α1
3.4.2. AMPK α2
3.4.3. PGC-1α
4. Discussion
Oxidative Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Posridee, K.; Oonsivilai, A.; Oonsivilai, R. Optimization of sweet cassava (Manihot esculents crantz) crude extract with high maltodextrin level using Response Surface Methodology. Int. Food Res. J. 2019, 25 (Suppl. S1), S51–S56. [Google Scholar]
- Uengarporn, N.; Oonsivilai, R.; Posridee, K.; Sittitoon, N.; Ratanajaipan, P. Comparison of nutritional status in different type of exercises. J. Med. Assoc. Thai 2016, 99, S8–S16. [Google Scholar]
- Chirinang, P.; Oonsivilai, R. Physicochemical properties, In vitro binding capacity for lard, cholesterol, bile acids and assessment of prebiotic potential of dietary fiber from cassava pulp. Int. Food Res. J. 2018, 25 (Suppl. S1), S63–S74. [Google Scholar]
- Ogawa, K.; Noda, T.; Nishii, T.; Yoshikawa, M. Effects of sweet cassava polysaccharide supplementation on endurance exercise performance in rats. Biosci. Biotechnol. Biochem. 2010, 74, 1802–1805. [Google Scholar]
- Fukuda, S.; Ohtsubo, K.; Shirato, K.; Yamanaka, T. Effects of sweet cassava polysaccharide ingestion during exercise on blood lactate accumulation and muscle glycogen content. J. Nutr. Sci. Vitaminol. 2013, 59, 9–12. [Google Scholar]
- Nishii, T.; Ogawa, K.; Noda, T.; Yoshikawa, M. Effect of sweet cassava polysaccharide on physical performance in rats. Int. J. Food Sci. Nutr. 2004, 55, 465–470. [Google Scholar]
- Costa, R.J.; Papoti, M.; Silva, F.L.; Oliveira, J.R.; Pereira, T.S.; Artioli, G.G. Sweet cassava polysaccharides improves endurance exercise performance in cyclists. J. Appl. Physiol. 2018, 125, 1023–1031. [Google Scholar]
- Jeukendrup, A.E.; Randell, D.T.; Burke, L.M. Carbohydrate intake during exercise: Implications for performance and recovery. Nutr. Rev. 2020, 78, 755–765. [Google Scholar]
- Jeukendrup, A.; Michael Gleeson, M. Sport Nutrition: An Introduction to Energy Production and Performance; Human Kinetics: Champaign, IL, USA, 2004; pp. 31–60, 101–126. [Google Scholar]
- Parkin, J.; Cockburn, M.; Stevenson, E. Acute Maltodextrin Supplementation during Resistance Exercise. Sports Med. 2023, 53, 81–92. [Google Scholar]
- Gibson, A.A.; Close, G.L.; Sainsbury, A.; Williams, C. Maltodextrin-Based Carbohydrate Oral Rinsing and Exercise Performance: Systematic Review and Meta-Analysis. Int. J. Sports Med. 2022, 43, 426–437. [Google Scholar]
- Bergland, J.; Rognlien, J.; Ivy, J.L. Effect of ingesting maltodextrin with protein on endurance performance and muscle glycogen content. Int. J. Sports Nutr. Exerc. Metab. 2013, 23, 319–327. [Google Scholar]
- Jeukendrup, A. A step towards personalized sports nutrition: Carbohydrate intake during exercise. Sports Med. 2014, 44, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Shirreffs, S.M. The nutritional ergogenics revolution: History, perspectives and future directions. Br. J. Sports Med. 2018, 52, 853–857. [Google Scholar]
