Ethanol Extracts from Torreya grandis Seed Have Potential to Reduce Hyperuricemia in Mouse Models by Influencing Purine Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Detection and Quantification of Total Flavonoids and Phenols in EST
2.2.3. Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis of EST
2.2.4. 1-Diphenyl-2-Picrylhydrazyl (DPPH) Radical-Scavenging Analysis
2.2.5. Determination of Xanthine Oxidase Activity Inhibition In Vitro
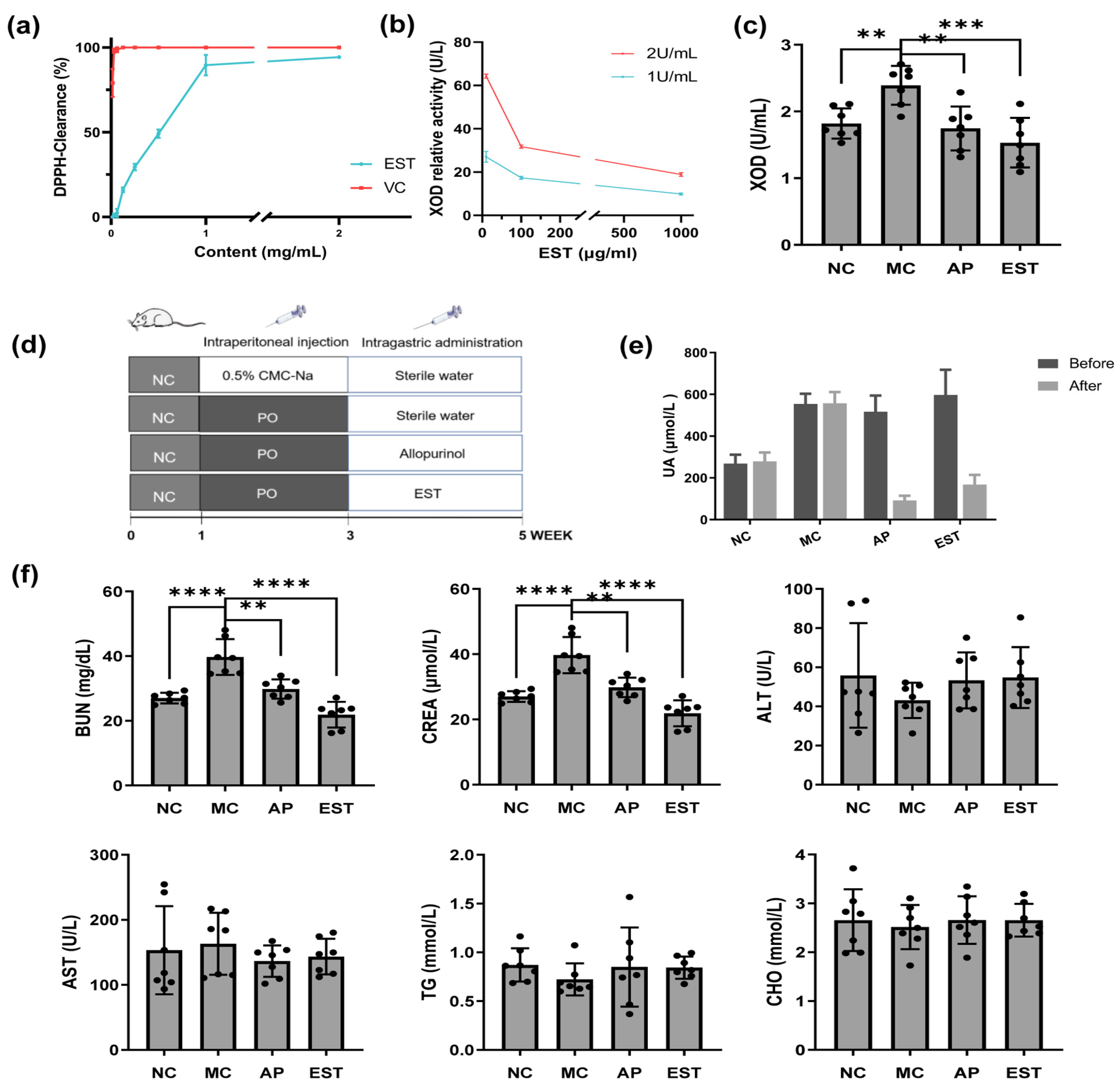
2.2.6. Animal Experimental Designs
2.2.7. Biochemical Analysis of Mouse Serum, Liver, and Kidney
2.2.8. Histopathological Examination
2.2.9. Determination of Xanthine Oxidase Inhibition In Vivo
2.2.10. Western Blotting Analysis
2.2.11. Gut Microbiota Analysis
2.2.12. Statistical Analysis
3. Results
3.1. Chemical Constituents in EST
3.2. In Vitro Activity of EST as ROS Scavenger and Xanthine Oxidase Inhibitor
3.3. EST Treatment Attenuated Hyperuricemia Symptoms in Model Mice
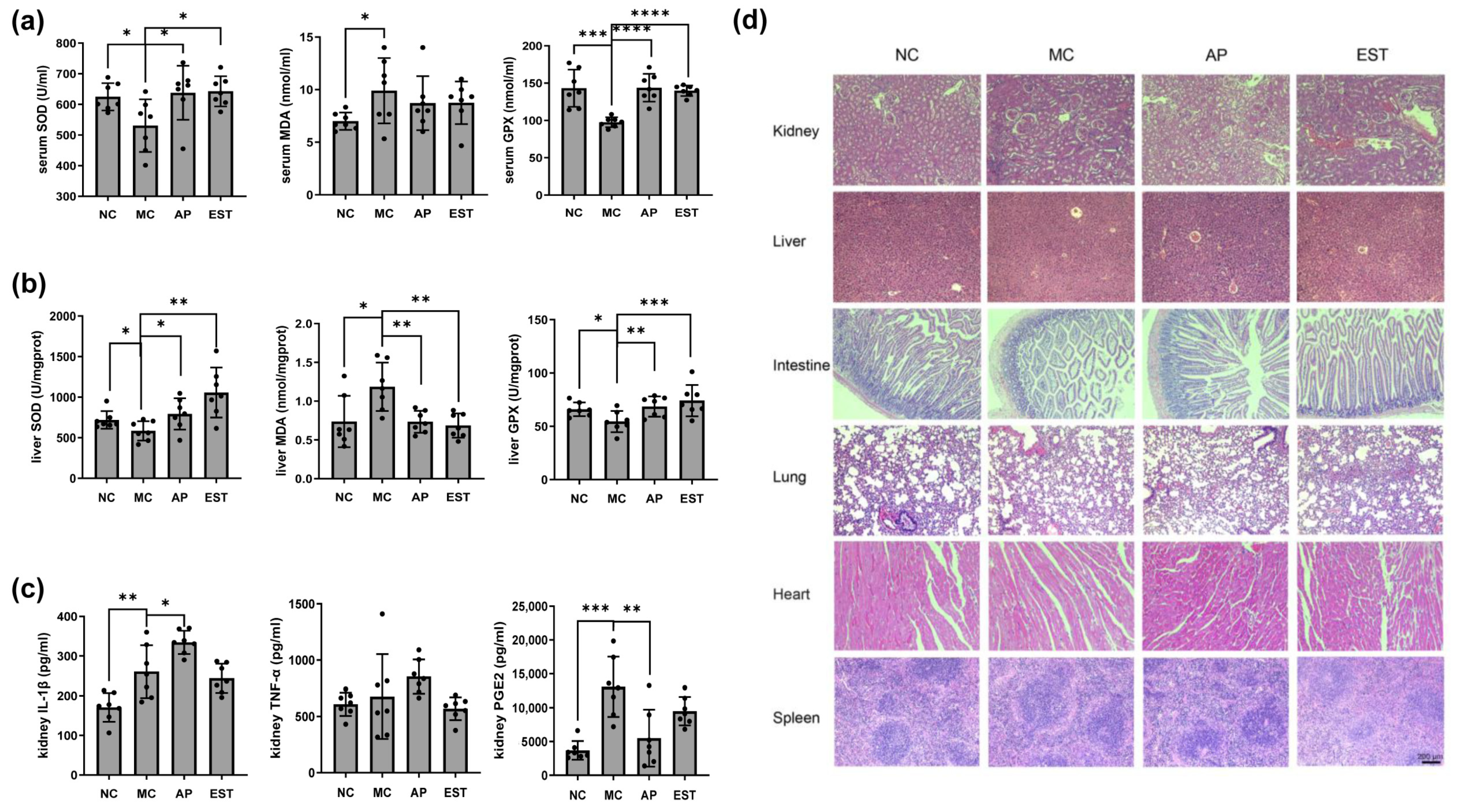
3.4. EST Improved the Liver and Kidney Enteritis Index IL-1β, TNF-α, PGE2, and Histopathological Changes
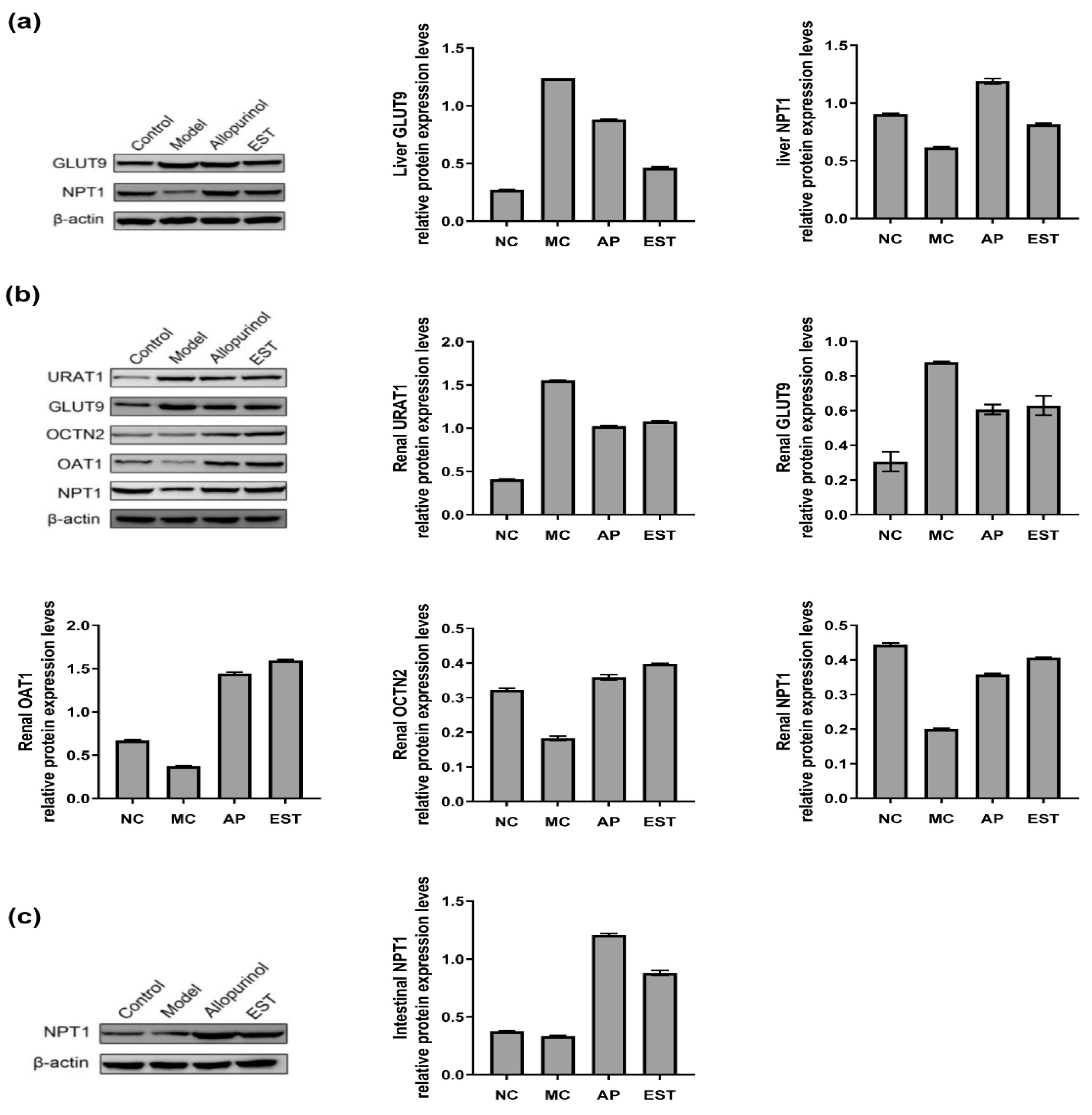
3.5. The Effects of EST on Protein Levels of Liver, Kidney and Intestine URAT1, GLUT9, OAT1, OCTN2 and NPT1 in Hyperuricemia Mice
3.6. Regulate the Effect of Intestinal Flora in Hyperuricemia Model Mice
3.6.1. Impact on Flora Diversity
3.6.2. Impact on the Classification and Composition of Intestinal Flora
3.6.3. Impact on Key Systemic Types of Intestinal Flora
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Company | Description | Catalog No. |
|---|---|---|
| Bioworld Technology (Nanjing, China) | Rabbit β-Actin antibody | KL002-1-1 |
| Goat anti-rabbit IgG antibody | BS13278 | |
| ABclonal Technology (Nanjing, China) | Rabbit mGLUT9 antibody | A14592 |
| Rabbit mURAT1 antibody | A5118 | |
| Rabbit mOCTN2 antibody | A1676 | |
| Rabbit mOAT1 antibody | A3184 | |
| Rabbit mNPT1 antibody | A16642 |
| Name | Molecular Weight | Precursor Ion | Product Ion | Cone V | Collision V | Calculated Concentration (ng/mL) |
|---|---|---|---|---|---|---|
| Geniposidic acid | 374.34 | 374.8 | 149 | 41.8 | 30.1 | 3430.00 ± 2090.14 |
| Caffeine | 194.19 | 195 | 138.2 | 68.8 | 28 | 2289.33 ± 2347.51 |
| Trans-cinnamic acid | 148.16 | 148.94 | 51.07 | 18 | 42 | 1970.33 ± 1540.95 |
| β-Sitosterol | 414.71 | 415 | 73 | 138.4 | 54.5 | 1476.33 ± 759.03 |
| Sinapic acid | 224.21 | 225.15 | 119.2 | 20 | 20 | 1310.00 ± 467.65 |
| Phenylalanine | 165.19 | 165.9 | 77.02 | 24 | 32 | 1002.00 ± 308.31 |
| Resveratrol | 228.25 | 229 | 107.1 | 10 | 30 | 995.33 ± 4.04 |
| Sinapine | 310.36 | 310.29 | 175.1 | 30 | 30 | 988.33 ± 0.58 |
| Syringate | 198.18 | 199.12 | 140.07 | 30 | 15 | 952.67 ± 280.30 |
| Vanillic acid | 168.15 | 169 | 93 | 25 | 40 | 881.67 ± 258.67 |
| Caffeic acid | 180.15 | 181 | 117 | 56.72 | 33 | 878.00 ± 207.86 |
