Survival of Listeria Strains and Shelf Life Determination of Fresh Blueberries (Vaccinium corymbosum) Treated with Cold Atmospheric Plasma
Abstract
1. Introduction
2. Materials and Methods
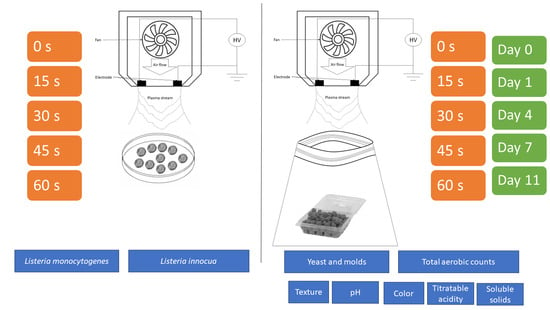
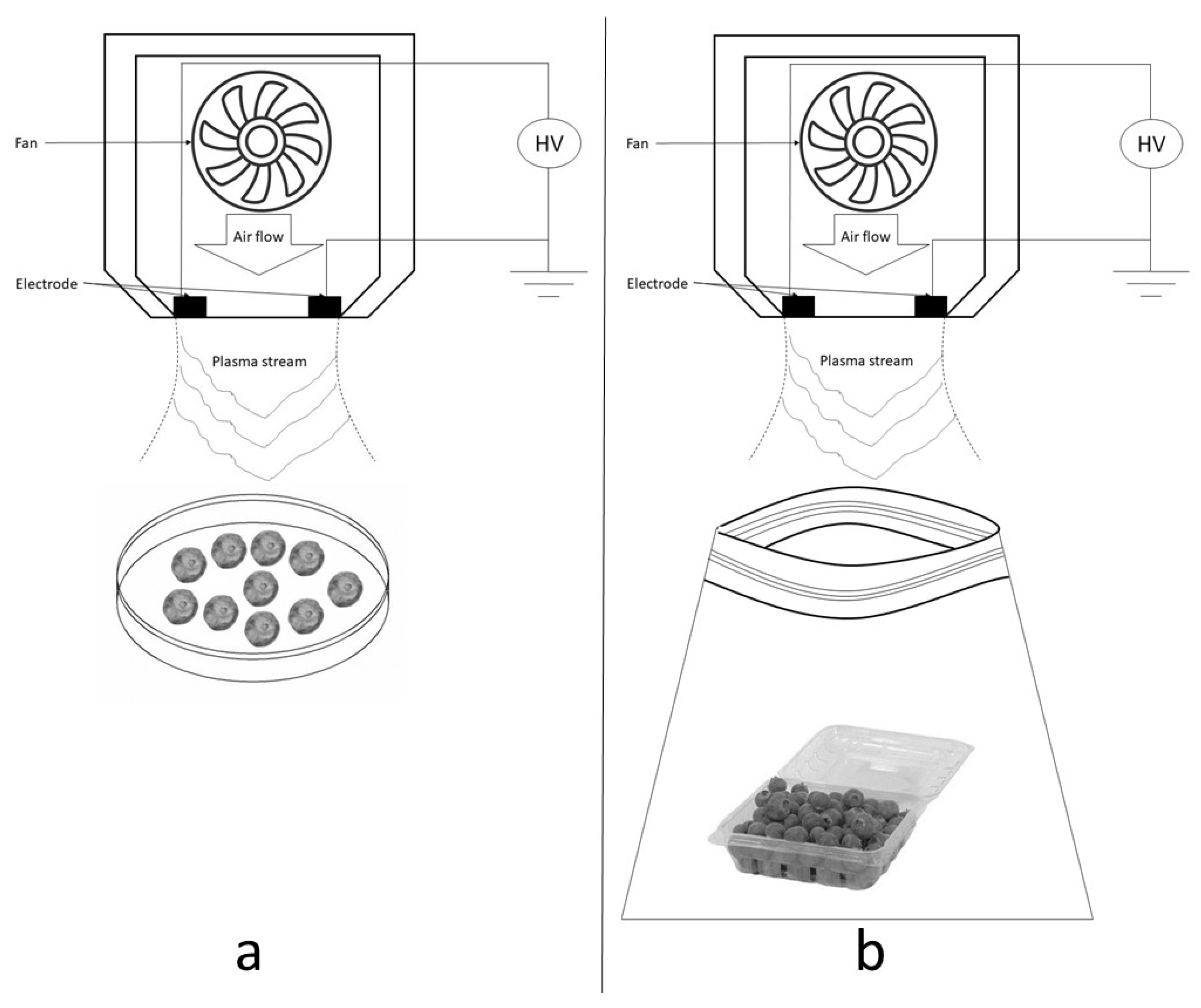
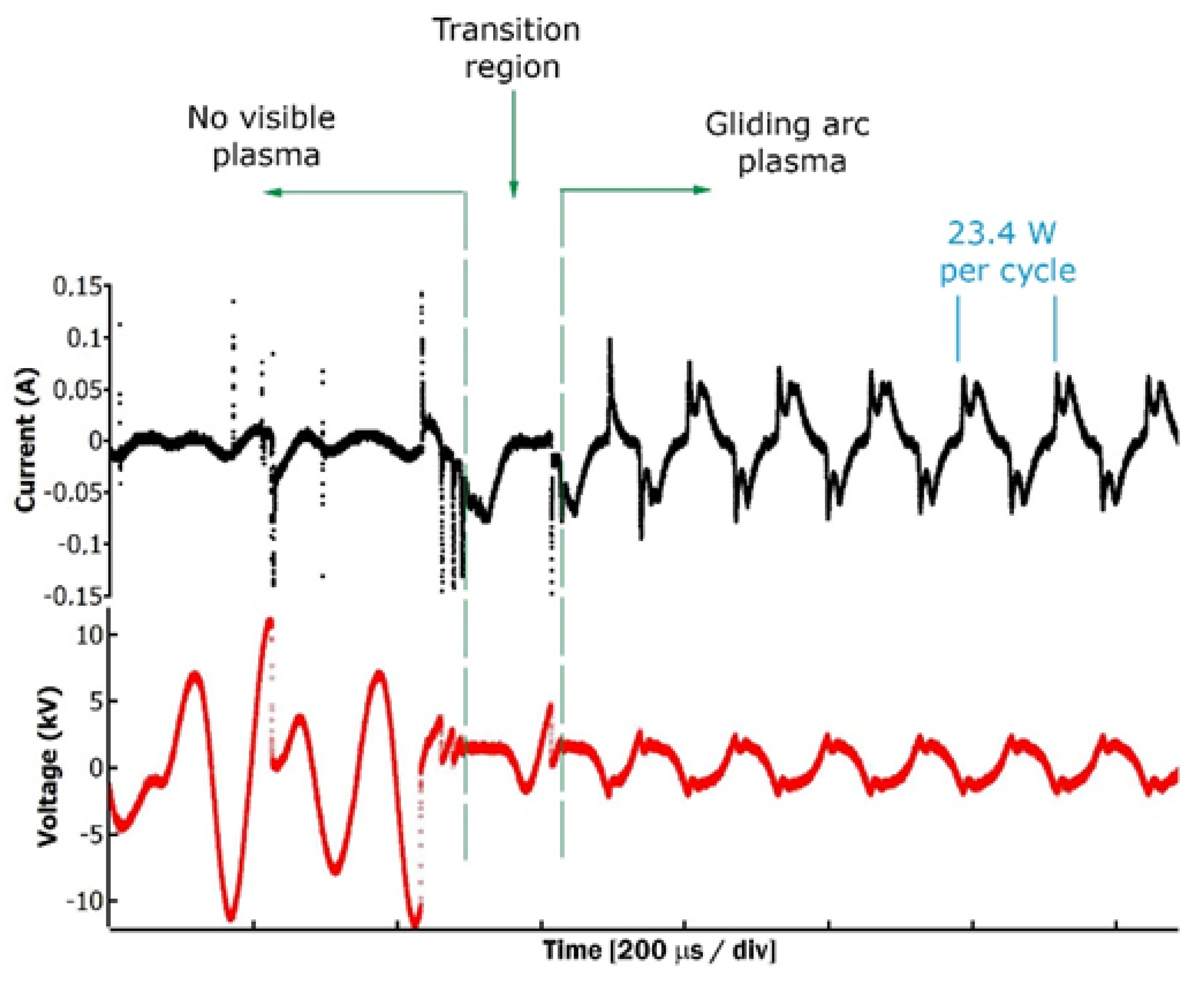
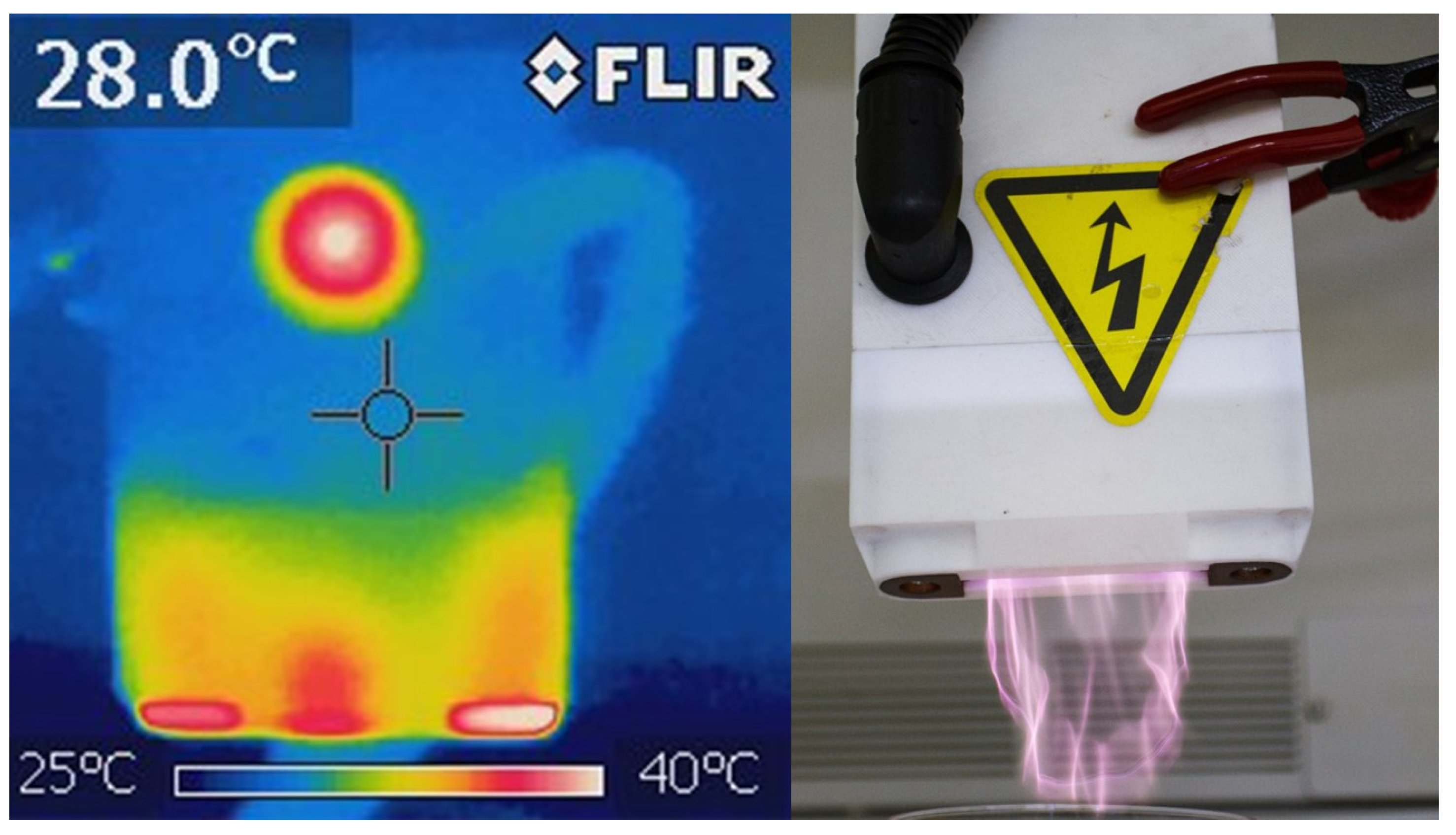
2.1. Cold Atmospheric Plasma Treatment Characterization
2.2. Cold Atmospheric Plasma In Vitro Antimicrobial Assay
2.2.1. Bacteria and Culture Conditions (Inocula Preparation)
2.2.2. Fruit Sample Inoculation
2.2.3. CAP In Vitro Antimicrobial Treatment
2.2.4. L. monocytogenes and L. innocua Recovery from Blueberries
2.3. CAP Shelf Life Extension Assay
2.3.1. Fruit Preparation
2.3.2. Cold Atmospheric Plasma Sample Treatment Conditions
2.3.3. Microbiological Shelf Life Evaluation of CAP Treated Blueberries
2.3.4. Quality Shelf Life Parameters Evaluation of CAP Treated Blueberries
2.4. Statistical Analysis
3. Results and Discussion
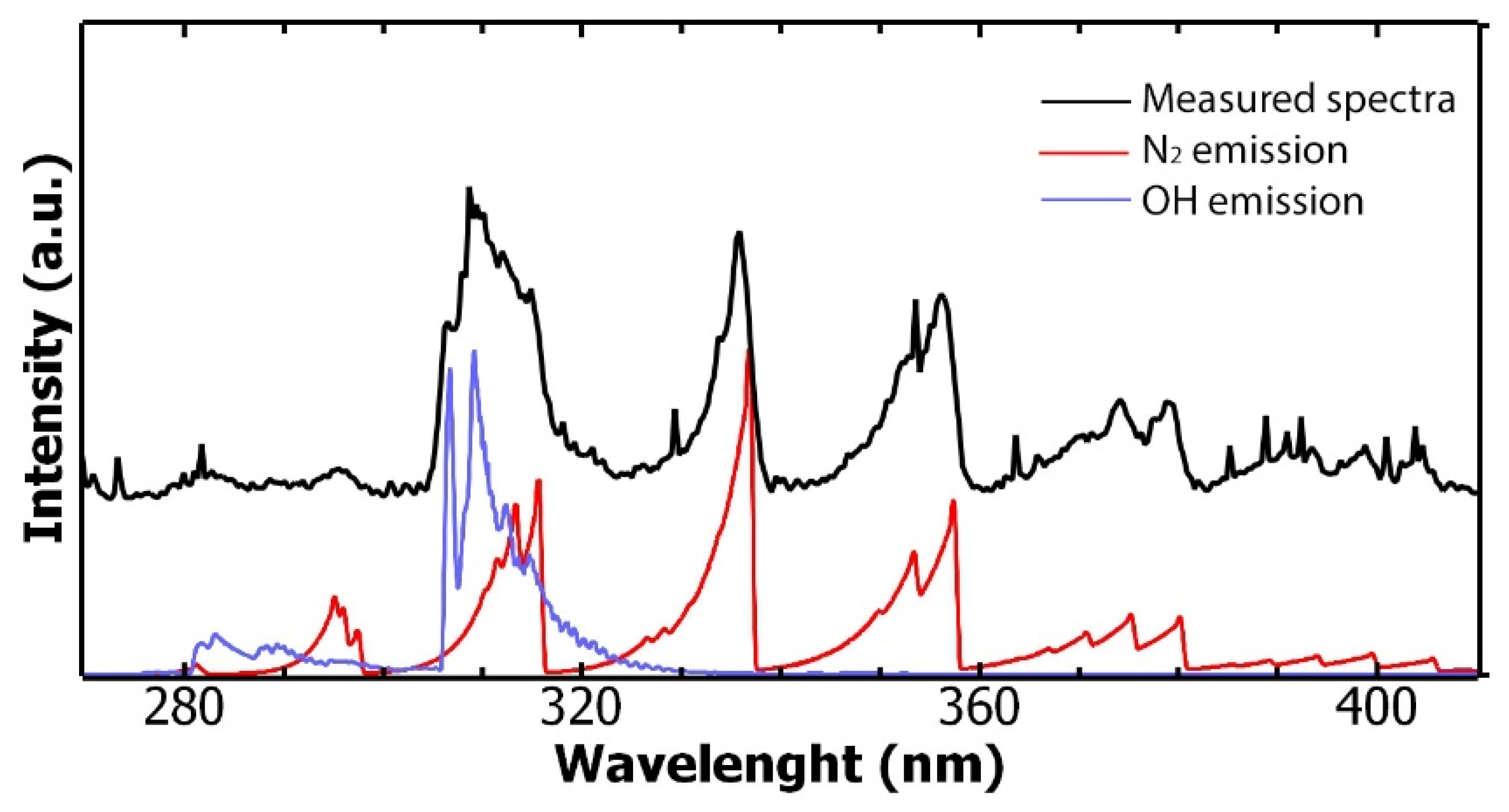
3.1. Cold Atmospheric Plasma Treatment Characterization
3.2. Cold Atmospheric Plasma (CAP) In Vitro Antimicrobial Assay
3.3. CAP Shelf Life Extension Assay
3.3.1. Microbiological Shelf Life Evaluation of CAP Treated Blueberries
3.3.2. Quality Shelf Life Parameters Evaluation of CAP Treated Blueberries
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ODEPA. Boletín de Fruta; Gobierno de Chile; ODEPA (Oficina de Estudios y Políticas Agrarias): Santiago, Chile, 2020; Available online: https://www.odepa.gob.cl/publicaciones/boletines/boletin-de-fruta-enero-de-2020 (accessed on 30 January 2020).
- Retamales, J.B.; Palma, M.J.; Morales, Y.A.; Lobos, G.A.; Moggia, C.E.; Mena, C.A. Blueberry production in Chile: Current status and future developments. Rev. Bras. Frutic. 2014, 36, 58–67. [Google Scholar] [CrossRef][Green Version]
- Mitcham, E.J.; Mitchell, F.G. Field Packing. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California Agriculture and Natural Resources: Davis, CA, USA, 2002; Volume 3311, p. 67. [Google Scholar]
- Rocourt, J.; BenEmbarek, P.; Toyofuku, H.; Schlundt, J. Quantitative risk assessment of Listeria monocytogenes in ready-to-eat foods: The FAO/WHO approach. FEMS Immunol. Med. Microbiol. 2003, 35, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Boland, J.A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Domínguez-Bernal, G.; Goebel, W.; González-Zorn, B.; Wehland, J.; Kreft, J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef]