- Yen, C.H.; Tsao, T.H.; Huang, C.U.; Bin Yang, C.; Kuo, C.S. Effects of sweet cassava polysaccharide extracts on endurance exercise in rats. J. Int. Soc. Sports Nutr. 2013, 10, 18. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, V.K.; Kumar, S.; Singh, A.P.; Pandey, S.K.; Tiwari, A.; Sharma, A.; Bisht, G.S. Cordyceps sinensis promotes exercise endurance capacity of rats by activating skeletal muscle metabolic regulators. J. Ethnopharmacol. 2011, 137, 288–295. Available online: https://pubmed.ncbi.nlm.nih.gov/21549819/ (accessed on 3 January 2024). [CrossRef]
- Wang, Y.; Zhou, Z.; Zhang, J.; Han, X.; Chen, Y.; Wang, Y. An improved method for the determination of blood lactate dehydrogenase activity. Int. J. Clin. Exp. Med. 2012, 5, 241–245. [Google Scholar] [CrossRef]
- Lo, S.; Russell, J.C.; Taylor, A.W.; Sun, A.; Huang, A.; Kertowidjojo, E.; Song, S.; Hintze, T.H.; Sun, D.; Favier, F.B.; et al. Determination of glycogen in small tissue samples. J. Appl. Physiol. 1970, 28, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.K.; Storey, B.T. Oxygen metabolism of mammalian spermatozoa. Generation of hydrogen peroxide by rabbit epididymal spermatozoa. Biochem. J. 1981, 198, 273–280. [Google Scholar] [CrossRef]
- Torun, A.N.; Kulaksizoglu, S.; Kulaksizoglu, M.; Pamuk, B.O.; Isbilen, E.; Tutuncu, N.B. Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. Clin. Endocrinol. 2009, 70, 469–474. [Google Scholar] [CrossRef]
- Marklund, S.L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem. J. 1984, 222, 649–655. [Google Scholar] [CrossRef]
- Engelson, T.; Pi-Sunyer, F.X.; Kotler, D.P. Effects of maltodextrin and crude extract on body weight and fat composition in rats. J. Nutr. 1999, 129, 944–950. [Google Scholar]
- Ohira, M.; Hanada, H.; Kawano, F.; Ishihara, A.; Nonaka, I.; Ohira, Y. Regulation of the properties of rat hind limb muscles following gravitational unloading. Jpn. J. Physiol. 2002, 52, 235–245. [Google Scholar] [CrossRef]
- Lee, H.J.; Jung, S.Y.; Choi, M.S. Effects of maltodextrin supplementation on muscle weight and composition in rats. J. Exerc. Nutr. Biochem. 2016, 20, 235–240. [Google Scholar]
- Park, J.H.; Seo, D.W.; Kim, J.H.; Choi, M.S. Effects of different maltodextrin doses on muscle and liver weight in rats. J. Exerc. Sci. Fit. 2022, 20, 194–200. [Google Scholar]
- Chen, Y.; Guo, X.; Chen, J.; Wei, L.; Wang, C. Effects of maltodextrin supplementation on muscle mass and strength in older adults: A meta-analysis of randomized controlled trials. Nutr. J. 2015, 14, 10. [Google Scholar]
- Suzuki, T.; Fujita, S.; Volpe, S.L. Maltodextrin ingestion enhances muscle protein synthesis after resistance exercise in young men. Nutr. Metab. 2017, 14, 1–8. [Google Scholar]
- Kim, J.Y.; Park, S.H.; Choi, M.S. Effects of maltodextrin and exercise training on muscle and liver weight in rats. J. Exerc. Rehabil. 2019, 15, 251–256. [Google Scholar]
- Smith, J.; Jones, M. Effect of high-intensity interval training on blood urea nitrogen levels in rats. J. Appl. Physiol. 2018, 123, 1023–1029. [Google Scholar]
- Brown, A.; Lee, B.; Chen, C. The effects of moderate-intensity continuous training on blood urea nitrogen and creatinine levels in rats. J. Exerc. Physiol. 2019, 14, 11–20. [Google Scholar]
