UV-C Treatment Impact on the Availability of Water-Soluble Carbohydrates, Polyphenols, and Antioxidant Capacity of an Algerian Underutilized Date Fruit (Phoenix dactylifera L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. UV-C Light Treatment
2.4. Techno-Functional Properties
2.5. Characterization of Dietary Fiber
2.6. Characterization of Soluble Carbohydrate Composition by HPLC-RID
2.7. Characterization of Phenolic Compounds by HPLC-QTOF
2.8. Multifunctional Antioxidant Capacity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Techno-Functional Properties
3.2. Characterization of Dietary Fiber Composition
3.3. Characterization of Carbohydrate Content by HPLC-RID
3.4. Characterization of Phenolic Compounds by HPLC-QTOF
3.5. Antioxidant Capacity In Vitro
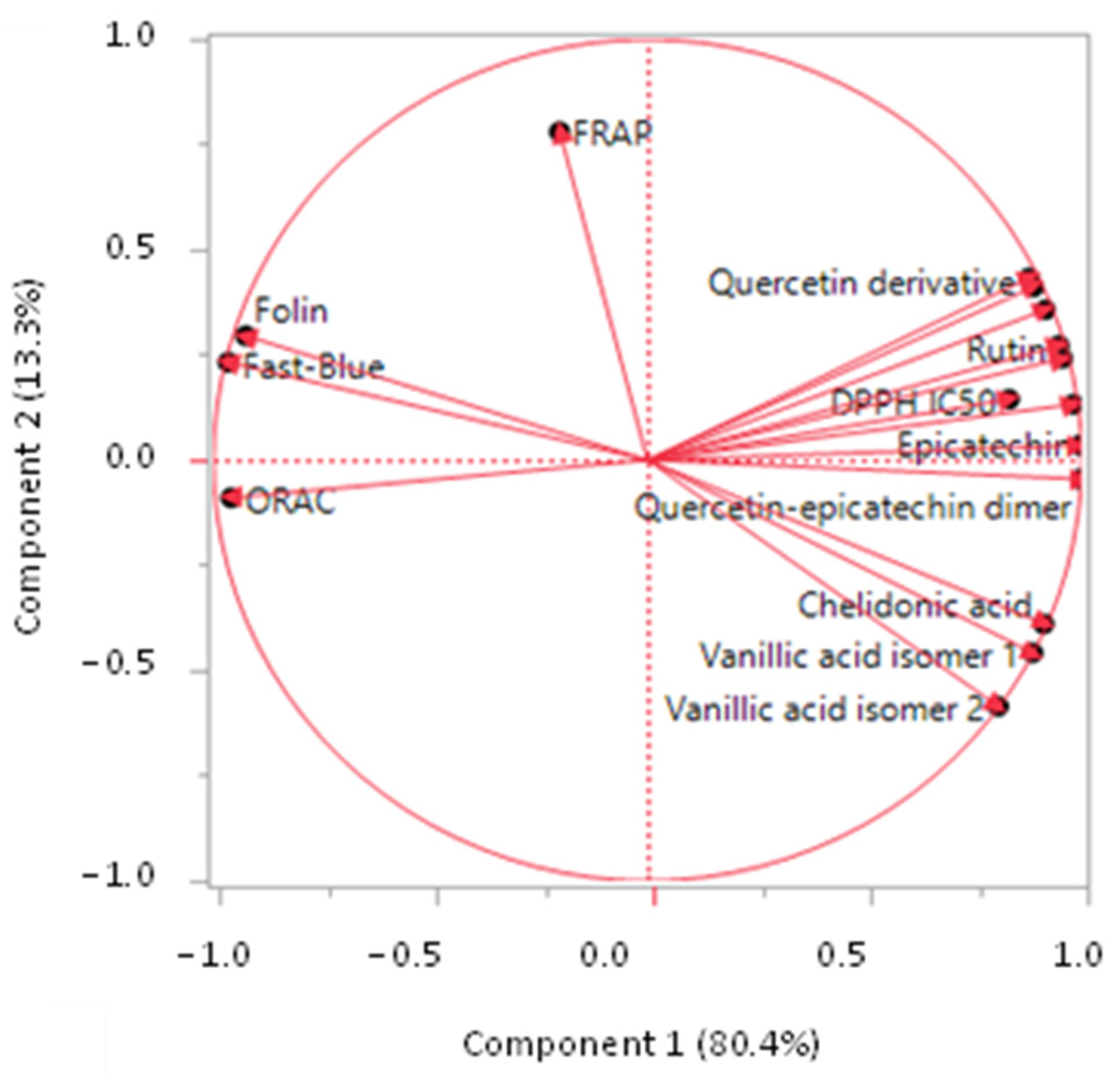
3.6. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallo, F.; Manzardo, A.; Camana, D.; Fedele, A.; Scipioni, A. Integration of a circular economy metric with life cycle assessment: Methodological proposal of compared agri-food products. Int. J. Life Cycle Assess. 2023. [Google Scholar] [CrossRef]
- Yontar, E. Critical success factor analysis of blockchain technology in agri-food supply chain management: A circular economy perspective. J. Environ. Manag. 2023, 330, 117173. [Google Scholar] [CrossRef]
- Bettaieb, I.; Kilani, A.; Ben Othman, K.; Benabderrahim, M.A.; Elfalleh, W. Phenolic Profile, Sugar Composition, and Antioxidant Capacities of Some Common Date Palm (Phoenix dactylifera L.) Cultivars as a Potential Nutraceutical and Functional Food Ingredients. J. Food Qual. 2023, 2023, 2474900. [Google Scholar] [CrossRef]
- Oladzad, S.; Fallah, N.; Mahboubi, A.; Afsham, N.; Taherzadeh, M.J. Date fruit processing waste and approaches to its valorization: A review. Bioresour. Technol. 2021, 340, 125625. [Google Scholar] [CrossRef]
- Taghian Dinani, S.; van der Goot, A.J. Challenges and solutions of extracting value-added ingredients from fruit and vegetable by-products: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 7749–7771. [Google Scholar] [CrossRef]
- Mandal, D.D.; Singh, G.; Majumdar, S.; Chanda, P. Challenges in developing strategies for the valorization of lignin—A major pollutant of the paper mill industry. Environ. Sci. Pollut. Res. 2023, 30, 11119–11140. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M.; Sayas-Barberá, E.; Navarro-Rodríguez de Vera, C.; Pérez-Álvarez, J.Á. Biological, nutritive, functional and healthy potential of date palm fruit (Phoenix dactylifera L.): Current research and future prospects. Agronomy 2022, 12, 876. [Google Scholar] [CrossRef]
- El-Far, A.H.; Oyinloye, B.E.; Sepehrimanesh, M.; Allah, M.A.G.; Abu-Reidah, I.; Shaheen, H.M.; Razeghian-Jahromi, I.; Noreldin, A.E.; Al Jaouni, S.K.; Mousa, S.A. Date palm (Phoenix dactylifera): Novel findings and future directions for food and drug discovery. Curr. Drug Discov. Technol. 2019, 16, 2–10. [Google Scholar] [CrossRef]