- Gombas, D.E.; Chen, Y.; Clavero, R.S.; Scott, V.N. Survey of Listeria monocytogenes in ready-to-eat foods. J. Food Prot. 2003, 66, 559–569. [Google Scholar] [CrossRef]
- Ryser, E.T.; Marth, E.H. Listeria, Listeriosis, and Food Safety, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 341. [Google Scholar]
- FDA. Frozen Blueberries Recalled due to Listeria monocytogenes Contamination. FDA Enforcement Report. 1998; pp. 52–98. Available online: https://webharvest.gov/peth04/20041118071029/http://www.fda.gov/bbs/topics/ENFORCE/ENF00570.html (accessed on 19 January 2024).
- Desai, A.N.; Anyoha, A.; Madoff, L.C.; Lassmann, B. Changing epidemiology of Listeria monocytogenes outbreaks sporadic cases and recalls globally: A review of ProMED reports from 1996 to 2018. Int. J. Infect. Dis. 2019, 84, 48–53. [Google Scholar] [CrossRef]
- Kniel, K.E.; Shearer, A.E. Berry Contamination: Outbreaks and Contamination Issues. In The Produce Contamination Problem: Causes and Solutions, 1st ed.; Sapers, G., Solomon., E., Matthews, K.R., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 271–297. [Google Scholar]
- Concha-Meyer, A.; Eifert, J.; Williams, R.; Marcy, J.; Welbaum, G. Survival of Listeria monocytogenes on Fresh Blueberries (Vaccinium corymbosum) Stored under Controlled Atmosphere and Ozone. J. Food Prot. 2014, 77, 832–836. [Google Scholar] [CrossRef]
- Sheng, L.; Tsai, H.C.; Zhu, H.; Zhu, M.J. Survival of Listeria monocytogenes on blueberries post-sanitizer treatments and subsequent cold storages. Food Control 2019, 100, 138–143. [Google Scholar] [CrossRef]
- Beaudry, R.M.; Moggia, C.E.; Retamales, J.B.; Hancock, J.F. Quality of ‘Ivanhoe’ and ‘Bluecrop’ blueberry fruit transported by air and sea from Chile to North America. HortScience 1998, 33, 313–317. [Google Scholar]
- Lobos, G.A.; Bravo, C.; Valdés, M.; Graell, J.; Ayala, I.L.; Beaudry, R.M.; Moggia, C. Within-plant variability in blueberry (Vaccinium corymbosum L.): Maturity at harvest and position within the canopy influence fruit firmness at harvest and postharvest. Postharvest Biol. Technol. 2018, 146, 26–35. [Google Scholar] [CrossRef]
- Moggia, C.; Graell, J.; Lara, I.; Schmeda-Hirschmann, G.; Thomas-Valdés, S.; Lobos, G.A. Fruit characteristics and cuticle triterpenes as related to postharvest quality of highbush blueberries. Sci. Hortic. 2016, 211, 449–457. [Google Scholar] [CrossRef]