| Vanillin | 152.17 | 152.88 | 93.07 | 26 | 14 | 718.67 ± 273.54 |
| Apigenin-7-o-glucoside | 432.38 | 432.2 | 119.2 | 44 | 25 | 698.67 ± 12.70 |
| Liquiritin | 418.4 | 418.99 | 137.12 | 10 | 40 | 625.00 ± 339.48 |
| Coumarin | 146.14 | 146.92 | 77.09 | 34 | 22 | 264.00 ± 251.17 |
| Deacetylasperulosidic acid | 390.34 | 391.1 | 149 | 46 | 45 | 239.00 ± 17.32 |
| 1-deoxynojirimycin | 163.17 | 164.14 | 68.89 | 30 | 16 | 227.33 ± 40.99 |
| Ligustilide | 190.24 | 191 | 173.2 | 73 | 20 | 223.00 ± 17.32 |
| Arecoline hydrobromide | 155.19 | 156 | 44 | 31.2 | 31.2 | 222.00 ± 6.93 |
| Arecoline standard | 155.19 | 156.1 | 44 | 50.3 | 31 | 214.00 ± 36.37 |
| Naringenin chalcone | 272 | 272.97 | 147.1 | 20 | 20 | 173.40 ± 89.37 |
| Name | Molecular Weight | Precursor Ion | Product Ion | Cone V | Collision V | Calculated Concentration (ng/mL) |
|---|---|---|---|---|---|---|
| Biochanin A | 284.26 | 283.1 | 239 | −55 | −38 | 68,779.33 ± 59,271.93 |
| Catechin | 290.27 | 289.1 | 245 | −84.7 | −18.8 | 15,470.00 ± 12,522.73 |
| Epicatechin gallate | 442.37 | 440.89 | 124.97 | −44 | −40 | 15,416.67 ± 12,441.90 |
| 4-hydroxybenzoic acid | 138.12 | 136.9 | 93 | −44.7 | −19.9 | 11,282.50 ± 15,722.52 |
| Xanthophyll | 568.87 | 567.9 | 35 | −25 | −33 | 8750.00 ± 4070.32 |
| Dihydromyricetin | 320.25 | 319.1 | 175 | −120 | −23.7 | 6716.67 ± 1737.82 |
| Emodin | 270.24 | 268.9 | 255 | −71.1 | −36.5 | 4126.67 ± 3071.50 |
| Carnosic acid | 332.43 | 331.02 | 215.04 | −56 | −54 | 3855.33 ± 3222.12 |
| 4-methoxycinnamic acid | 178.18 | 177.1 | 132.9 | −58 | −16.2 | 2158.00 ± 1423.75 |
| Procyanidin b1 | 578.5 | 576.89 | 288.99 | −48 | −24 | 1916.67 ± 1587.71 |
| Danshensu | 198.17 | 197 | 134.9 | −72.1 | −26 | 1026.67 ± 5.77 |
| Gallocatechin | 306.27 | 304.98 | 124.98 | −62 | −18 | 954.00 ± 550.79 |
| Chlorogenic acid | 354.31 | 353 | 191 | −24 | −20.1 | 950.00 ± 50.23 |
| Protocatechuic acid | 154.12 | 152.86 | 80.9 | −30 | −20 | 949.67 ± 31.75 |
| Geniposidic acid | 374.34 | 373.1 | 123.1 | −50 | −30.92 | 858.67 ± 5.77 |
| EGCG | 458.37 | 456.89 | 125.04 | −34 | −40 | 768.33 ± 382.21 |
| Epicatechin | 290.27 | 288.97 | 109.04 | −40 | −22 | 738.00 ± 420.23 |
| Isoquercitrin | 464.38 | 463 | 254.9 | −60 | −38 | 704.03 ± 547.27 |
| Hyperoside | 464.38 | 462.84 | 254.94 | −66 | −42 | 665.00 ± 322.07 |
| Isoliquiritin | 418.39 | 417.2 | 255 | −82.1 | −19.9 | 568.33 ± 210.73 |
| Ethyl vanillin | 166.17 | 164.95 | 92.05 | −8 | −18 | 565.33 ± 267.89 |
| Guaiaverin | 434.35 | 432.87 | 270.94 | −72 | −40 | 543.33 ± 154.09 |
| Baicalein | 270.24 | 268.89 | 195.06 | −52 | −26 | 513.67 ± 42.15 |
| Taxifolin | 304.25 | 302.84 | 124.98 | −2 | −20 | 450.33 ± 22.05 |
| Kaempferol | 286.23 | 285.1 | 150.9 | −95 | −30 | 444.67 ± 206.69 |
| 2-Bromophenol | 173.01 | 172.81 | 80.9 | −24 | −14 | 433.67 ± 206.69 |
| Benzoic acid | 122.12 | 121 | 77 | −49.2 | −16.9 | 421.33 ± 170.90 |
| Salicylic acid | 138.12 | 136.96 | 65.05 | −8 | −24 | 419.67 ± 494.79 |