- Echegaray, N.; Gullón, B.; Pateiro, M.; Amarowicz, R.; Misihairabgwi, J.M.; Lorenzo, J.M. Date fruit and its by-products as promising source of bioactive components: A review. Food Rev. Int. 2023, 39, 1411–1432. [Google Scholar] [CrossRef]
- Hussain, M.I.; Farooq, M.; Syed, Q.A. Nutritional and biological characteristics of the date palm fruit (Phoenix dactylifera L.)–A review. Food Biosci. 2020, 34, 100509. [Google Scholar] [CrossRef]
- Al-Qarni, S.S.M.; Bazzi, M.D. Date fruit ripening with degradation of chlorophylls, carotenes, and other pigments. Int. J. Fruit Sci. 2020, 20 (Suppl. S2), S827–S839. [Google Scholar] [CrossRef]
- Muñoz-Bas, C.; Muñoz-Tebar, N.; Candela-Salvador, L.; Pérez-Alvarez, J.A.; Lorenzo, J.M.; Viuda-Martos, M.; Fernández-López, J. Quality characteristics of fresh date palm fruits of “Medjoul” and “Confitera” cv. from the Southeast of Spain (Elche Palm Grove). Foods 2023, 12, 2659. [Google Scholar] [CrossRef]
- Muñoz-Tebar, N.; Viuda-Martos, M.; Lorenzo, J.M.; Fernandez-Lopez, J.; Perez-Alvarez, J.A. Strategies for the Valorization of Date Fruit and Its Co-Products: A New Ingredient in the Development of Value-Added Foods. Foods 2023, 12, 1456. [Google Scholar] [CrossRef]
- Abdul-Hamid, N.A.; Mustaffer, N.H.; Maulidiani, M.; Mediani, A.; Ismail, I.S.; Tham, C.L.; Shadid, K.; Abas, F. Quality evaluation of the physical properties, phytochemicals, biological activities and proximate analysis of nine Saudi date palm fruit varieties. J. Saudi Soc. Agric. Sci. 2020, 19, 151–160. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Lante, A. Environmentally friendly techniques for the recovery of polyphenols from food by-products and their impact on polyphenol oxidase: A critical review. Appl. Sci. 2022, 12, 1923. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Shokramraji, Z.; Tavakkoli, S.; Mihaylova, D.; Lante, A. Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review. Plants 2023, 12, 1533. [Google Scholar] [CrossRef]
- Koutchma, T.; Bissonnette, S.; Popović, V. An Update on Research, Development and Implementation of UV and Pulsed Light Technologies for Nonthermal Preservation of Milk and Dairy Products; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Singh, H.; Bhardwaj, S.K.; Khatri, M.; Kim, K.-H.; Bhardwaj, N. UVC radiation for food safety: An emerging technology for the microbial disinfection of food products. Chem. Eng. J. 2021, 417, 128084. [Google Scholar] [CrossRef]
- EFSA on Dietetic Products, Nutrition and Allergies. Scientific opinion on the safety of UV-treated milk as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2016, 14, 4370. [Google Scholar]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef]
- Gil, M.I.; Selma, M.V.; López-Gálvez, F.; Allende, A. Fresh-cut product sanitation and wash water disinfection: Problems and solutions. Int. J. Food Microbiol. 2009, 134, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, S.; Xu, J.; Liu, S.; Li, G. Effects of combined aqueous chlorine dioxide and UV-C on shelf-life quality of blueberries. Postharvest Biol. Technol. 2016, 117, 125–131. [Google Scholar] [CrossRef]