- Ziuzina, D.; Patil, S.; Cullen, P.J.; Keener, K.M.; Bourke, P. Atmospheric cold plasma inactivation of Escherichia coli, Salmonella enterica serovar Typhimurium and Listeria monocytogenes inoculated on fresh produce. Food Microbiol. 2014, 42, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, A.; Niemira, B.A.; Gurtler, J.B.; Fan, X.; Sites, J.; Boyd, G.; Chen, H. Atmospheric cold plasma inactivation of aerobic microorganisms on blueberries and effects on quality attributes. Food Microbiol. 2015, 46, 479–484. [Google Scholar] [CrossRef]
- Calvo, T.; Alvarez-Ordóñez, A.; Prieto, M.; Bernardo, A.; López, M. Stress adaptation has a minor impact on the effectivity of Non-Thermal Atmospheric Plasma (NTAP) against Salmonella spp. Food Res. Int. 2017, 102, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Niemira, B.A. Cold plasma decontamination of foods. Annu. Rev. Food Sci. Technol. 2012, 3, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Noriega, E.; Shama, G.; Laca, A.; Díaz, M.; Kong, M.G. Cold atmospheric gas plasma disinfection of chicken meat and chicken skin contaminated with Listeria innocua. Food Microbiol. 2011, 28, 1293–1300. [Google Scholar] [CrossRef]
- Rana, S.; Mehta, D.; Bansal, V.; Shivhare, U.S.; Yadav, S.K. Atmospheric cold plasma (ACP) treatment improved in-package shelf-life of strawberry fruit. J. Food Sci. Technol. 2020, 57, 102–112. [Google Scholar] [CrossRef]
- Concha-Meyer, A.; Eifert, J.D.; Williams, R.C.; Marcy, J.E.; Welbaum, G.E. Shelf life determination of fresh blueberries (Vaccinium corymbosum) stored under controlled atmosphere and ozone. Int. J. Food Sci. 2015, 2015, 164143. [Google Scholar] [CrossRef]
- Donoso, W.; Castro, R.I.; Guzmán, L.; López-Cabaña, Z.; Nachtigall, F.M.; Santos, L.S. Fast detection of Listeria monocytogenes through a nanohybrid quantum dot complex. Anal. Bioanal. Chem. 2017, 409, 5359–5371. [Google Scholar] [CrossRef]
- Caggia, C.; Scifo, G.O.; Restuccia, C.; Randazzo, C.L. Growth of acid-adapted Listeria monocytogenes in orange juice and in minimally processed orange slices. Food Control 2009, 20, 59–66. [Google Scholar] [CrossRef]
- Concha-Meyer, A.; Eifert, J.D.; Williams, R.C.; Marcy, J.E.; Welbaum, G.E. Listeria monocytogenes survival in the presence of malic acid, lactic acid or blueberry extract. J. Berry Res. 2017, 7, 33–41. [Google Scholar] [CrossRef]