| Ethyl gallate | 198.17 | 196.95 | 124.08 | −46 | −22 | 418.00 ± 55.00 |
| Myricetin | 318.2 | 316.79 | 150.97 | −52 | −24 | 417.33 ± 136.25 |
| Luteolin | 286.23 | 285.11 | 133.09 | −58 | −30 | 393.67 ± 78.52 |
| Morin | 302.24 | 300.83 | 124.98 | −42 | −20 | 387.67 ± 225.74 |
| Licochalcone-A | 338.4 | 337.3 | 120 | −86.1 | −39.8 | 361.67 ± 26.56 |
| Pterostilbene | 256.3 | 255.1 | 239.9 | −76.9 | −28.4 | 327.67 ± 144.81 |
| Quercetin | 302.24 | 300.83 | 150.99 | −10 | −20 | 317.00 ± 189.60 |
| Aromadendrin | 288.25 | 286.84 | 124.98 | −2 | −20 | 310.33 ± 11.55 |
| Ginsenoside F1 | 638.87 | 637.6 | 475.3 | −103.1 | −33 | 260.00 ± 77.94 |
| P-coumaric acid | 164.16 | 162.87 | 65.04 | −18 | −38 | 255.33 ± 44.41 |
| Lithospermic acid B | 718.6 | 717.5 | 519.2 | −62.7 | −29.8 | 226.60 ± 173.41 |
| Apigenin | 270.24 | 268.89 | 107.03 | −62 | −28 | 223.33 ± 23.67 |
| 18β-Glycyrrhetinic Acid | 470.69 | 469.4 | 425.4 | −98.1 | −49.8 | 203.90 ± 90.24 |
| Quinic acid | 192.16 | 191 | 85.08 | −48 | −20 | 208.30 ± 144.97 |
| Ginsenoside Rg2 | 785.03 | 783.5 | 637.4 | −141.2 | −42 | 208.33 ± 68.70 |
| Cynaroside | 448.37 | 446.89 | 107.03 | −82 | −56 | 202.00 ± 36.37 |
| Daidzein | 254.24 | 252.89 | 91.1 | −62 | −38 | 96.83 ± 48.79 |
| Genistein | 270.24 | 268.89 | 132.91 | −52 | −32 | 63.50 ± 1.21 |
References
- Feng, S.; Wu, S.; Xie, F.; Yang, C.S.; Shao, P. Natural compounds lower uric acid levels and hyperuricemia: Molecular mechanisms and prospective. Trends Food Sci. Technol. 2022, 123, 87–102. [Google Scholar] [CrossRef]
- Bardin, T.; Richette, P. Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, H.; Lan, Q.; Chen, Y.; Liao, W.; Guo, X. Composite Dietary Antioxidant Index Is Negatively Associated with Hyperuricemia in US Adults: An Analysis of NHANES 2007–2018. Int. J. Endocrinol. 2023, 2023, 6680229. [Google Scholar] [CrossRef]
- Almuqrin, A.; Alshuweishi, Y.A.; Alfaifi, M.; Daghistani, H.; Al-Sheikh, Y.A.; Alfhili, M.A. Prevalence and association of hyperuricemia with liver function in Saudi Arabia: A large cross-sectional study. Ann. Saudi Med. 2024, 44, 18–25. [Google Scholar] [CrossRef]
- Otaki, Y.; Konta, T.; Ichikawa, K.; Fujimoto, S.; Iseki, K.; Moriyama, T.; Yamagata, K.; Tsuruya, K.; Narita, I.; Kondo, M.; et al. Possible burden of hyperuricaemia on mortality in a community-based population: A large-scale cohort study. Sci. Rep. 2021, 11, 8999. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-F.; Grainge, M.J.; Mallen, C.; Zhang, W.; Doherty, M. Rising burden of gout in the UK but continuing suboptimal management: A nationwide population study. Ann. Rheum. Dis. 2015, 74, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Dehlin, M.; Jacobsson, L.; Roddy, E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 2020, 16, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, Z.; Huang, Z.; Zhou, W.; Li, Z.; Li, X.; Sun, F. The prevalence of gout in mainland China from 2000 to 2016: A systematic review and meta-analysis. J. Public Health 2017, 25, 521–529. