Fatty Acid Release and Gastrointestinal Oxidation Status: Different Methods of Processing Flaxseed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment of Flaxseed and Preparation of Flaxseed-Based Milk
2.3. In Vitro Simulated Digestion
2.4. Particle Size Measurement
2.5. Lipid Content Determination
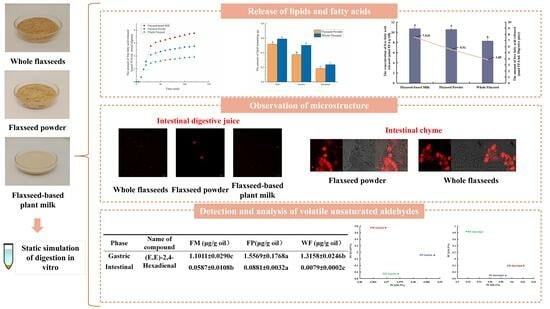
2.6. Observation of Microstructure
2.7. Determination of Volatile Unsaturated Aldehydes in Secondary Oxidation Products
2.8. Data Analysis
3. Results and Discussion
3.1. Changes in FM, FP, and WF during Oral In Vitro Digestion
3.2. Changes of FM, FP, and WF during Gastric In Vitro Digestion
3.3. Changes in FM, FP, and WF during Intestinal In Vitro Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef]
- Lu, M.; Tan, L.; Zhou, X.; Yang, Z.; Zhu, Q.; Chen, J.; Luo, H.; Wu, G. Secoisolariciresinol diglucoside delays the progression of aging-related diseases and extendsthe lifespan of Caenorhabditis elegans via DAF-16 and HSF-1. Oxid. Med. Cell. Longev. 2020, 13, 1293935. [Google Scholar] [CrossRef]
- Askarpour, M.; Karimi, M.; Hadi, A.; Ghaedi, E.; Symonds, M.E.; Miraghajani, M.; Javadian, P. Effect of flflaxseed supplementation on markers of inflflammation and endothelial function: A systematic review and meta-analysis. Cytokine 2020, 126, 154922. [Google Scholar] [CrossRef]
- Hadi, A.; Askarpour, M.; Ziaei, R.; Venkatakrishnan, K.; Ghaedi, E.; Ghavami, A. Impact of flflaxseed supplementation on plasma lipoprotein (a) concentrations: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2020, 34, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Charles, G.; Palanisamy, G.; Hesham, E.; Hui, T. A review on advanced microencapsulation technology to enhance bioavailability of phenolic compounds: Based on its activity in the treatment of Type 2 Diabetes. Trends Food Sci. Technol. 2019, 85, 149–162. [Google Scholar] [CrossRef]
- Srmhs, A.; Barbara, J.M. Stunting is a recognized problem: Evidence for the potential benefits of omega-3 long-chain polyunsaturated fatty acids. Nutrition 2020, 73, 110564. [Google Scholar] [CrossRef]
- Rovere, M.T.L.; Christensen, J.H. The autonomic nervous system and cardiovascular disease: Role of n-3 PUFAs. Vasc. Pharmacol. 2015, 71, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, Y.; McClements, D.J.; Hou, T.; Geng, F.; Chen, P.; Chen, H.; Xie, B.; Sun, Z.; Tang, H.; et al. Composition, processing, and quality control of whole flaxseed products used to fortify foods. Compr. Rev. Food Sci. Food Saf. 2022, 22, 587–614. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Ma, F.; Wei, X.; Zang, X.; Chen, P.; Zhang, Y.; Huang, F.; Huang, Q. Progress of studies on quality characteristics of flaxseed processing. Chin. J. Oil Crop Sci. 2016, 38, 126–134. [Google Scholar] [CrossRef]
- Marpalle, P.; Sonawane, S.K.; Arya, S.S. Effect of flaxseed flour addition on physicochemical and sensory properties of functional bread. LWT-Food Sci. Technol. 2014, 58, 614–619. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for Special Dietary Needs: Non-dairy Plant-based Milk Substitutes and Fermented Dairy-type Products. Crit. Rev. Food Sci. Nurition 2016, 56, 39–49. [Google Scholar] [CrossRef]
- Gallier, S.; Singh, H. Behavior of almond oil bodies during in vitro gastric and intestinal digestion. Food Funct. 2012, 3, 547–555. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Arendt, E.K. Past, present and future: The strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res. Int. 2018, 110, 42–51. [Google Scholar] [CrossRef]
- Meng, C.; Chen, Y.; Wang, X.; Chen, H.; Deng, Q. Effect of Different Temperatures on the Storage Stability of Flaxseed Milk. Foods 2023, 12, 3571. [Google Scholar] [CrossRef]
