Lupin as Ingredient in Durum Wheat Breadmaking: Physicochemical Properties of Flour Blends and Bread Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Flours
2.2. Content of Phenolic Compounds and Antioxidant Activity
2.3. Water Binding Capacity and Oil Binding Capacity
2.4. Rheological Tests and Falling Number
2.5. Leavening Test
2.6. Baking Test
2.7. Colour Determination
2.8. Preparation of Breads for Sensory Analysis
2.9. Descriptive Sensory Analysis
2.10. Statistical Analyses
3. Results and Discussion
3.1. Phenolic Compounds and Antioxidant Activity in Pure Flours and Their Blends
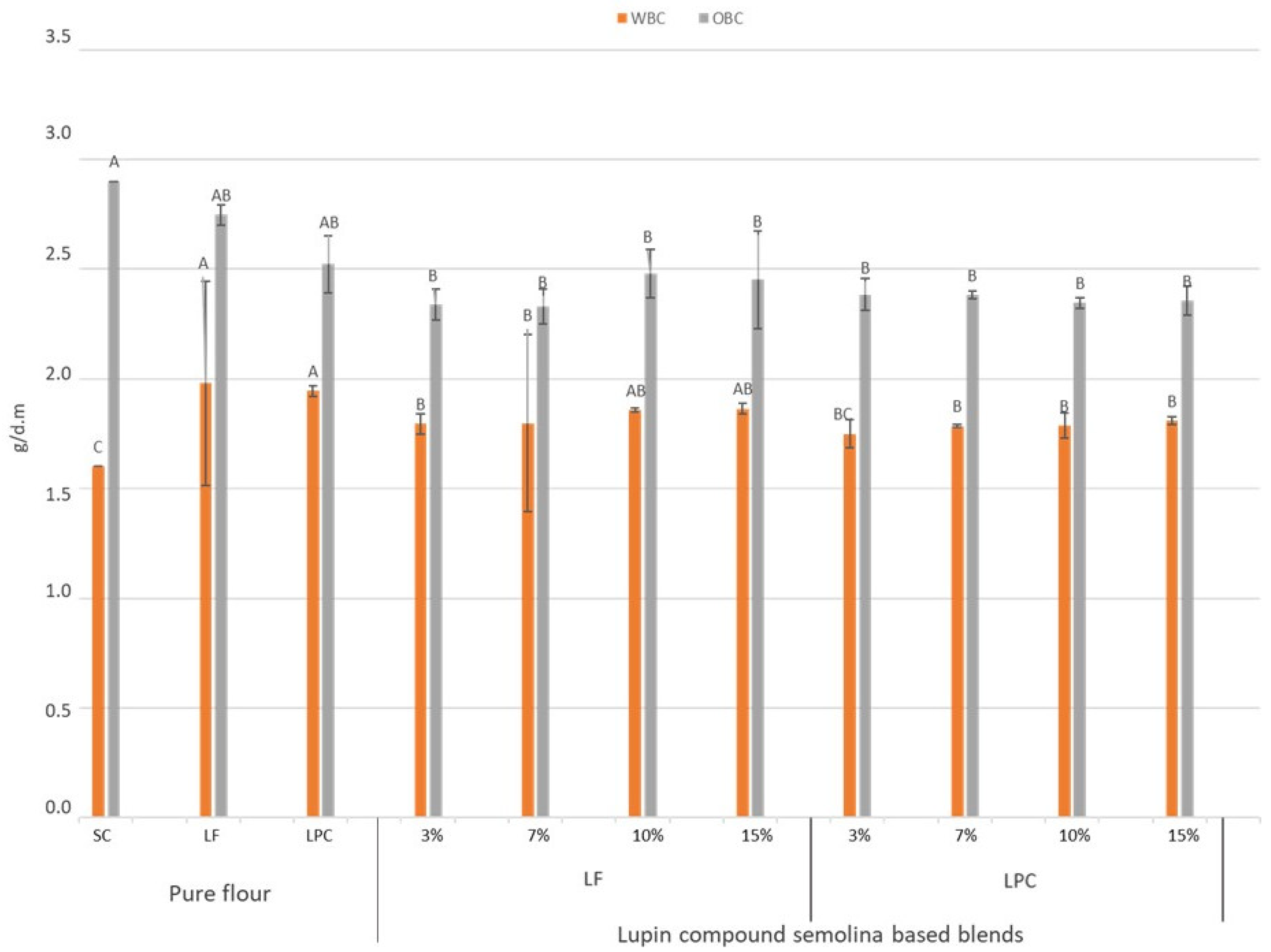
3.2. Water Binding and Oil Binding Capacities of Flour Blends
3.3. Rheological Testing of Dough and Falling Number
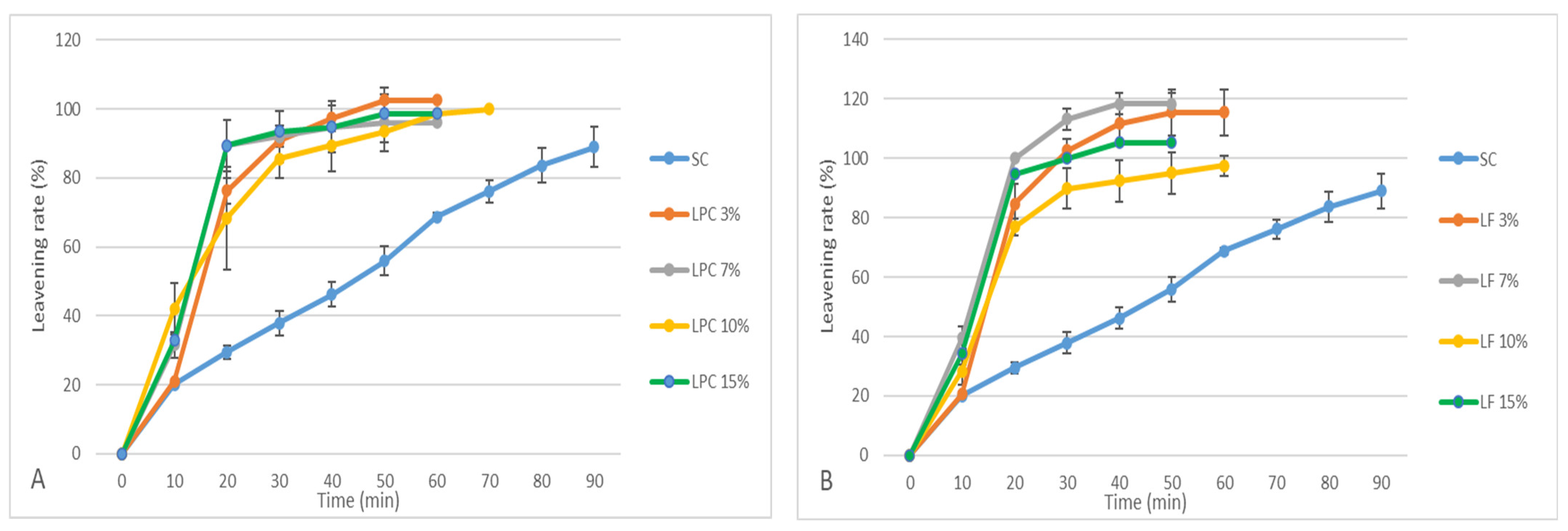
3.4. Dough Fermentation Test
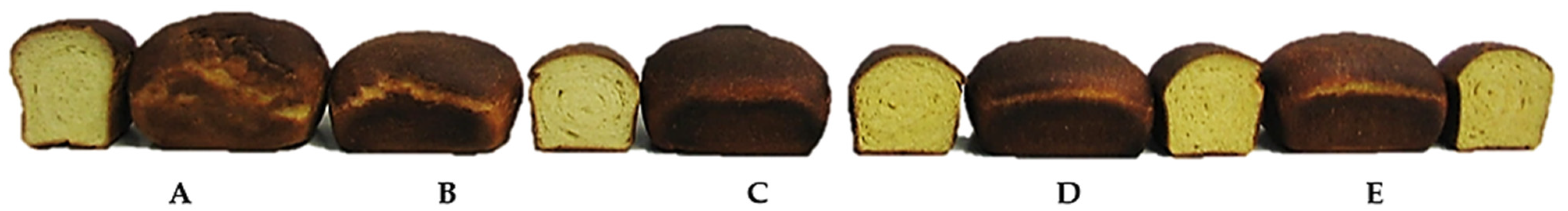
3.5. Physicochemical Characteristics of Bread
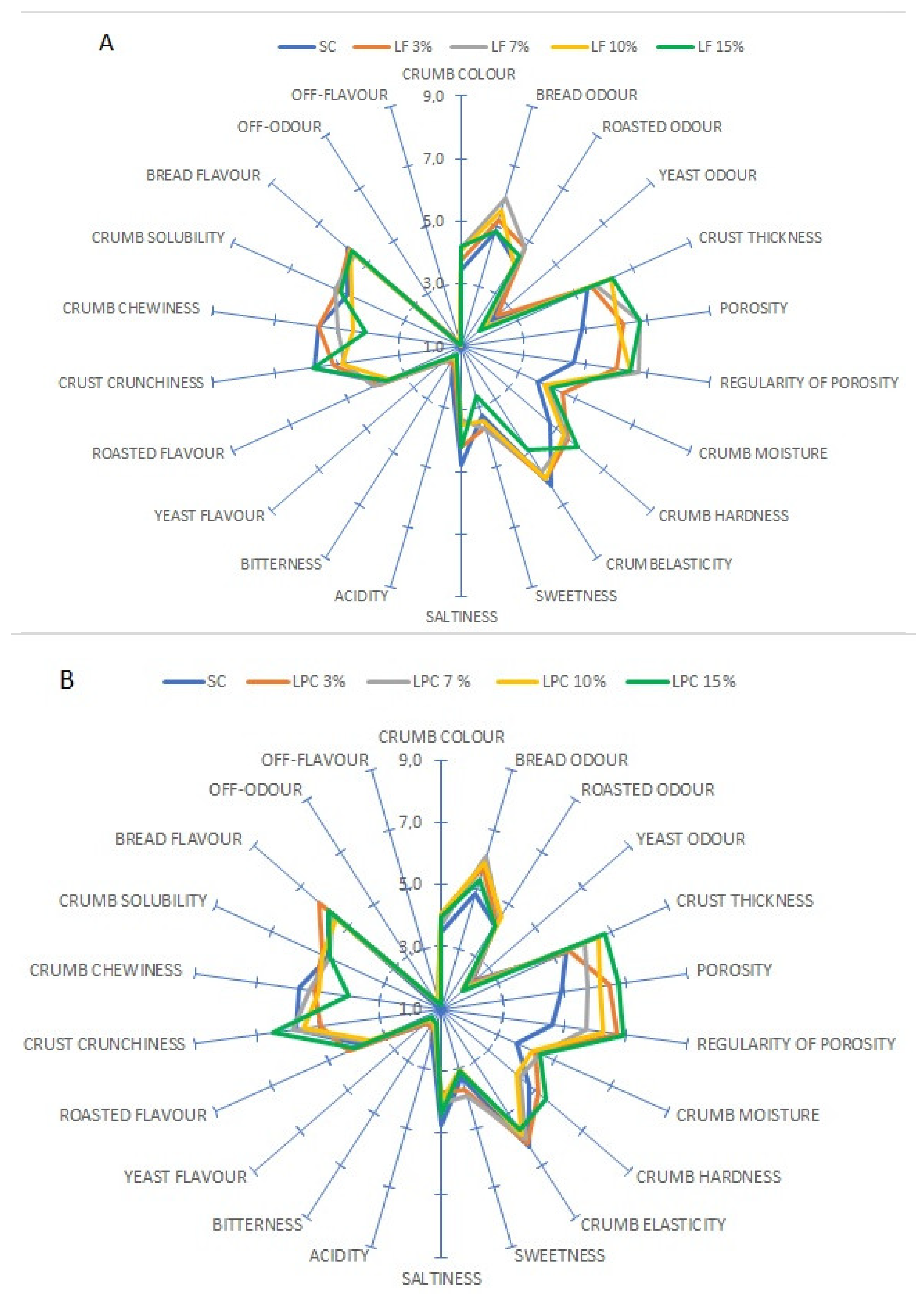
3.6. Sensory Characteristics of the Breads
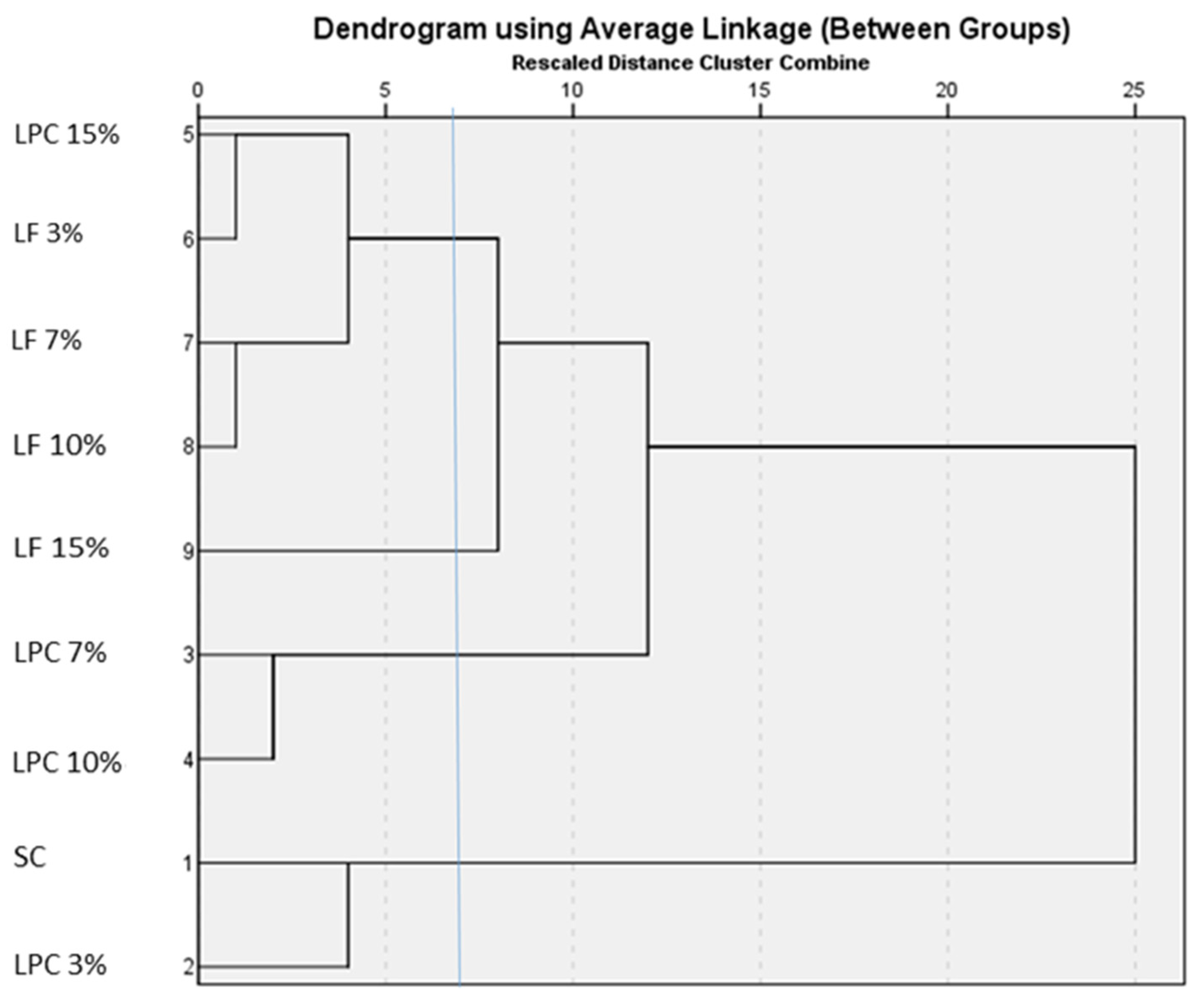
3.7. Cluster Analysis (Hierarchical Cluster Analysis and K-Means Cluster Analysis) of Flours, Doughs, and Breads
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angioloni, A.; Collar, C. High legume-wheat matrices: An alternative to promote bread nutritional value meeting dough viscoelastic restrictions. Eur. Food Res. Technol. 2012, 234, 273–284. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; Gallagher, E. Recent advances in the development of high fibre baked products. Trends Food Sci. Technol. 2012, 28, 4–14. [Google Scholar] [CrossRef]
- Dziki, D.; Róyło, R.; Gawlik-Dziki, U.; Swieca, M. Current trends in the enhancement of antioxidant activity of wheat bread by the addition of plant materials rich in phenolic compounds. Trends Food Sci. Technol. 2014, 40, 48–61. [Google Scholar] [CrossRef]
- Costantini, M.; Summo, C.; Faccia, M.; Caponio, F.; Pasqualone, A. Kabuli and Apulian black chickpea milling by-products as innovative ingredients to provide high levels of dietary fibre and bioactive compounds in gluten-free fresh pasta. Molecules 2021, 26, 4442. [Google Scholar] [CrossRef] [PubMed]