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kwak, S.G.; Lee, H.; Kim, S.-K.; Choe, J.-Y.; Park, S.-H. Prevalence and incidence of gout in Korea: Data from the national health claims database 2007–2015. Rheumatol. Int. 2017, 37, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Brucato, A.; Cianci, F.; Carnovale, C. Management of hyperuricemia in asymptomatic patients: A critical appraisal. Eur. J. Intern. Med. 2020, 74, 8–17. [Google Scholar] [CrossRef]
- Vareldzis, R.; Perez, A.; Reisin, E. Hyperuricemia: An Intriguing Connection to Metabolic Syndrome, Diabetes, Kidney Disease, and Hypertension. Curr. Hypertens. Rep. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Li, M.; Hou, W.; Zhang, X.; Hu, L.; Tang, Z. Hyperuricemia and risk of stroke: A systematic review and meta-analysis of prospective studies. Atherosclerosis 2014, 232, 265–270. [Google Scholar] [CrossRef]
- Kaewput, W.; Thongprayoon, C.; Rangsin, R.; Ruangkanchanasetr, P.; Bathini, T.; Mao, M.A.; Cheungpasitporn, W. Association between serum uric acid and chronic kidney disease in patients with hypertension: A multicenter nationwide cross-sectional study. J. Evid.-Based Med. 2019, 12, 235–242. [Google Scholar] [CrossRef]
- Flores, M.; Rodríguez, J.A.; Delgado, A.; García-Trabanino, R. Prevalence and association of chronic kidney disease, diabetes, hypertension, and hyperuricemia in an adult urban population of El Salvador. Nefrol. Latinoam. 2017, 14, 137–143. [Google Scholar] [CrossRef]
- Bach, M.H.; Simkin, P.A. Uricosuric drugs: The once and future therapy for hyperuricemia? Curr. Opin. Rheumatol. 2014, 26, 169–175. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Pirro, M.; Watts, G.F.; Mikhailidis, D.P.; Banach, M.; Sahebkar, A. Effects of Allopurinol on Endothelial Function: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. Drugs 2018, 78, 99–109. [Google Scholar] [CrossRef]
- Liu, Y.; Jarman, J.B.; Low, Y.S.; Augustijn, H.E.; Huang, S.; Chen, H.; DeFeo, M.E.; Sekiba, K.; Hou, B.-H.; Meng, X.; et al. A widely distributed gene cluster compensates for uricase loss in hominids. Cell 2023, 186, 3400–3413.e20. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, Z.; Lu, Y. The potential of probiotics in the amelioration of hyperuricemia. Food Funct. 2022, 13, 2394–2414. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, X.; Zhang, L.; Meng, F.; Zhou, L.; Pang, X.; Lu, Z.; Lu, Y. Lacticaseibacillus rhamnosus Fmb14 prevents purine induced hyperuricemia and alleviate renal fibrosis through gut-kidney axis. Pharmacol. Res. 2022, 182, 106350. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, P.; Hu, X.; Cao, W.; Liu, P.; Han, H.; Jin, W.; Li, X. Probiotic Limosilactobacillus fermentum GR-3 ameliorates human hyperuricemia via degrading and promoting excretion of uric acid. iScience 2022, 25, 105198. [Google Scholar] [CrossRef]