- Wan, L.; Yu, X.; Geng, F.; Cheng, C.; Yang, J.; Deng, Q. Effects of tocopherols on the stability of flaxseed oil-in-water emulsions stabilized by different emulsifiers: Interfacial partitioning and interaction. Food Chem. 2022, 16, 131691. [Google Scholar] [CrossRef]
- Kanner, J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol. Nutr. Food Res. 2007, 51, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, V.N.; Leitinger, N. Anti-inflammatory properties of lipid oxidation products. J. Mol. Med. 2003, 81, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, V.N.; Kadl, A.; Huber, J.; Gruber, F.; Binder, B.R.; Leitinger, N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature 2002, 419, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Klooster, S.; Schroën, K.; Berton-Carabin, C. Lipid oxidation products in model food emulsions: Do they stay in or leave droplets, that’s the question. Food Chem. 2023, 405, 134992. [Google Scholar] [CrossRef]
- Gouvêa, F.J.; Oliveira, V.S.; Mariano, B.J.; Takenaka, N.A.R.; Gamallo, O.D.; Ferreira, M.S.; Saldanha, T. Natural antioxidants as strategy to minimize the presence of lipid oxidation products in canned fish: Research progress, current trends and future perspectives. Food Res. Int. 2023, 173, 113314. [Google Scholar] [CrossRef] [PubMed]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Food lipid oxidation under gastrointestinal digestion conditions: A review. Crit. Rev. Food Sci. Nutr. 2018, 60, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, X.; Jiao, Y.; Wang, Y.; Dong, J.; Yang, N.; Yang, Q.; Qu, W.; Wang, W. Free iron rather than heme iron mainly induces oxidation of lipids and proteins in meat cooking. Food Chem. 2022, 382, 132345. [Google Scholar] [CrossRef] [PubMed]
- Grundy, M.M.L.; Grassby, T.; Mandalari, G.; Waldron, K.W.; Butterworth, P.J.; Berry, S.E.E.; Ellis, P.R. Effffect of mastication on lipid bioaccessibility of almonds in a randomized human study and its implications for digestion kinetics, metabolizable energy, and postprandial lipemia. Am. J. Clin. Nutr. 2015, 101, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.K.; Koley, H.; Ghosh, M.; Dey, S.; Dhar, P. Effects of nano-sizing on lipid bioaccessibility and ex vivo bioavailability from EPA-DHA rich oil in water nanoemulsion. Food Chem. 2019, 275, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kauser, S.; Hussain, A.; Ashraf, S.; Fatima, G.; Ambreen; Javaria, S.; Abideen, Z.U.I.; Kabir, K.; Yaqub, S.; Akram, S.; et al. Flaxseed (Linum usitatissimum); phytochemistry, pharmacological characteristics and functional food applications. Food Chem. Adv. 2024, 4, 100573. [Google Scholar] [CrossRef]
- Yang, Y.N.; Deng, Q.C.; Jia, X.; Shi, J.; Wan, C.; Zhou, Q.; Wang, Q. Characterization of key odorants in peeled and unpeeled flaxseed powders using solvent-assisted flavor evaporation and odor activity value calculation. LWT 2021, 138, 110724. [Google Scholar] [CrossRef]
- Mandalari, G.; Grundy, M.M.L.; Grassby, T.; Parker, M.L.; Cross, K.L.; Chessa, S.; Bisignano, C.; Barreca, D.; Bellocco, E.; Laganà, G.; et al. The effects of processing and mastication on almond lipid bioaccessibility using novel methods of in vitro digestion modelling and micro-structural analysis. Br. J. Nutr. 2014, 112, 1521–1529. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Lorrain, B.; Dangles, O.; Loonis, M. Dietary Iron-Initiated Lipid Oxidation and Its Inhibition by Polyphenols in Gastric Conditions. J. Agric. Food Chem. 2012, 60, 9074–9081. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, H.J.; Geng, F. Natural oil bodies from typical oilseeds: Structural characterization and their potentials as natural delivery system for curcumin. Food Hydrocoll. 2022, 128, 107521. [Google Scholar] [CrossRef]