- Ranalli, P.; Spina, A.; Parisi, B.; Torricelli, R. Leguminose Minori: Lupino, Cicerchia, Roveja. Collana Edagricole ‘Tecnica & Pratica’; Edagricole: Bologna, Italy; Edizioni Agricole di New Business Media Srl: Milan, Italy, 2018; p. 180. [Google Scholar]
- Spina, A. Prodotti alimentari innovativi a base di farine di legumi per celiaci, vegetariani e vegani. In I Libri dell’Accademia—I Legumi in Italia: Attualità e Prospettive; Annali CXXXIX (Anno 2019); Accademia Nazionale di Agricoltura (ANA): Bologna, Italy, 2020; pp. 525–540. [Google Scholar]
- Pasqualone, A.; De Angelis, D.; Squeo, G.; Difonzo, G.; Caponio, F.; Summo, C. The effect of the addition of Apulian black chickpea flour on the nutritional and qualitative properties of durum wheat-based bakery products. Foods 2019, 8, 504. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; Muccilli, S.; Padalino, L.; Giannone, V.; Lecce, L.; Giovanniello, V.; Del Nobile, M.A.; De Vita, P.; Spina, A. Durum wheat breads ‘high in fibre’ and with reduced in vitro glycaemic response obtained by partial semolina replacement with minor cereals and pulses. J. Food Sci. Technol. 2018, 55, 4458–4467. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Rooney, L.W.; Ali, R.; Riaz, M.N. Application and opportunities of pulses in food system: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 1168–1179. [Google Scholar] [CrossRef]
- Previtali, M.A.; Mastromatteo, M.; De Vita, P.; Ficco, D.B.M.; Conte, A.; Del Nobile, M.A. Effect of the lentil flour and hydrocolloids on baking characteristics of wholemeal durum wheat bread. Int. J. Food Sci. Technol. 2014, 49, 2382–2390. [Google Scholar] [CrossRef]
- Juntunen, K.S.; Laaksonen, D.E.; Autio, K.; Niskanen, L.K.; Holst, J.J.; Savolainen, K.E.; Liukkonen, K.-H.; Poutanen, K.S.; Mykkänen, H.M. Structural differences between rye and wheat breads but not total fiber content may explain the lower postprandial insulin response to rye bread. Am. J. Clin. Nutr. 2003, 78, 957–964. [Google Scholar] [CrossRef]