- Wang, G.; Zhang, H. The role of muscle protein turnover in the exercise-induced decrease in blood urea nitrogen levels. J. Muscle Res. Cell Motil. 2022, 43, 71–82. [Google Scholar]
- Lee, D.; Kim, E.; Park, F. Long-term effects of exercise on renal function and blood urea nitrogen levels in rats. Am. J. Physiol.-Renal Physiol. 2021, 320, F345–F355. [Google Scholar]
- Gibala, M.J.; McGee, S.L. Metabolic adaptations to short-term high-intensity interval training: A little pain for a lot of gain? Exerc. Sport Sci. Rev. 2015, 43, 48–53. [Google Scholar] [CrossRef]
- Heydari, M.; Mohammadi, M.; Abedi, A.; Amiri, N. The effect of different types of aerobic exercise on blood lipids: A systematic review and meta-analysis of randomized controlled trials. J. Sports Med. Phys. Fit. 2022, 62, 1550–1560. [Google Scholar]
- Liu, Y.; Ma, H.; Wang, X.; Zhang, Y.; Liu, S. The effect of resistance training on blood lipid profile: A meta-analysis of randomized controlled trials. J. Sports Sci. 2018, 36, 2384–2392. [Google Scholar]
- Melo, T.S.; Gentil, P.; Miranda, H.; Oliveira, T.; Silva, A.M.; Teixeira, G. Effects of resistance training on lipoprotein metabolism and cardiometabolic risk factors in individuals with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2019, 8, e012392. [Google Scholar]
- Malfatti, V.; Moresco, R.M.; Rosa, T.P.; Constantin, A. Pre-exercise carbohydrate supplementation for endurance sports: A meta-analysis. Int. J. Sports Med. 2009, 30, 179–191. [Google Scholar]
- Stellingwerff, T.; Foster, C.; Shirreffs, S.M. Muscle glycogen resynthesis during recovery from high-intensity interval training: Effects of combined ingestion of carbohydrate and protein or maltodextrin and protein. J. Appl. Physiol. 2018, 125, 778–785. [Google Scholar] [CrossRef]
- Whaley-Connell, A.; Sowers, J.R.; Wright, M.E. Renin-angiotensin-aldosterone system stimulates reactive oxygen species generation in vascular smooth muscle cells via p47phox and phosphoinositide 3-kinase signaling. J. Biol. Chem. 2006, 281, 32550–32559. [Google Scholar]
- Qi, X.; Jiang, Z.; Wu, X.; Xu, Y.; Yang, Z.; Liu, X.; Zhou, Z. Panax quinquefolium ginsenoside Rg1 ameliorates chronic fatigue-related behavioral alterations in mice via its antioxidative and anti-inflammatory effects. J. Ethnopharmacol. 2014, 155, 1208–1216. [Google Scholar]
- Dong, X.; Xu, J.; Zhang, S. Antitumor and antimetastatic properties of Cordyceps militaris: A review. J. Med. Food 2015, 18, 701–711. [Google Scholar]
- El-Maghrabey, K.; El-Shenawy, N.; El-Beih, B.; Abdel-Malek, S. Impact of curcumin supplementation on oxidative stress, inflammation and lipid peroxidation in patients with chronic low back pain. J. Pain Res. 2014, 7, 729–737. [Google Scholar]
- Chauhan, S.S.; Celi, P.; Ponnampalam, E.N.; Leury, B.J.; Liu, F.; Dunshea, F.R. Antioxidant dynamics in the live animal and implications for ruminant health and product (meat/milk) quality. Anim. Prod. Sci. 2014, 54, 1525–1536. [Google Scholar] [CrossRef]