- Cheng, C.; Yu, X.; Huang, F.H.; Peng, D.; Chen, H.; Chen, Y.; Huang, Q.; Deng, Q. Effect of different structural flflaxseed lignans on the stability of flflaxseed oil-inwater emulsion: An interfacial perspective. Food Chem. 2021, 357, 129522. [Google Scholar] [CrossRef]
- AOAC (Association of Offificial Analytical Chemists). Official Methods of Analysis, 16th ed.; AOAC International: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Dickinson, E. Food emolsions and foams: Stabilization by particle. Curr. Opin. Colloid Interface Sci. 2021, 15, 40–49. [Google Scholar] [CrossRef]
- Li, X.W.; Huang, Q.D.; Wang, X.T.; Zhang, M.; Quan, S.; Geng, F.; Chen, H.; Deng, Q. Exploration of suitable in vitro simulated digestion model for lipid oxidation of flflaxseed oil emulsion during digestion. J. Sci. Food Agric. 2022, 102, 5495–5501. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, E.; Damerau, A.; Suomela, J.; Kortesniemi, M.; Linderborg, K.M. Oxidative stability, oxidation pattern and α-tocopherol response of docosahexaenoic acid (DHA, 22:6n-3)-containing triacylglycerols and ethyl esters. Food Chem. 2022, 387, 132882. [Google Scholar] [CrossRef]
- Ghnimi, S.; Budilarto, E.; Kamal-Eldin, A. The New Paradigm for Lipid Oxidation and Insights to Microencapsulation of Omega-3 Fatty Acids. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Tullberg, C.; Vegarud, G.; Undeland, I. Oxidation of marine oils during in vitro gastrointestinal digestion with human digestive fluids-role of oil origin, added tocopherols and lipolytic activity. Food Chem. 2018, 270, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Ardabilchi, M.; Amjadi, S.; Ardabilchi, M.; Roufegarinejad, L.; Jafari, S.M. Fortification of yogurt with flaxseed powder and evaluation of its fatty acid profile, physicochemical, antioxidant, and sensory properties. Powder Technol. 2019, 359, 76–84. [Google Scholar] [CrossRef]
- Chen, T.; He, J.; Zhang, J.; Li, X.; Zhang, H.; Hao, J.; Li, L. The isolation and identification of two compounds with predominant radical scavenging activity in hempseed (seed of Cannabis sativa L.). Food Chem. 2012, 134, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Herchi, W.; Arráez-Román, D.; Trabelsi, H.; Bouali, I.; Boukhchina, S.; Kallel, H.; Segura-Carretero, A.; Fernández-Gutierrez, A. Phenolic compounds in flaxseed:a review of their properties and analytical methods. an overview of the lastdecade. J. Oleo Sci. 2014, 63, 7–14. [Google Scholar] [CrossRef]
- Ramsay, A.; Fliniaux, O.; Quéro, A.; Molinie, R.; Mesnard, F. Kinetics of the incorporation of the main phenolic compounds into the lignan macromolecule during flaxseed development. Food Chem. 2017, 217, 1–8. [Google Scholar] [CrossRef]
- Giang, T.M.; Gaucel, S.; Brestaz, P.; Anton, M.; Meynier, A.; Trelea, I.C.; Feunteun, S.L. Dynamic modeling of in vitro lipid digestion: Individual fatty acid release and bioaccessibility kinetics. Food Chem. 2016, 194, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- He, S.H.; Liu, C.H. Comparison of two different natural oil body emulsions: In vitro gastrointestinal digestion. J. Oleo Sci. 2020, 69, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, N.; Robert, P.; Troncoso, E.; Arancibia, C. Inflfluence of the particle size and hydrocolloid type on lipid digestion of thickened emulsions. Food Funct. 2020, 11, 5955–5964. [Google Scholar] [CrossRef] [PubMed]
- Golding, M.; Wooster, T.J. The inflfluence of emulsion structure and stability on lipid digestion. Curr. Opin. Colloid Interface Sci. 2010, 15, 90–101. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, Y. Review of in vitro digestion models for rapid screening of emulsion-based systems. Food Funct. 2010, 1, 32–59. [Google Scholar] [CrossRef]