- Spina, A.; Scarangella, M.; Canale, M.; Sanfilippo, R.; Giannone, V.; Summo, C.; Pasqualone, A. Nutritional features of flour blends composed of durum wheat and lupin. Int. J. Food Sci. Technol. 2023, 58, 4812–4819. [Google Scholar] [CrossRef]
- Spina, A.; Cambrea, M.; Scarangella, M. Prime osservazioni sui caratteri merceologici e sulla composizione chimica di Lupinus albus per consumo umano. In Proceedings of the Atti del IV Convegno Nazionale Piante Mediterranee ‘Le Potenzialità del Territorio e dell’Ambiente’, Nova Siri, Italy, 7–10 October 2009; Lulu Press, Inc.: Morrisville, NC, USA, 2010; pp. 702–706. (In Italian). [Google Scholar]
- Boukid, F.; Pasqualone, A. Lupine (Lupinus spp.) proteins: Characteristics, safety and food applications. Eur. Food Res. Technol. 2022, 248, 345–356. [Google Scholar] [CrossRef]
- Brandolini, A.; Glorio-Paulet, P.; Estivi, L.; Locatelli, N.; Cordova-Ramos, J.S.; Hidalgo, A. Tocopherols, carotenoids and phenolics changes during Andean lupin (Lupinus mutabilis Sweet) seeds processing. J. Food Compos. Anal. 2022, 106, 104335. [Google Scholar] [CrossRef]
- Rumiyati; Jayasena, V.; James, A.P. Total phenolic and phytosterol compounds and the radical scavenging activity of germinated Australian sweet lupin flour. Plant Foods Hum. Nutr. 2013, 68, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Ötles, S.; Ozgoz, S. Health effects of dietary fiber. Acta Sci. Pol. Technol. Aliment. 2014, 13, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-López, M.A.; Barrientos-Ramírez, L.; García-López, P.M.; Valdés-Miramontes, E.H.; Zamora-Natera, J.F.; Rodríguez-Macias, R.; Salcedo-Pérez, E.; Bañuelos-Pineda, J.; Vargas-Radillo, J.J. Nutritional and bioactive compounds in Mexican lupin beans species: A mini-review. Nutrients 2019, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Villarino, C.B.J.; Jayasena, V.; Coorey, R.; Chakrabarti-Bell, S.; Johnson, S.K. The effects of Australian sweet lupin (ASL) variety on physical properties of flours and breads. LWT-Food Sci. Technol. 2015, 60, 435–443. [Google Scholar] [CrossRef]