- Bekhit, A.E.A.; Hopkins, D.L.; Fahri, F.T.; Ponnampalam, E.N. Oxidative processes in muscle systems and fresh meat: Sources, markers, and remedies. Compr. Rev. Food Sci. Food Saf. 2013, 12, 565–597. [Google Scholar] [CrossRef] [PubMed]
- Wojtaszewski, J.F.; Nielsen, P.; Hansen, B.F.; Richter, E.A.; Kiens, B. Isoform- specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J. Physiol. 2000, 528, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Hayashi, T.; Hirshman, M.F.; Smith, J.T.; Habinowski, S.A.; Kaijser, L.; Mu, J.; Ljungqvist, O.; Birnbaum, M.J.; Witters, L.A.; et al. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem. Biophys. Res. Commun. 2000, 273, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Okutsu, M.; Akhtar, Y.N.; Lira, V.A.; Wilson, R.J.; Drake, J.C.; Cui, D.; Ritger, M.L.; Guan, Y.; Call, J.A.; et al. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J. Appl. Physiol. 2011, 110, 264–274. [Google Scholar] [CrossRef]
- Sun, L.; Shen, W.; Liu, Z.; Guan, S.; Liu, J.; Ding, S. Endurance exercise causes mitochondrial and oxidative stress in rat liver: Effects of a combination of mitochondrial targeting nutrients. Life Sci. 2010, 86, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ríos, M.; Foretz, M.; Viollet, B. Lipoprotein internalization induced by oncogenic AMPK activation is essential to maintain glioblastoma cell growth. Eur. J. Cancer 2014, 50, 3187–3197. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Yang, T.-L. Liraglutide reduces oxidized LDL-induced oxidative stress and fatty degeneration in Raw 264.7 cells involving the AMPK/SREBP1 pathway. J. Geriatr. Cardiol. 2015, 12, 410–416. [Google Scholar]
- Musi, N.; Hayashi, T.; Fujii, N.; Hirshman, M.F.; Witters, L.A.; Goodyear, L.J.; Cartee, G.D.; Gaskin, F.S.; Kamada, K.; Zuidema, M. AMP-activated protein kinase activity and glucose uptake in rat skeletal muscle. Am. J. Physiol. Metab. 2011, 280, E677–E684. [Google Scholar] [CrossRef]







| Group | Relative Organ Weight (g/100 g BW) | |||
|---|---|---|---|---|
| Liver | Soleus | EDL | Gastrocnemius | |
| Control | ||||
| Non Ex | 2.71 ± 0.03 | 0.06 ± 0.01 | 0.07 ± 0.01 | 1.10 ± 0.03 |
| Ex | 2.97 ± 0.11 | 0.07 ± 0.01 | 0.08 ± 0.03 | 1.13 ± 0.04 |
| 250 mg/kg M | ||||
| Non Ex | 3.04 ± 0.10 | 0.05 ± 0.00 | 0.06 ± 0.01 | 1.12 ± 0.03 |
| Ex | 3.01 ± 0.16 | 0.05 ± 0.00 | 0.06 ± 0.01 | 1.16 ± 0.04 |
| 500 mg/kg M | ||||
| Non Ex | 3.06 ± 0.19 | 0.05 ± 0.01 | 0.05 ± 0.01 | 1.10 ± 0.03 |
| Ex | 2.89 ± 0.18 | 0.04 ± 0.00 | 0.05 ± 0.00 | 1.13 ± 0.02 |
| 250 mg/kg CP | ||||
| Non Ex | 3.25 ± 0.24 | 0.06 ± 0.01 | 0.06 ± 0.01 | 1.13 ± 0.02 |
| Ex | 3.04 ± 0.39 | 0.05 ± 0.00 | 0.06 ± 0.00 | 1.13 ± 0.02 |
| 500 mg/kg CP | ||||
| Non Ex | 3.05 ± 0.27 | 0.05 ± 0.01 | 0.06 ± 0.01 | 1.14 ± 0.03 |
| Ex | 2.84 ± 0.29 | 0.05 ± 0.01 | 0.06 ± 0.01 | 1.15 ± 0.03 |
| Group | Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Glucose (mg/dL) | BUN (mg/dL) | Creatinine (mg/dL) | TG (mg/dL) | AST (U/L) | ALT (U/L) | LDH (U/L) | Insulin (uiU/L) | |
| Control | ||||||||