- Pasquali, F.; Stratakos, A.C.; Koidis, A.; Berardinelli, A.; Cevoli, C.; Ragni, L.; Mancusi, R.; Manfreda, G.; Trevisani, M. Atmospheric cold plasma process for vegetable leaf decontamination: A feasibility study on radicchio (red chicory. Cichorium intybus L.). Food Control 2016, 60, 552–559. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 15th ed.; AOAC International: Gaithersburg, MD, USA, 2000; ISBN 978-0-935584-83-7. [Google Scholar]
- Roth, J.R. Industrial Plasma Engineering, 1st ed.; IOP Publishing Ltd.: Bristol, UK, 1995; ISBN 0-7503-0318-2. [Google Scholar]
- Fridman, A.; Nester, S.; Kennedy, L.A.; Saveliev, A.; Mutaf-Yardimci, O. Gliding arc gas discharge. Prog. Energy Combust. Sci. 1999, 25, 211–231. [Google Scholar] [CrossRef]
- Niemira, B.A.; Gutsol, A.; Fridman, A. Cold, atmospheric pressure plasma reduces Listeria innocua on the surface of apples. In Proceedings of the International Association for Food Protection Annual Meeting, Baltimore, MD, USA, 16 August 2005. [Google Scholar]
- Ziuzina, D.; Patil, S.; Cullen, P.J.; Keener, K.M.; Bourke, P. Atmospheric cold plasma inactivation of Escherichia coli in liquid media inside a sealed package. J. Appl. Microbiol. 2013, 114, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Moldgy, A.; Nayak, G.; Aboubakr, H.A.; Goyal, S.M.; Bruggeman, P.J. Inactivation of virus and bacteria using cold atmospheric pressure air plasmas and the role of reactive nitrogen species. J. Phys. D Appl. Phys. 2020, 53, 434004. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ryan, S.; Gahan, C.G.; Hill, C. Presence of GadD1 glutamate decarboxylase in selected Listeria monocytogenes strains is associated with an ability to grow at low pH. Appl. Environ. Microbiol. 2005, 71, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ordóñez, A.; Broussolle, V.; Colin, P.; Nguyen-The, C.; Prieto, M. The adaptive response of bacterial food-borne pathogens in the environment, host and food: Implications for food safety. Int. J. Food Microbiol. 2015, 213, 99–109. [Google Scholar] [CrossRef]
- Leistner, L. Basic aspects of food preservation by hurdle technology. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Wesche, A.M.; Gurtler, J.B.; Marks, B.P.; Ryser, E.T. Stress, sublethal injury, resuscitation, and virulence of bacterial foodborne pathogens. J. Food Prot. 2009, 72, 1121–1138. [Google Scholar] [CrossRef]