- Pleming, D.; Farahnaky, A.; Majzoobi, M. Effects of bread making methods, lupin variety and gluten powder on the quality of bread enriched with high percentage of lupin flour. Int. J. Food Sci. 2021, 56, 6707–6718. [Google Scholar] [CrossRef]
- Pasqualone, A. Italian Durum Wheat Breads; Nova Science Publisher Inc.: Hauppauge, NY, USA, 2012; pp. 57–79. [Google Scholar]
- Pasqualone, A.; Summo, C.; Bilancia, M.T.; Caponio, F. Variations of the sensory profile of durum wheat Altamura PDO (Protected Designation of Origin) bread during staling. J. Food Sci. 2007, 72, S191–S196. [Google Scholar] [CrossRef]
- Giannone, V.; Giarnetti, M.; Spina, A.; Todaro, A.; Pecorino, B.; Summo, C.; Caponio, F.; Paradiso, V.M.; Pasqualone, A. Physico-chemical properties and sensory profile of durum wheat Dittaino PDO (Protected Designation of Origin) bread and quality of re-milled semolina used for its production. Food Chem. 2018, 241, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.; Crombag, J.J.; Senden, J.M.; Waterval, W.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidant by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Vignolini, P.; Urciuoli, S.; Heimler, D.; Romani, A. Carotenoids, Polyphenols and Antioxidant Activity Evaluation in Stone-Grinded Wheat Semolina. J. Health Sci. 2018, 6, 432–438. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, R.; Canale, M.; Dugo, G.; Oliveri, C.; Scarangella, M.; Strano, M.C.; Amenta, M.; Crupi, A.; Spina, A. Effects of Partial Replacement of Durum Wheat Re-Milled Semolina with Bean Flour on Physico-Chemical and Technological Features of Doughs and Breads during Storage. Plants 2023, 12, 1125. [Google Scholar] [CrossRef] [PubMed]
- AACC Official Approved Methods of Analysis, 11th ed.; AACC: St. Paul, MN, USA, 2000.