- Kasahara, K.; Kerby, R.L.; Zhang, Q.; Pradhan, M.; Mehrabian, M.; Lusis, A.J.; Bergström, G.; Bäckhed, F.; Rey, F.E. Gut bacterial metabolism contributes to host global purine homeostasis. Cell Host Microbe 2023, 31, 1038–1053.e10. [Google Scholar] [CrossRef]
- Yin, H.; Liu, N.; Chen, J. The Role of the Intestine in the Development of Hyperuricemia. Front. Immunol. 2022, 13, 845684. [Google Scholar] [CrossRef]
- Tamura, Y.; Morimoto, C.; Kuribayashi-Okuma, E.; Uchida, S.; Hosoyamada, M.; Nakagawa, T.; Shibata, S. Melinjo seed extract stimulates intestinal ABCG2 expression to reduce serum uric acid levels in hyperuricemic rats. J. Funct. Foods 2021, 87, 104849. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.; Xu, B.; Wang, Y.; Zhang, C.; Cao, Y.; Xing, X. Research progress on classification, sources and functions of dietary polyphenols for prevention and treatment of chronic diseases. J. Futur. Foods 2023, 3, 289–305. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef]
- Liu, X.; Chen, R.; Shang, Y.; Jiao, B.; Huang, C. Superoxide radicals scavenging and xanthine oxidase inhibitory activity of magnesium lithospermate B from Salvia miltiorrhiza. J. Enzym. Inhib. Med. Chem. 2009, 24, 663–668. [Google Scholar] [CrossRef]
- Wu, Z.; Tian, E.; Chen, Y.; Dong, Z.; Peng, Q. Gut microbiota and its roles in the pathogenesis and therapy of endocrine system diseases. Microbiol. Res. 2023, 268, 127291. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, P.-G.; Liang, W.-Q.; Hu, Y.-J.; Xu, P.; Zhou, J.; Pu, J.-B.; Zhang, H.-J. Luteolin-4′-O-glucoside and its aglycone, two major flavones of Gnaphalium affine D. Don, resist hyperuricemia and acute gouty arthritis activity in animal models. Phytomedicine 2018, 41, 54–61. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chang, T.-C.; Chang, S.-T. A review of antioxidant and pharmacological properties of phenolic compounds in Acacia confusa. J. Tradit. Complement. Med. 2018, 8, 443–450. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.-C.; Zhou, Q.; Yan, J.-X.; Zhang, J.-L.; Su, G.-H. Hypouricemic effect in hyperuricemic mice and xanthine oxidase inhibitory mechanism of dietary anthocyanins from purple sweet potato (Ipomoea batatas L.). J. Funct. Foods 2020, 73, 104151. [Google Scholar] [CrossRef]
- Yuan, L.; Bao, Z.; Ma, T.; Lin, S. Hypouricemia effects of corn silk flavonoids in a mouse model of potassium oxonated-induced hyperuricemia. J. Food Biochem. 2021, 45, e13856. [Google Scholar] [CrossRef]
- Umer, M.; Nisa, M.U.; Ahmad, N.; Rahim, M.A.; Kasankala, L.M. Quantification of quercetin from red onion (Allium cepa L.) powder via high-performance liquid chromatography-ultraviolet (HPLC-UV) and its effect on hyperuricemia in male healthy Wistar albino rats. Food Sci. Nutr. 2023, 12, 1067–1081. [Google Scholar] [CrossRef]