- Pathak, N.; Grossi Bovi, G.; Limnaios, A.; Fröhling, A.; Brincat, J.P.; Taoukis, P.; Valdramidis, V.P.; Schlüter, O. Impact of cold atmospheric pressure plasma processing on storage of blueberries. J. Food Process. Preserv. 2020, 44, e14581. [Google Scholar] [CrossRef]
- Dong, X.Y.; Yang, Y.L. A novel approach to enhance blueberry quality during storage using cold plasma at atmospheric air pressure. Food Bioprocess Technol. 2019, 12, 1409–1421. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, W.; Liao, J.; Jiang, A.; Xiu, Z.; Gaowa, S.; Guan, Y.; Yang, X.; Feng, K.; Liu, C. Effect of atmospheric cold plasma treatment on antioxidant activities and reactive oxygen species production in postharvest blueberries during storage. J. Sci. Food Agric. 2020, 100, 5586–5595. [Google Scholar] [CrossRef]
- Sarangapani, C.; O’Toole, G.; Cullen, P.J.; Bourke, P. Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries. Innov. Food Sci. Emerg. Technol. 2017, 44, 235–241. [Google Scholar] [CrossRef]
- Tappi, S.; Gozzi, G.; Vannini, L.; Berardinelli, A.; Romani, S.; Ragni, L.; Rocculi, P. Cold plasma treatment for fresh-cut melon stabilization. Innov. Food Sci. Emerg. Technol. 2016, 33, 225–233. [Google Scholar] [CrossRef]
- Hu, X.; Sun, H.; Yang, X.; Cui, D.; Wang, Y.; Zhuang, J.; Wang, X.; Ma, R.; Jiao, Z. Potential use of atmospheric cold plasma for postharvest preservation of blueberries. Postharvest Biol. Technol. 2021, 179, 111564. [Google Scholar] [CrossRef]
- Limnaios, A.; Pathak, N.; Grossi Bovi, G.; Fröhling, A.; Valdramidis, V.P.; Taoukis, P.S.; Schlüter, O. Effect of cold atmospheric pressure plasma processing on quality and shelf life of red currants. LWT-Food Sci. Technol. 2021, 151, 112213. [Google Scholar] [CrossRef]
- Silva, J.L.; Marroquin, E.; Matta, F.B.; Garner, J.O.; Stojanovic, J., Jr. Physicochemical, carbohydrate and sensory characteristics of highbush and rabbiteye blueberry cultivars. J. Sci. Food Agric. 2005, 85, 1815–1821. [Google Scholar] [CrossRef]
- Saftner, R.; Polashock, J.; Ehlenfeldt, M.; Vinyard, B. Instrumental and sensory quality characteristics of blueberry fruit from twelve cultivars. Postharvest Biol. Technol. 2008, 49, 19–26. [Google Scholar] [CrossRef]
- Retamales, J.B.; Hancock, J.F. Blueberries, 2nd ed.; Cabi: Oxfordshire, UK, 2018; pp. 343–393. [Google Scholar]
- Won, M.Y.; Lee, S.J.; Min, S.C. Mandarin preservation by microwave-powered cold plasma treatment. Innov. Food Sci. Emerg. Technol. 2017, 39, 25–32. [Google Scholar] [CrossRef]