- UNI 10453; Durum Heat and Semolina—Determination of Rheological Properties Using an Alveograph. Ente Nazionale Italiano di Unificazione (UNI): Milan, Italy, 1995.
- ISO 3093:2009; Wheat, Rye and Their Flours, Durum Wheat and Durum Wheat Semolina—Determination of the Falling Number According to Hagberg-Perten. International Organization for Standardization: Geneva, Switzerland, 2009.
- Canale, M.; Spina, A.; Summo, C.; Strano, M.C.; Bizzini, M.; Allegra, M.; Sanfilippo, R.; Amenta, M.; Pasqualone, A. Waste from Artichoke Processing Industry: Reuse in Bread-Making and Evaluation of the Physico-Chemical Characteristics of the Final Product. Plants 2022, 11, 3409. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; Canale, M.; Giannone, V.; Strano, M.C.; Allegra, M.; Zingale, S.; Spina, A. Durum Wheat Bread with a Potentially High Health Value through the Addition of Durum Wheat Thin Bran or Barley Flour. Plants 2023, 12, 397. [Google Scholar] [CrossRef]
- Bot, B.; Sánchez, H.; de la Torre, M.; Osella, C. Mother dough in bread making. Food Sci. Nutr. 2014, 2, 24–29. [Google Scholar] [CrossRef][Green Version]
- Dallmann, H. Porentabelle, 4th ed.; Verlag Moritz Schäfer: Detmold, Germany, 1981. [Google Scholar]
- ISO 13299:2016; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 8586:2023; Sensory Analysis—Selection and Training of Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2023.
- ISO 8589:2014; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2014.
- Comendador, F.J. Atlante Sensoriale dei Prodotti Alimentari Book; Società Italiana di Scienze Sensoriali, Ed.; Tecniche Nuove: Milan, Italy, 2012. [Google Scholar]
- Ferchichi, N.; Toukabri, W.; Vrhovsek, U.; Nouairi, I.; Angeli, A.; Masuero, D.; Mhamdi, R.; Trabelsi, D. Proximate composition, lipid and phenolic profiles, and antioxidant activity of different ecotypes of Lupinus albus, Lupinus luteus and Lupinus angustifolius. J. Food Meas. Charact. 2021, 15, 1241–1257. [Google Scholar] [CrossRef]
- Canale, M.; Sanfilippo, R.; Strano, M.C.; Amenta, M.; Allegra, M.; Proetto, I.; Papa, M.; Palmeri, R.; Todaro, A.; Spina, A. Artichoke Industrial Waste in Durum Wheat Bread: Effects of Two Different Preparation and Drying Methods of Flours and Evaluation of Quality Parameters during Short Storage. Foods 2023, 12, 3419. [Google Scholar] [CrossRef] [PubMed]
- Pasarin, D.; Lavric, V.; Enascuta, C.E.; Ghizdareanu, A.I.; Matei, C.B. Optimal Enzymatic Hydrolysis of Sweet Lupine Protein towards Food Ingredients. Fermentation 2023, 9, 203. [Google Scholar] [CrossRef]
- Khalid, I.I.; Elharadallou, S.B. Functional properties of Cowpea (Vigna ungiculata L. Walp), and Lupin (Lupinus termis) flour and protein isolates. J. Nutr. Food Sci. 2013, 3, 1. [Google Scholar]
- Borchani, C.; Masmoudi, M.; Besbes, S.; Attia, H.; Deroanne, C.; Blecker, C. Effect of date flesh fiber concentrate addition on dough performance and bread quality. J. Texture Stud. 2011, 42, 300–308. [Google Scholar] [CrossRef]
- Rosell, C.M.; Rojas, J.A.; De Barber, C.B. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll. 2001, 15, 75–81. [Google Scholar] [CrossRef]
- Wang, J.; Rosell, C.M.; de Barber, C.B. Effect of the addition of different fibres on wheat dough performance and bread quality. Food Chem. 2002, 79, 221–226. [Google Scholar] [CrossRef]
- Makri, E.; Papalamprou, E.; Doxastakis, G. Study of functional properties of seed storage proteins from indigenous European legume crops (lupin, pea, broad bean) in admixture with polysaccharides. Food Hydrocoll. 2005, 19, 583–594. [Google Scholar] [CrossRef]
- Des Marchais, L.P.; Foisy, M.; Mercier, S.; Villeneuve, S.; Mondor, M. Bread-making potential of pea protein isolate produced by a novel ultrafiltration/diafiltration process. Procedia Food Sci. 2011, 1, 1425–1430. [Google Scholar] [CrossRef]
- Yellavila, S.B.; Agbenorhevi, J.K.; Asibuo, J.Y.; Sampson, G.O. Proximate composition, minerals content and functional properties of five lima bean accessions. J. Food Secur. 2015, 3, 69–74. [Google Scholar]