- Lin, X.; Zhou, Q.; Zhou, L.; Sun, Y.; Han, X.; Cheng, X.; Wu, M.; Lv, W.; Wang, J.; Zhao, W. Quinoa (Chenopodium quinoa Willd) Bran Saponins Alleviate Hyperuricemia and Inhibit Renal Injury by Regulating the PI3K/AKT/NFκB Signaling Pathway and Uric Acid Transport. J. Agric. Food Chem. 2023, 71, 6635–6649. [Google Scholar] [CrossRef]
- Zhang, W.; Du, W.; Li, G.; Zhang, C.; Yang, W.; Yang, S.; Feng, Y.; Chen, H. Constituents and Anti-Hyperuricemia Mechanism of Traditional Chinese Herbal Formulae Erding Granule. Molecules 2019, 24, 3248. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Yan, H.; Jiang, X.; Hu, W.; Han, N.; Wang, D. Anti-gouty arthritis and anti-hyperuricemia properties of celery seed extracts in rodent models. Mol. Med. Rep. 2019, 20, 4623–4633. [Google Scholar] [CrossRef]
- Zhang, Z.-C.; Su, G.-H.; Luo, C.-L.; Pang, Y.-L.; Wang, L.; Li, X.; Wen, J.-H.; Zhang, J.-L. Effects of anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8) on the serum uric acid level and xanthine oxidase activity in hyperuricemic mice. Food Funct. 2015, 6, 3045–3055. [Google Scholar] [CrossRef]
- Li, X.; Yan, Z.; Carlström, M.; Tian, J.; Zhang, X.; Zhang, W.; Wu, S.; Ye, F. Mangiferin Ameliorates Hyperuricemic Nephropathy Which Is Associated With Downregulation of AQP2 and Increased Urinary Uric Acid Excretion. Front. Pharmacol. 2020, 11, 49. [Google Scholar] [CrossRef]
- Beatrice, G.; Francesco, G.; Virginia, L.; Domenico, M.; Raffaele, R.; Claudio, V.; He, G.; Ma, Z.; Yin, W. Grandione, a New Heptacyclic Dimeric Diterpene from Torreya grandis Fort. Tetrahedron 1999, 55, 11385–11394. [Google Scholar] [CrossRef]
- He, Z.; Zhu, H.; Li, W.; Zeng, M.; Wu, S.; Chen, S.; Qin, F.; Chen, J. Chemical components of cold pressed kernel oils from different Torreya grandis cultivars. Food Chem. 2016, 209, 196–202. [Google Scholar] [CrossRef]
- Yu, M.; Zeng, M.; Qin, F.; He, Z.; Chen, J. Physicochemical and functional properties of protein extracts from Torreya grandis seeds. Food Chem. 2017, 227, 453–460. [Google Scholar] [CrossRef]
- Chen, B.-Q.; Cui, X.-Y.; Zhao, X.; Zhang, Y.-H.; Piao, H.-S.; Kim, J.-H.; Lee, B.-C.; Pyo, H.-B.; Yun, Y.-P. Antioxidative and acute antiinflammatory effects of Torreya grandis. Fitoterapia 2006, 77, 262–267. [Google Scholar] [CrossRef]
- Huang, Y.J.; Wang, J.; Li, G.; Zheng, Z.; Su, W. Antitumor and antifungal activities in endophytic fungi isolated from pharmaceutical plants Taxus mairei, Cephalataxus fortunei and Torreya grandis. FEMS Immunol. Med. Microbiol. 2001, 31, 163–167. [Google Scholar] [CrossRef]
- Intisar, R.A.; Mufeed, J.E.; Maha, F.M. Novel Natural Anti Gout Medication Extract from Momdica charantia. J. Nat. Sci. Res. 2014, 4, 2224–3186. [Google Scholar]
- Zou, F.; Li, X.; Yang, R.; Zhang, R.; Zhao, X. Effects and underlying mechanisms of food polyphenols in treating gouty arthritis: A review on nutritional intake and joint health. J. Food Biochem. 2022, 46, e14072. [Google Scholar] [CrossRef]