- Kader, A.A.; Ben-Yehoshua, S. Effects of superatmospheric oxygen levels on postharvest physiology and quality of fresh fruits and vegetables. Postharvest Biol. Technol. 2000, 20, 1–13. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Li, J.; Liu, S.; Zhang, H.; Bai, Y. Effects of dielectric barrier discharge plasma on the inactivation of Zygosaccharomyces rouxii and quality of apple juice. Food Chem. 2018, 254, 201–207. [Google Scholar] [CrossRef] [PubMed]


| Listeria innocua (Log CFU mL−1) | Listeria monocytogenes (Log CFU mL−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| CAP Treatment (s) | ATR pH 5.5 | ATR pH 6.0 | ATR pH 5.5 | ATR pH 6.0 | ||||
| 0 | 5.85 ± 0.02 | A a | 5.90 ± 0.02 | B a | 6.38 ± 0.01 | C a | 6.42 ± 0.02 | D a |
| 15 | 5.67 ± 0.03 | A ab | 5.86 ± 0.04 | B a | 6.30 ± 0.01 | C a | 6.40 ± 0.01 | C ab |
| 30 | 5.58 ± 0.04 | A bc | 5.67 ± 0.02 | B b | 6.15 ± 0.02 | C b | 6.22 ± 0.04 | C bc |
| 45 | 5.57 ± 0.02 | A c | 5.83 ± 0.01 | B b | 5.94 ± 0.02 | C b | 6.17 ± 0.01 | C cd |
| 60 | 5.45 ± 0.01 | A c | 5.57 ± 0.02 | A c | 5.84 ± 0.02 | B c | 6.14 ± 0.01 | C d |
| CAP Treatment (s) | Day 0 | Day 1 | Day 4 | Day 7 | Day 11 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2.48 ± 0.20 | A | a | 2.85 ± 0.06 | A | a | 2.17 ± 0.03 | A | a | 2.25 ± 0.08 | A | a | 2.42 ± 0.07 | A | a |
| 15 | 2.76 ± 0.22 | A | a | 1.45 ± 0.15 | B | b | <1.00 ± 0.00 | B | b | 1.30 ± 0.10 | B | bc | 3.01 ± 0.09 | A | a |
| 30 | 1.30 ± 0.10 | A | b | 2.60 ± 0.04 | C | a | 1.70 ± 0.17 | ABC | a | 1.30 ± 0.10 | B | bc | 1.07 ± 0.97 | B | b |
| 45 | <1.00 ± 0.00 | A | b | 1.20 ± 0.10 | A | b | <1.00 ± 0.00 | A | b | <1.00 ± 0.00 | A | c | 2.00 ± 0.00 | B | ab |
| 60 | <1.00 ± 0.00 | A | b | 2.33 ± 0.06 | B | a | <1.00 ± 0.00 | A | b | 1.40 ± 0.17 | B | ab | 2.27 ± 0.03 | B | ab |
| CAP Treatment (s) | Day 0 | Day 1 | Day 4 | Day 7 | Day 11 |
|---|---|---|---|---|---|
| 0 | <1.00 ± 0.00 | 1.30 ± 0.10 | 1.69 ± 0.09 | <1.00 ± 0.00 | 1.30 ± 0.00 |
| 15 | <1.00 ± 0.00 | <1.00 ± 0.00 | <1.00 ± 0.00 | 1.20 ± 0.10 | 1.40 ± 0.17 |
| 30 | <1.00 ± 0.00 | 1.20 ± 0.10 | 1.60 ± 0.10 | 1.30 ± 0.10 | 1.30 ± 0.10 |
| 45 | <1.00 ± 0.00 | <1.00 ± 0.00 | <1.00 ± 0.00 | <1.00 ± 0.00 | 1.30 ± 0.10 |
| 60 | 1.45 ± 0.15 | 1.30 ± 0.10 | <1.00 ± 0.00 | 1.30 ± 0.10 | 1.30 ± 0.10 |
| CAP Treatment (s) | Day 0 | Day 1 | Day 4 | Day 7 | Day 11 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 186.59 ± 65.54 | A | a | 177.55 ± 64.93 | A | a | 215.97 ± 61.37 | AB | a | 200.11 ± 79.92 | A | a | 296.57 ± 80.87 | B | a |