- Turfani, V.; Narducci, V.; Durazzo, A.; Galli, V.; Carcea, M. Technological, nutritional and functional properties of wheat bread enriched with lentil or carob flours. LWT-Food Sci. Technol. 2017, 78, 361–366. [Google Scholar] [CrossRef]
- Bresciani, A.; Marti, A. Using pulses in baked products: Lights, shadows, and potential solutions. Foods 2019, 8, 451. [Google Scholar] [CrossRef] [PubMed]
- Dervas, G.; Doxastakis, G.; Hadjisavva-Zinoviadi, S.; Triantafillakos, N. Lupin flour addition to wheat flour doughs and effect on rheological properties. Food Chem. 1999, 66, 67–73. [Google Scholar] [CrossRef]
- Doxastakis, G.; Zafiriadis, I.; Irakli, M.; Marlani, H.; Tananaki, C. Lupin, soya and triticale addition to wheat flour doughs and their effect on rheological properties. Food Chem. 2002, 77, 219–227. [Google Scholar] [CrossRef]
- El-Adawy, T.A. Effect of sesame seed protein supplementation on the nutritional, physical, chemical and sensory properties of wheat flour bread. Food Chem. 1997, 59, 7–14. [Google Scholar] [CrossRef]
- Sathe, S.K.; Deshpande, S.S.; Salunkhe, D.K. Functional properties of winged bean [Psophocarpus tetragonolobus (L.) DC] proteins. J. Food Sci. 1982, 47, 503–509. [Google Scholar] [CrossRef]
- Du, S.K.; Jiang, H.; Yu, X.; Jane, J.L. Physicochemical and functional properties of whole legume flour. LWT-Food Sci. Technol. 2014, 55, 308–313. [Google Scholar] [CrossRef]
- Jitngarmkusol, S.; Hongsuwankul, J.; Tananuwong, K. Chemical compositions, functional properties, and microstructure of defatted macadamia flours. Food Chem. 2008, 110, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Singh, S.; Kumari, D. Evaluation of functional properties of composite flours and sensorial attributes of composite flour biscuits. J. Food Sci. Technol. 2015, 52, 3681–3688. [Google Scholar] [CrossRef]
- Culetu, A.; Susman, I.E.; Duta, D.E.; Belc, N. Nutritional and functional properties of gluten-free flours. Appl. Sci. 2021, 11, 6283. [Google Scholar] [CrossRef]
- Siddiq, M.; Ravi, R.; Harte, J.B.; Dolan, K.D. Physical and functional characteristics of selected dry bean (Phaseolus vulgaris L.) flours. LWT-Food Sci. Technol. 2010, 43, 232–237. [Google Scholar] [CrossRef]
- Villacrés, E.; Cueva, P.; Díaz, M.; Rosell, C.M. Replacing Wheat Flour with Debittered and Fermented Lupin: Effects on Bread’s Physical and Nutritional Features. Plant Foods Hum. Nutr. 2020, 75, 569–575. [Google Scholar] [CrossRef]
- Correia, P.M.R.; Gonzaga, M.; Batista, L.M.; Beirão-Costa, L.; Guiné, R.F.P. Development and Characterization of Wheat Bread with Lupin Flour. Int. J. Agric. Biol. Eng. 2015, 9, 923–927. [Google Scholar]
- Guardianelli, L.M.; Carbas, B.; Brites, C.; Puppo, M.C.; Salinas, M.V. White Lupine (Lupinus albus L.) Flours for Healthy Wheat Breads: Rheological Properties of Dough and the Bread Quality. Foods 2023, 12, 1645. [Google Scholar] [CrossRef] [PubMed]
- Carbas, B.; Salinas, M.V.; Puppo, M.C.; Brites, C. Influence of Misak lupine (Lupinus albus L.) flour on dough rheology and texture of bread. In Proceedings of the 10th AISTEC Conference Grains for Feeding the World, Milan, Italy, 1–3 July 2015; pp. 1–3. [Google Scholar]
- López, E.P. Influence of the addition of lupine protein isolate on the protein and technological characteristics of dough and fresh bread with added Brea Gum. Food Sci. Technol. 2014, 34, 195–203. [Google Scholar] [CrossRef]
- Pasqualone, A.; Laddomada, B.; Centomani, I.; Paradiso, V.M.; Minervini, D.; Caponio, F.; Summo, C. Bread making aptitude of mixtures of re-milled semolina and selected durum wheat milling by-products. LWT-Food Sci. Technol. 2017, 78, 151–159. [Google Scholar] [CrossRef]
- Almeida, E.L.; Chang, Y.K.; Steel, C.J. Dietary fibre sources in bread: Influence on technological quality. LWT-Food Sci. Technol. 2013, 50, 545–553. [Google Scholar] [CrossRef]