- Ao, G.-Z.; Zhou, M.-Z.; Li, Y.-Y.; Li, S.-N.; Wang, H.-N.; Wan, Q.-W.; Li, H.-Q.; Hu, Q.-H. Discovery of novel curcumin derivatives targeting xanthine oxidase and urate transporter 1 as anti-hyperuricemic agents. Bioorganic Med. Chem. 2017, 25, 166–174. [Google Scholar] [CrossRef]
- Sun, Z.-R.; Liu, H.-R.; Hu, D.; Fan, M.-S.; Wang, M.-Y.; An, M.-F.; Zhao, Y.-L.; Xiang, Z.-M.; Sheng, J. Ellagic Acid Exerts Beneficial Effects on Hyperuricemia by Inhibiting Xanthine Oxidase and NLRP3 Inflammasome Activation. J. Agric. Food Chem. 2021, 69, 12741–12752. [Google Scholar] [CrossRef]
- Tanaka, T.; Milaneschi, Y.; Zhang, Y.; Becker, K.G.; Zukley, L.; Ferrucci, L. A double blind placebo controlled randomized trial of the effect of acute uric acid changes on inflammatory markers in humans: A pilot study. PLoS ONE 2017, 12, e0181100. [Google Scholar] [CrossRef]
- Ejaz, A.A.; Dass, B.; Kambhampati, G.; Ejaz, N.I.; Maroz, N.; Dhatt, G.S.; Arif, A.A.; Faldu, C.; Lanaspa, M.A.; Shah, G.; et al. Lowering serum uric acid to prevent acute kidney injury. Med. Hypotheses 2012, 78, 796–799. [Google Scholar] [CrossRef]
- Hakoda, M.; Ichida, K. Genetic Basis of the Epidemiological Features and Clinical Significance of Renal Hypouricemia. Biomedicines 2022, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, G. Drug transport by Organic Anion Transporters (OATs). Pharmacol. Ther. 2012, 136, 106–130. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, T.; Ci, X.; Zhao, F.; Sun, Y.; Li, Y.; Liu, R.; Wu, W.; Yi, X.; Liu, C. The effect of polymorphism of uric acid transporters on uric acid transport. J. Nephrol. 2019, 32, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Das Gupta, E.; Sakthiswary, R.; Lee, S.L.; Wong, S.F.; Hussein, H.; Gun, S.C. Clinical significance of SLC2A9/GLUT9 rs11722228 polymorphisms in gout. Int. J. Rheum. Dis. 2016, 25, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Matsuo, H.; Kawamura, Y.; Nagamori, S.; Nishiyama, T.; Wei, L.; Nakayama, A.; Nakamura, T.; Sakiyama, M.; Takada, T.; et al. NPT1/SLC17A1 is a renal urate exporter in humans and its common gain-of-function variant decreases the risk of renal underexcretion gout. Arthritis Rheumatol. 2015, 67, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Han, J.; Tang, S.; Bao, W.; Lu, C.; Zhou, J.; Ming, T.; Li, Y.; Su, X. Comparisons of protective effects between two sea cucumber hydrolysates against diet induced hyperuricemia and renal inflammation in mice. Food Funct. 2020, 11, 1074–1086. [Google Scholar] [CrossRef]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef] [PubMed]
- Purnell, D.M.; Bartlett, G.L.; Kreider, J.W.; Biro, T.G.; Kontra, J. Comparative Antitumor Effects of Corynebacterium parvum, Bordetella pertussis, Bacillus Calmette-Guerin, and Levamisole Alone or in Combination with Cyclophosphamide in the CaD2 Murine Mammary Adenocarcinoma System. Cancer Res. 1979, 39, 4838–4842. [Google Scholar]