| 15 | 207.41 ± 56.19 | AB | a | 193.61 ± 47.60 | A | a | 268.77 ± 65.99 | AB | a | 264.88 ± 82.43 | AB | a | 285.45 ± 94.11 | B | a |
| 30 | 218.95 ± 56.83 | A | a | 185.56 ± 58.11 | AB | a | 231.42 ± 63.20 | ABC | a | 276.23 ± 65.02 | BC | a | 316.03 ± 61.10 | C | a |
| 45 | 179.60 ± 54.05 | A | a | 138.89 ± 40.57 | A | a | 200.85 ± 46.54 | AB | a | 279.62 ± 98.92 | B | a | 283.87 ± 84.11 | B | a |
| 60 | 158.04 ± 35.01 | A | a | 145.95 ± 46.24 | A | a | 203.73 ± 70.85 | AB | a | 271.72 ± 70.72 | BC | a | 315.59 ± 51.48 | C | a |
| Color Dimension | CAP Treatment (s) | Day 0 | Day 1 | Day 4 | Day 7 | Day 11 |
|---|---|---|---|---|---|---|
| L* | 0 | 43.70 ± 0.59 | 43.60 ± 0.10 | 43.54 ± 0.44 | 43.36 ± 0.51 | 42.42 ± 0.41 |
| 15 | 43.70 ± 0.43 | 44.92 ± 0.32 | 42.94 ± 0.30 | 44.80 ± 0.58 | 42.56 ± 0.40 | |
| 30 | 44.77 ± 0.57 | 43.64 ± 0.16 | 43.27 ± 0.12 | 43.13 ± 0.27 | 40.81 ± 0.85 | |
| 45 | 44.07 ± 0.16 | 43.21 ± 0.45 | 42.58 ± 0.37 | 43.17 ± 0.67 | 40.03 ± 0.59 | |
| 60 | 43.30 ± 0.10 | 43.06 ± 0.16 | 39.61 ± 0.23 | 40.66 ± 0.49 | 38.79 ± 0.46 | |
| a* | 0 | 0.04 ± 0.17 | 0.09 ± 0.23 | 0.82 ± 0.1 | 0.63 ± 0.07 | 0.2 ± 0.1 |
| 15 | 0.23 ± 0.07 | −0.09 ± 0.10 | 0.41 ± 0.12 | 0.27 ± 0.10 | 0.28 ± 0.12 | |
| 30 | −0.17 ± 0.10 | 0.07 ± 0.10 | 0.21 ± 0.06 | 0.47 ± 0.19 | 0.40 ± 0.21 | |
| 45 | −0.07 ± 0.03 | −0.09 ± 0.17 | 0.19 ± 0.17 | 0.19 ± 0.15 | 0.76 ± 0.37 | |
| 60 | 0.43 ± 0.23 | 0.25 ± 0.03 | 1.15 ± 0.14 | 0.56 ± 0.22 | 0.89 ± 0.15 | |
| b* | 0 | −2.65 ± 0.19 | −2.55 ± 0.20 | −2.5 ± 0.44 | −2.42 ± 0.1 | −2.49 ± 0.04 |
| 15 | −2.23 ± 0.21 | 2.82 ± 0.04 | −1.91 ± 0.26 | −2.05 ± 0.08 | −2.09 ± 0.24 | |
| 30 | −2.39 ± 0.16 | −2.40 ± 0.22 | −1.66 ± 0.38 | −1.56 ± 0.11 | −1.94 ± 0.25 | |
| 45 | −2.32 ± 0.06 | −2.23 ± 0.11 | −1.84 ± 0.17 | −1.55 ± 0.17 | −1.03 ± 0.50 | |
| 60 | −2.14 ± 0.18 | −1.75 ± 0.11 | −0.79 ± 0.48 | −0.76 ± 0.06 | −1.16 ± 0.14 | |
| 0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| 15 | 0.68 ± 0.32 | 1.40 ± 0.11 | 0.96 ± 0.16 | 1.53 ± 0.34 | 0.45 ± 0.18 | |
| * | 30 | 1.38 ± 0.27 | 0.78 ± 0.70 | 0.93 ± 0.44 | 1.31 ± 0.61 | 1.82 ± 0.35 |
| 45 | 0.72 ± 0.22 | 0.68 ± 0.25 | 1.34 ± 0.13 | 1.02 ± 0.05 | 2.89 ± 0.15 | |
| 60 | 0.84 ± 0.34 | 1.01 ± 0.27 | 4.34 ± 0.42 | 3.17 ± 0.04 | 3.93 ± 0.28 | |
| 0 | 2.65 ± 0.19 | 2.56 ± 0.20 | 2.64 ± 0.39 | 2.50 ± 0.08 | 2.50 ± 0.04 | |