- Renzetti, S.; Theunissen, M.; Horrevorts, K. A systematic comparison of the intrinsic properties of wheat and oat bran fractions and their effects on dough and bread properties: Elucidation of chemical mechanisms, water binding, and steric hindrance. Foods 2021, 10, 2311. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.S.; Izydorczyk, M.S.; Preston, K.R.; Dexter, J.E. Evaluation of baking procedures for incorporation of barley roller milling fractions containing high levels of dietary fibre into bread. J. Sci. Food Agric. 2008, 88, 558–568. [Google Scholar] [CrossRef]
- Villarino, C.B.J.; Jayasena, V.; Coorey, R.; Chakrabarti-Bell, S.; Foley, R.; Fanning, K.; Johnson, S.K. The effects of lupin (Lupinus angustifolius) addition to wheat bread on its nutritional, phytochemical and bioactive composition and protein quality. Food Res. Int. 2015, 76, 58–65. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef]
- Pollard, N.J.; Stoddard, F.L.; Popineau, Y.; Wrigley, C.W.; MacRitchie, F. Lupin flours as additives: Dough mixing, breadmaking, emulsifying, and foaming. Cereal Chem. 2002, 79, 662–669. [Google Scholar] [CrossRef]
- Roland, W.S.; Pouvreau, L.; Curran, J.; van de Velde, F.; de Kok, P.M. Flavor aspects of pulse ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef]


| Bread Type | Semolina | LPC | LF | Yeast | NaCl | Sunflower Oil | Water |
|---|---|---|---|---|---|---|---|
| SC | 100 | - | - | 0.5 | 1.7 | 3.33 | 62.3 |
| LF 3% | 97 | - | 3 | 0.5 | 1.7 | 3.33 | 65.5 |
| LF 7% | 93 | - | 7 | 0.5 | 1.7 | 3.33 | 66.3 |
| LF 10% | 90 | - | 10 | 0.5 | 1.7 | 3.33 | 66.7 |
| LF 15% | 85 | - | 15 | 0.5 | 1.7 | 3.33 | 69.1 |
| LPC 3% | 97 | 3 | - | 0.5 | 1.7 | 3.33 | 62.1 |
| LPC 7% | 93 | 7 | - | 0.5 | 1.7 | 3.33 | 63.8 |
| LPC 10% | 90 | 10 | - | 0.5 | 1.7 | 3.33 | 63.9 |
| LPC 15% | 85 | 15 | - | 0.5 | 1.7 | 3.33 | 64.1 |
| Sample | Polyphenols (mg GAE/100 g d.m.) | Antioxidant Activity (µmol TE/g d.m.) |
|---|---|---|
| Pure flours | ||
| SC | 21.88 ± 2.19 c | 56.67 ± 0.20 b |
| LF | 33.58 ± 0.18 ab | 61.00 ± 1.06 b |
| LPC | 37.91 ± 1.48 a | 76.33 ± 1.69 a |
| Blends | ||
| LF 3% | 22.17 ± 0.16 c | 56.80 ± 0.16 b |
| LF 7% | 26.32 ± 0.11 bc | 56.97 ± 0.11 b |
| LF 10% | 27.15 ± 1.07 bc | 57.11 ± 0.07 b |
| LF 15% | 29.59 ± 0.18 ab | 57.32 ± 0.01 b |
| LPC 3% | 22.36 ± 2.08 c | 57.26 ± 0.24 b |
| LPC 7% | 23.00 ± 1.93 c | 58.77 ± 0.61 b |
| LPC 10% | 24.85 ± 0.13 c | 59.35 ± 0.83 b |
| LPC 15% | 25.91 ± 0.02 bc | 60.69 ± 2.49 b |
| Sample | Farinograph | Alveograph | Falling Number (s) | ||||
|---|---|---|---|---|---|---|---|
| Dough Development Time (min) | Stability (min) | Softening Degree (B.U.) | Water Absorption at 500 B.U. (%) | W (10−4 J) | P/L | ||
| SC | 1.36 ± 0.02 e | 5.23 ± 0.05 b | 45 ± 0.7 f | 62.3 ± 0.4 e | 225 ± 4 a | 2.40 ± 0.03 bc | 419 ± 14 d |
| LF 3% | 1.62 ± 0.04 c | 5.13 ± 0.40 b | 62 ± 2.1 d | 65.5 ± 0.1 c | 114 ± 1 cd | 1.77 ± 0.06 c | 503 ± 20 ac |
| LF 7% | 1.72 ± 0.03 b | 4.93 ± 0.03 b | 54 ± 2.8 e | 66.3 ± 0.1 b | 96 ± 3 ef | 1.81 ± 0.13 c | 487 ± 9 bcd |
| LF 10% | 1.59 ± 0.01 c | 4.87 ± 0.08 b | 60 ± 2.1 e | 66.7 ± 0.1 b | 82 ± 1 fg | 2.06 ± 0.03 bc | 461 ± 8 bcd |
| LF 15% | 1.48 ± 0.03 d | 1.83 ± 0.04 e | 107 ± 3.5 ab | 69.1 ± 1.06 a | 76 ±1 g | 3.94 ± 0.06 a | 439 ± 28 cd |
| LPC 3% | 1.69 ± 0.02 b | 6.05 ± 0.14 a | 6 ± 7.8 d | 62.1 ± 0.1 e | 147 ± 1 b | 2.89 ± 0.56 abc | 488 ± 12 bcd |
| LPC 7% | 1.52 ± 0.04 d | 2.61 ± 0.02 d | 83 ± 2.1 c | 63.8 ± 1.2 d | 127 ± 2 c | 2.27 ± 0.15 bc | 566 ± 28 a |
| LPC 10% | 1.61 ± 0.07 c | 3.83 ± 0.08 c | 78 ± 7.1 cd | 63.9 ± 0.1 d | 122 ± 1 cd | 2.49 ± 0.78 abc | 530 ± 9 ab |
| LPC 15% | 3.08 ± 0.05 a | 1.72 ± 0.01 e | 119 ± 6.4 a | 64.1 ± 0.1 d | 108 ± 3 de | 3.49 ± 0.08 ab | 518 ± 26 ab |
| Bread Type | Moisture (g/100 g) | Specific Volume (cm3/g) | Density (g/cm3) | Height (mm) | Hardness (N) | Porosity (1–8) * |
|---|---|---|---|---|---|---|