- Durand, G.A.; Pham, T.; Ndongo, S.; Traore, S.I.; Dubourg, G.; Lagier, J.-C.; Michelle, C.; Armstrong, N.; Fournier, P.-E.; Raoult, D.; et al. Blautia massiliensis sp. nov., isolated from a fresh human fecal sample and emended description of the genus Blautia. Anaerobe 2017, 43, 47–55. [Google Scholar] [CrossRef]
- Miller, J.C.; Babu, A.K.S.; Petersen, C.; Wankhade, U.D.; Robeson, M.S.; Putich, M.N.; Mueller, J.E.; O’Farrell, A.S.; Cho, J.M.; Chintapalli, S.V.; et al. Gut Microbes Are Associated with the Vascular Beneficial Effects of Dietary Strawberry on Metabolic Syndrome-Induced Vascular Inflammation. Mol. Nutr. Food Res. 2022, 66, e2200112. [Google Scholar] [CrossRef]


| Polyphenols | Calculated Concentration (mg/L) |
|---|---|
| Biochanin A | 68.78 ± 59.27 |
| Catechin | 15.47 ± 12.52 |
| Epicatechin gallate | 15.42 ± 12.44 |
| 4-hydroxybenzoic acid | 11.28 ± 15.72 |
| Xanthophyll | 8.75 ± 4.07 |
| Dihydromyricetin | 6.72 ± 1.74 |
| Emodin | 4.13 ± 3.07 |
| Carnosic acid | 3.86 ± 3.22 |
| Geniposidic acid | 3.43 ± 2.09 |
| Caffeine | 2.29 ± 2.35 |
| 4-methoxycinnamic acid | 2.16 ± 1.42 |
| Trans-cinnamic acid | 1.97 ± 1.54 |
| Procyanidin b1 | 1.92 ± 1.59 |
| β-Sitosterol | 1.48 ± 0.76 |
| Sinapic acid | 1.31 ± 0.47 |
| Salvianic acid A | 1.03 ± 0.06 |
| Phenylalanine | 1.00 ± 0.03 |
| Resveratrol | 0.99 ± 0.04 |
| Sinapine | 0.98 ± 0.01 |
| Gallocatechin | 0.95 ± 0.01 |
| Group | Heart (%) | Liver (%) | Spleen (%) | Lung (%) | Kidney (%) |
|---|---|---|---|---|---|
| NC | 0.52 ± 0.06 * | 4.40 ± 0.39 | 0.36 ± 0.07 | 0.61 ± 0.06 | 1.30 ± 0.09 * |
| MC | 0.61 ± 0.04 | 4.39 ± 0.44 | 0.42 ± 0.23 | 0.59 ± 0.09 | 1.45 ± 0.13 |
| AP | 0.54 ± 0.04 * | 4.39 ± 0.56 | 0.39 ± 0.07 | 0.53 ± 0.04 | 1.37 ± 0.18 |
| EST | 0.57 ± 0.08 ** | 4.18 ± 0.10 | 0.35 ± 0.18 | 0.59 ± 0.06 | 1.40 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Bai, E.; Duan, Y.; Huang, Y. Ethanol Extracts from Torreya grandis Seed Have Potential to Reduce Hyperuricemia in Mouse Models by Influencing Purine Metabolism. Foods 2024, 13, 840. https://doi.org/10.3390/foods13060840
Yao J, Bai E, Duan Y, Huang Y. Ethanol Extracts from Torreya grandis Seed Have Potential to Reduce Hyperuricemia in Mouse Models by Influencing Purine Metabolism. Foods. 2024; 13(6):840. https://doi.org/10.3390/foods13060840
Chicago/Turabian StyleYao, Jianghui, Enhe Bai, Yanwen Duan, and Yong Huang. 2024. "Ethanol Extracts from Torreya grandis Seed Have Potential to Reduce Hyperuricemia in Mouse Models by Influencing Purine Metabolism" Foods 13, no. 6: 840. https://doi.org/10.3390/foods13060840
APA StyleYao, J., Bai, E., Duan, Y., & Huang, Y. (2024). Ethanol Extracts from Torreya grandis Seed Have Potential to Reduce Hyperuricemia in Mouse Models by Influencing Purine Metabolism. Foods, 13(6), 840. https://doi.org/10.3390/foods13060840