| 15 | 2.24 ± 0.20 | 2.82 ± 0.04 | 1.96 ± 0.23 | 2.07 ± 0.06 | 2.11 ± 0.23 | |
| C* | 30 | 2.40 ± 0.17 | 2.40 ± 0.22 | 1.68 ± 0.37 | 1.64 ± 0.04 | 1.99 ± 0.20 |
| 45 | 2.32 ± 0.06 | 2.24 ± 0.11 | 1.86 ± 0.15 | 1.57 ± 0.15 | 1.37 ± 0.20 | |
| 60 | 2.19 ± 0.13 | 1.77 ± 0.11 | 1.44 ± 0.21 | 0.96 ± 0.09 | 1.47 ± 0.03 |
| CAP Treatment (s) | Day 0 | Day 1 | Day 4 | Day 7 | Day 11 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.58 ± 0.01 | AB | a | 0.64 ± 0.02 | A | a | 0.57 ± 0.01 | AB | a | 0.65 ± 0.06 | A | a | 0.48 ± 0.02 | B | a |
| 15 | 0.53 ± 0.02 | A | ab | 0.57 ± 0.04 | A | a | 0.59 ± 0.10 | A | a | 0.53 ± 0.03 | A | abc | 0.54 ± 0.02 | A | a |
| 30 | 0.47 ± 0.01 | C | ab | 0.67 ± 0.01 | A | a | 0.53 ± 0.01 | B | a | 0.59 ± 0.06 | AB | ab | 0.50 ± 0.09 | BC | a |
| 45 | 0.48 ± 0.02 | B | ab | 0.60 ± 0.05 | A | a | 0.60 ± 0.03 | A | a | 0.43 ± 0.02 | B | c | 0.51 ± 0.02 | AB | a |
| 60 | 0.43 ± 0.03 | C | b | 0.65 ± 0.03 | A | a | 0.63 ± 0.08 | A | a | 0.50 ± 0.03 | BC | bc | 0.59 ± 0.04 | AB | a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concha-Meyer, A.A.; González-Esparza, A.; Cullen, P.J.; Veloso, F.; Favre, M.; Valenzuela, J.C.; Toloza, L.; Niemira, B.A. Survival of Listeria Strains and Shelf Life Determination of Fresh Blueberries (Vaccinium corymbosum) Treated with Cold Atmospheric Plasma. Foods 2024, 13, 822. https://doi.org/10.3390/foods13060822
Concha-Meyer AA, González-Esparza A, Cullen PJ, Veloso F, Favre M, Valenzuela JC, Toloza L, Niemira BA. Survival of Listeria Strains and Shelf Life Determination of Fresh Blueberries (Vaccinium corymbosum) Treated with Cold Atmospheric Plasma. Foods. 2024; 13(6):822. https://doi.org/10.3390/foods13060822
Chicago/Turabian StyleConcha-Meyer, Anibal A., Alexandra González-Esparza, Patrick J. Cullen, Felipe Veloso, Mario Favre, Julio C. Valenzuela, Lorena Toloza, and Brendan A. Niemira. 2024. "Survival of Listeria Strains and Shelf Life Determination of Fresh Blueberries (Vaccinium corymbosum) Treated with Cold Atmospheric Plasma" Foods 13, no. 6: 822. https://doi.org/10.3390/foods13060822
APA StyleConcha-Meyer, A. A., González-Esparza, A., Cullen, P. J., Veloso, F., Favre, M., Valenzuela, J. C., Toloza, L., & Niemira, B. A. (2024). Survival of Listeria Strains and Shelf Life Determination of Fresh Blueberries (Vaccinium corymbosum) Treated with Cold Atmospheric Plasma. Foods, 13(6), 822. https://doi.org/10.3390/foods13060822