| SC | 29.98 ± 0.18 a | 2.85 ± 0.05 a | 0.35 ± 0.07 c | 76.0 ± 0.0 a | 16.85 ± 0.27 abc | 5 |
| LF 3% | 28.64 ± 0.01 b | 2.14 ± 0.05 bc | 0.47 ± 0.19 ab | 63.4 ± 0.7 ab | 16.36 ± 0.82 c | 6 |
| LF 7% | 28.83 ± 0.02 b | 2.21 ± 0.03 bc | 0.45 ± 0.21 b | 64.1 ± 0.2 ab | 16.81 ± 0.99 abc | 6 |
| LF 10% | 29.22 ± 0.01 ab | 2.32 ± 0.03 b | 0.43 ± 0.12 b | 63.1 ± 0.0 ab | 18.49 ± 1.75 abc | 6 |
| LF 15% | 29.22 ±0.03 ab | 1.93 ± 0.02 c | 0.52 ± 0.10 a | 60.1 ± 1.8 b | 21.17 ± 1.59 ab | 8 |
| LPC 3% | 29.33 ± 0.02 ab | 2.16 ± 0.01 bc | 0.46 ± 0.07 ab | 63.2 ± 0.1 ab | 15.79 ± 0.89 c | 6 |
| LPC 7% | 29.53 ± 0.01 ab | 2.24 ± 0.07 bc | 0.45 ± 0.07 b | 65.7 ± 1.4 ab | 16.65 ± 1.68 bc | 6 |
| LPC 10% | 28.60 ± 0.42 b | 2.19 ± 0.03 bc | 0.46 ± 0.05 b | 64.6 ± 0.4 ab | 18.51 ± 1.36 abc | 7 |
| LPC 15% | 28.41 ± 0.01 b | 2.32 ± 0.01 b | 0.43 ± 0.07 b | 65.6 ± 0.1 ab | 21.34 ± 1.64 a | 7 |
| Sample | Polyphenols (mg GAE/g d.m.) | Antioxidant Activity (µmol/g d.m.) |
|---|---|---|
| SC | 12.08 ± 0.05 g | 17.66 ± 1.34 d |
| LF 3% | 15.36 ± 0.09 f | 18.35 ± 0.47 d |
| LF 7% | 16.30 ± 0.08 ef | 21.96 ± 0.89 cd |
| LF 10% | 17.33 ± 0.20 de | 24.38 ± 0.68 c |
| LF 15% | 18.20 ± 0.04 cd | 24.47 ± 0.43 c |
| LPC 3% | 16.26 ± 0.26 ef | 26.59 ± 0.38 bc |
| LPC 7% | 19.12 ± 0.15 c | 30.67 ± 0.07 ab |
| LPC 10% | 22.38 ± 0.02 b | 31.05 ± 0.29 ab |
| LPC 15% | 25.41 ± 0.30 a | 36.00 ± 1.05 a |
| Bread Type | Crumb | Crust | ||||||
|---|---|---|---|---|---|---|---|---|
| Brown Index (100 − L*) | Red Index (a*) | Yellow Index (b*) | ∆E | Brown Index (100 − L*) | Red Index (a*) | Yellow Index (b*) | ∆E | |
| SC | 25.8 ± 0.1 ab | −3.0 ± 0.1 d | 21.8 ± 0.3 e | - | 64.0 ± 1.2 c | 12.4 ± 0.1 a | 17.0 ± 0.6 a | - |
| LF 3% | 27.0 ± 0.1 ab | −3.0 ± 0.1 d | 26.1 ± 1.6 cde | 3.9 | 64.8 ± 1.3 bc | 9.7 ± 0.4 abc | 14.1 ± 0.1 b | 3.7 |
| LF 7% | 25.8 ± 0.0 ab | −3.1 ± 0.1 d | 28.3 ± 0.8 bcd | 6.1 | 65.7 ± 0.3 bc | 9.7 ± 0.0 abc | 13.0 ± 0.9 bc | 4.9 |
| LF 10% | 29.6 ± 0.0 ab | −3.0 ± 0.1 d | 32.6 ± 1.0 ab | 11.7 | 70.4 ± 1.4 a | 7.0 ± 0.0 c | 8.1 ± 0.1 d | 10.9 |
| LF 15% | 27.3 ± 0.0 ab | −2.8 ± 0.2 cd | 36.4 ± 1.8 a | 14.1 | 68.5 ± 0.7 ab | 8.5 ± 0.0 bc | 11.1 ± 0.4 c | 8.7 |
| LPC 3% | 25.3 ± 0.0 b | −2.1 ± 0.1 bcd | 23.0 ± 1.6 de | 1.7 | 62.8 ± 2.2 c | 10.4 ± 0.3 ab | 15.3 ± 1.7 ab | 2.1 |
| LPC 7% | 28.9 ± 0.1 ab | −1.5 ± 0.2 bc | 31.0 ± 1.7 abc | 7.7 | 67.0 ± 0.9 abc | 8.7 ± 0.0 bc | 11.2 ± 0.9 c | 4.2 |
| LPC 10% | 28.6 ± 0.0 ab | −1.1 ± 0.3 ab | 31.5 ± 1.4 abc | 9.5 | 65.8 ± 0.6 bc | 10.6 ± 0.2 ab | 13.0 ± 0.1 bc | 4.7 |
| LPC 15% | 29.9 ± 0.0 a | −0.1 ± 0.1 a | 32.8 ± 0.9 ab | 12.1 | 65.4 ± 0.8 bc | 10.9 ± 0.0 ab | 13.0 ± 0.4 bc | 7.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spina, A.; Summo, C.; Timpanaro, N.; Canale, M.; Sanfilippo, R.; Amenta, M.; Strano, M.C.; Allegra, M.; Papa, M.; Pasqualone, A. Lupin as Ingredient in Durum Wheat Breadmaking: Physicochemical Properties of Flour Blends and Bread Quality. Foods 2024, 13, 807. https://doi.org/10.3390/foods13050807
Spina A, Summo C, Timpanaro N, Canale M, Sanfilippo R, Amenta M, Strano MC, Allegra M, Papa M, Pasqualone A. Lupin as Ingredient in Durum Wheat Breadmaking: Physicochemical Properties of Flour Blends and Bread Quality. Foods. 2024; 13(5):807. https://doi.org/10.3390/foods13050807
Chicago/Turabian StyleSpina, Alfio, Carmine Summo, Nicolina Timpanaro, Michele Canale, Rosalia Sanfilippo, Margherita Amenta, Maria Concetta Strano, Maria Allegra, Martina Papa, and Antonella Pasqualone. 2024. "Lupin as Ingredient in Durum Wheat Breadmaking: Physicochemical Properties of Flour Blends and Bread Quality" Foods 13, no. 5: 807. https://doi.org/10.3390/foods13050807
APA StyleSpina, A., Summo, C., Timpanaro, N., Canale, M., Sanfilippo, R., Amenta, M., Strano, M. C., Allegra, M., Papa, M., & Pasqualone, A. (2024). Lupin as Ingredient in Durum Wheat Breadmaking: Physicochemical Properties of Flour Blends and Bread Quality. Foods, 13(5), 807. https://doi.org/10.3390/foods13050807