- Golding, M.; Wooster, T.J.; Day, L.; Xu, M.; Lundin, L.; Keoghc, J.; Clifton, P. Impact of gastric structuring on the lipolysis of emulsified lipids. Soft Matter 2021, 7, 3513–3523. [Google Scholar] [CrossRef]
- Pilosof, A.M.R. Potential impact of interfacial composition of proteins and polysaccharides stabilized emulsions on the modulation of lipolysis. The role of bile salts. Food Hydrocoll. 2017, 68, 178–185. [Google Scholar] [CrossRef]
- Grundy, M.M.L.; Carrière, F.; Mackie, A.R.; Gray, D.A.; Butterwortha, P.J.; Ellis, P.R. The role of plant cell wall encapsulation and porosity in regulating lipolysis during the digestion of almond seeds. Food Funct. 2016, 7, 69–78. [Google Scholar] [CrossRef]
- Berry, S.E.; Tydeman, E.A.; Lewis, H.B.; Phalora, R.; Rosborough, J.; Picout, D.R.; Ellis, P.R. Manipulation of lipid bioaccessibility of almond seeds inflfluences postprandial lipemia in healthy human subjects. Am. J. Clin. Nutr. 2008, 88, 922–929. [Google Scholar] [CrossRef] [PubMed]






| Phase | Name of Compound | FM (ng/g Oil) | FP (ng/g Oil) | WF (ng/g Oil) |
|---|---|---|---|---|
| Original Sample | Hexanal | 21.1 ± 4.1 b | 34.1 ± 2.9 b | 58.8 ± 10.4 a |
| Nonanal | - | - | 19.9 ± 3.4 | |
| Oral | Hexanal | 34.7 ± 2.5 b | 46.3 ± 2.8 b | 71.8 ± 2.2 a |
| Octanal | 4.5 ± 0.3 | - | - | |
| Nonanal | 7.9 ± 0.8 | - | 38.0 ± 8.7 | |
| Gastric | Propanal | - | 17.3 ± 2.2 | 17.8 ± 0.1 |
| Pentanal | - | 22.5 ± 0.8 | 24.0 ± 1.7 | |
| 2-Butenal | - | 55.2 ± 1.3 | 52.9 ± 3.6 | |
| Hexanal | 166.0 ± 7.3 c | 663.6 ± 43.6 b | 865.7 ± 40.8 a | |
| (E)-2-Pentenal | 213.2 ± 9.0 a | 166.3 ± 11.3 b | 155.1 ± 7.7 b | |
| 3-Hexenal | - | 18.8 ± 3.7 | 0.018.0 ± 2.5 | |
| Heptanal | 6.2 ± 2.1 b | 27.6 ± 0.4 a | 35.2 ± 4.9 a | |
| (E)-2-Hexenal | 56.6 ± 8.1 b | 126.7 ± 0.7 a | 167.5 ± 8.5 a | |
| Octanal | - | 22.7 ± 1.4 | 32.0 ± 2.9 | |
| (E)-2-Heptenal | 205.2 ± 23.8 b | 341.9 ± 2.0 a | 371.5 ± 21.0 a | |
| Nonanal | 12.8 ± 0.7 c | 39.8 ± 2.8 b | 61.5 ± 10.0 a | |
| (E,E)-2,4-Hexadienal | - | 42.9 ± 11.1 | 10.0 ± 13.8 | |
| (E)-2-Octenal | 42.1 ± 7.4 b | 132.3 ± 27.8 a | 158.1 ± 10.7 a | |
| (E,E)-2,4-Heptadienal | 1101.1 ± 29.0 c | 1556.9 ± 176.8 a | 1315.8 ± 24.6 b | |
| Benzaldehyde | 28.0 ± 6.3 b | 33.1 ± 5.1 a | 31.5 ± 3.9 a | |
| 2-Decenal | - | 15.0 ± 1.9 | 17.1 ± 5.0 | |
| (E,E)-2,4-Decadienal | - | 7.0 ± 0.9 | 10.6 ± 1.4 | |
| Intestinal | Hexanal | 36.8 ± 2.4 a | 33.9 ± 0.6 b | 19.9 ± 2.1 c |
| (E)-2-Pentenal | 10.8 ± 0.6 | 14.8 ± 0.2 | - | |
| Heptanal | 8.8 ± 0.1 | - | - | |
| Octanal | 16.0 ± 2.2 | - | - | |
| (E)-2-Hexenal | - | 7.4 ± 0.2 | - | |
| (E)-2-Heptenal | 4.6 ± 0.7 | 6.0 ± 0.9 | - | |
| Nonanal | 15.9 ± 0.8 a | 8.2 ± 0.2 b | 8.5 ± 0.8 b | |
| (E,E)-2,4-Heptadienal | 58.7 ± 10.8 b | 88.1 ± 3.2 a | 7.9 ± 0.2 c | |
| Decanal | 4.2 ± 0.3 | - | - | |
| Benzaldehyde | 6.4 ± 0.6 | 4.5 ± 0.4 | - | |
| (E)-2-Nonenal | 4.0 ± 0.5 | - | - | |
| (E,E)-2,6-Nonadienal | 5.1 ± 0.4 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Chen, Y.; Chen, H.; Deng, Q. Fatty Acid Release and Gastrointestinal Oxidation Status: Different Methods of Processing Flaxseed. Foods 2024, 13, 784. https://doi.org/10.3390/foods13050784
Zhang M, Chen Y, Chen H, Deng Q. Fatty Acid Release and Gastrointestinal Oxidation Status: Different Methods of Processing Flaxseed. Foods. 2024; 13(5):784. https://doi.org/10.3390/foods13050784
Chicago/Turabian StyleZhang, Mingkai, Yashu Chen, Hongjian Chen, and Qianchun Deng. 2024. "Fatty Acid Release and Gastrointestinal Oxidation Status: Different Methods of Processing Flaxseed" Foods 13, no. 5: 784. https://doi.org/10.3390/foods13050784