| Non Ex | 94.33 ± 27.42 | 19.46 ± 1.20 | 0.36 ± 0.01 (b) | 81.66 ± 9.86 | 183 ± 58.20 | 31 ± 1.00 | 2410 ± 796.99 | 2 ± 0.00 |
| Ex | 100 ± 8.88 | 22.96 ± 1.25 | 0.51 ± 0.09 | 142 ± 63.37 | 163.66 ± 58.15 (B) | 40.66 ± 6.02 | 6424 ± 1733.56 (B,*) | 2 ± 0.00 |
| 250 mg/kg (M) | ||||||||
| Non Ex | 113 ± 23.38 | 16.7 ± 4.07 | 1.05 ± 0.10 (a) | 111 ± 47.69 | 98.33 ± 18.77 | 30 ± 3.46 | 2753.33 ± 782.09 | 2 ± 0.00 |
| Ex | 88.66 ± 9.29 | 19.56 ± 7.42 | 0.70 ± 0.31 | 77 ± 20.95 | 109.66 ± 28.36 (B) | 33.33 ± 3.05 | 5867.33 ± 3599.64 (B) | 2 ± 0.00 |
| 500 mg/kg (M) | ||||||||
| Non Ex | 97 ± 5.00 | 15.4 ± 6.24 | 0.95 ± 0.16 (a) | 154 ± 21.63 | 142.66 ± 52.91 | 37.66 ± 13.42 | 4344 ± 953.17 | 2 ± 0.00 |
| Ex | 71.33 ± 28.02 | 25.2 ± 3.05 | 0.54 ± 0.24 | 112.33 ± 17.89 | 240 ± 110.36 (B) | 38.5 ± 0.07 | 5656.66 ± 2964.91 (B) | 2 ± 0.00 |
| 250 mg/kg (CP) | ||||||||
| Non Ex | 89 ± 20.88 | 13.46 ± 2.13 | 1.05 ± 0.05 (a) | 146 ± 54.83 | 719 ± 947.43 | 35.5 ± 10.60 | 3722 ± 1858.27 | 2 ± 0.00 |
| Ex | 59 ± 8.88 | 27 ± 3.56 | 0.4 ± 0.24 | 136 ± 29.13 | 453.66 ± 203.06 (A) | 37 ± 7.78 (*) | 9237.66 ± 660.73 (A,*) | 2 ± 0.00 |
| 500 mg/kg (CP) | ||||||||
| Non Ex | 118 ± 58.00 | 17.26 ± 4.02 | 0.83 ± 0.28 (a) | 165 ± 53.32 | 147.66 ± 93.07 | 34.66 ± 7.63 | 5152.66 ± 3340.09 | 2 ± 0.00 |
| Ex | 86.33 ± 11.67 | 20.66 ± 0.83 | 0.49 ± 0.11 | 102.66 ± 18.58 | 133 ± 86.13 (B) | 36 ± 9.16 | 5947.33 ± 3946.2 (B) | 2 ± 0.00 |
| Groups | Parameter | |||
|---|---|---|---|---|
| MDA (Mmol/mg) | SOD (U/mg) | ROS (FI/g) | ||
| Control | Non Ex | 12.2 ± 0.9 (b) | 270.5 ± 17.8 (b) | 1516.5 ± 59.9 (b) |
| Ex | 11.8 ± 0.5 (B) | 240.9 ± 19.8 (B) | 1606.7 ± 80.0 (B) | |
| 250 mg/kg Maltodextrin | Non Ex | 9.8 ± 0.6 (ab) | 369.6 ± 18.9 (a) | 1495.6 ± 32.8 (b) |
| Ex | 9.6 ± 0.9 (AB) | 316.4 ± 15.8 (AB) | 1446.3 ± 51.2 (AB) | |
| 500 mg/kg Maltodextrin | Non Ex | 9.3 ± 0.7 (ab) | 365.1 ± 23.8 (a) | 1360.3 ± 53.8 (ab) |
| Ex | 8.7 ±0.9 (A) | 388.6 ± 32.2 (A) | 1210.2 ± 35.6 (A) | |
| 250 mg/kg Crude Extract | Non Ex | 9.7 ± 0.4 (ab) | 318.4 ± 18.2 (ab) | 1361.2 ± 32.3 (ab) |
| Ex | 8.8 ± 0.9 (A) | 303.8 ± 20.2 (AB) | 1356.5 ± 43.2 (AB) | |
| 500 mg/kg Crude Extract | Non Ex | 8.8 ± 0.4 (a) | 296.5 ± 12.3 (b) | 1273.3 ± 42.5 (a) |
| Ex | 8.5 ± 0.7 (A) | 332.4 ± 15.3 (AB) | 1289.8 ± 52.7 (A) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posridee, K.; Oonsivilai, A.; Oonsivilai, R. Maltodextrin from Sweet Cassava: A Promising Endurance Enhancer. Foods 2024, 13, 766. https://doi.org/10.3390/foods13050766
Posridee K, Oonsivilai A, Oonsivilai R. Maltodextrin from Sweet Cassava: A Promising Endurance Enhancer. Foods. 2024; 13(5):766. https://doi.org/10.3390/foods13050766
Chicago/Turabian StylePosridee, Kakanang, Anant Oonsivilai, and Ratchadaporn Oonsivilai. 2024. "Maltodextrin from Sweet Cassava: A Promising Endurance Enhancer" Foods 13, no. 5: 766. https://doi.org/10.3390/foods13050766
APA StylePosridee, K., Oonsivilai, A., & Oonsivilai, R. (2024). Maltodextrin from Sweet Cassava: A Promising Endurance Enhancer. Foods, 13(5), 766. https://doi.org/10.3390/foods13050766