- Delorme, M.M.; Guimarães, J.T.; Coutinho, N.M.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Margalho, L.P.; Pimentel, T.C.; Silva, M.C.; Freitas, M.Q. Ultraviolet radiation: An interesting technology to preserve quality and safety of milk and dairy foods. Trends Food Sci. Technol. 2020, 102, 146–154. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Z.; Guo, C.; Wu, Y.; Liu, W.; Yu, J.; Menghe, B.; Yang, R.; Zhang, H. Genetic diversity and population structure of Lactobacillus delbrueckii subspecies bulgaricus isolated from naturally fermented dairy foods. Sci. Rep. 2016, 6, 22704. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Cao, J.; Jiang, W. UV-C treatment controls brown rot in postharvest nectarine by regulating ROS metabolism and anthocyanin synthesis. Postharvest Biol. Technol. 2021, 180, 111613. [Google Scholar] [CrossRef]
- Günter, E.A.; Kapustina, O.M.; Popeyko, O.V.; Ovodov, Y.S. Influence of ultraviolet-C on the compositions of cell-wall polysaccharides and carbohydrase activities of Silene vulgaris callus. Carbohydr. Res. 2007, 342, 182–189. [Google Scholar] [CrossRef]
- 28 Meléndez-Pizarro, C.O.; Calva-Quintana, A.; Espinoza-Hicks, J.C.; Sánchez-Madrigal, M.Á.; Quintero-Ramos, A. Continuous Flow UV-C Irradiation Effects on the Physicochemical Properties of Aloe vera Gel and Pitaya (S tenocereus spp.) Blend. Foods 2020, 9, 1068. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Robles, P.A.; Gómez, P.A.; Tomás-Callejas, A.; Artés, F.; Martínez-Hernández, G.B. Quality changes of fresh-cut watermelon during storage as affected by cut intensity and UV-C pre-treatment. Food Bioprocess Technol. 2021, 14, 505–517. [Google Scholar] [CrossRef]
- Rabelo, M.C.; Bang, W.Y.; Nair, V.; Alves, R.E.; Jacobo-Velázquez, D.A.; Sreedharan, S.; de Miranda, M.R.A.; Cisneros-Zevallos, L. UVC light modulates vitamin C and phenolic biosynthesis in acerola fruit: Role of increased mitochondria activity and ROS production. Sci. Rep. 2020, 10, 21972. [Google Scholar] [CrossRef]
- Djaoud, K.; Boulekbache-Makhlouf, L.; Yahia, M.; Mansouri, H.; Mansouri, N.; Madani, K.; Romero, A. Dairy dessert processing: Effect of sugar substitution by date syrup and powder on its quality characteristics. J. Food Process. Preserv. 2020, 44, e14414. [Google Scholar] [CrossRef]
- Delgado, A.; Unamuno, V.; Muñiz, J.L.; Correcher, V.; Gomez-Ros, J.M. A simple UV irradiator for low dose reassessment with LiF TLD-100. Radiat. Prot. Dosim. 1996, 67, 303–306. [Google Scholar] [CrossRef]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Esteban, R.M. Effect of sterilisation on dietary fibre and physicochemical properties of onion by-products. Food Chem. 2011, 127, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Rupérez, P. High hydrostatic pressure improves the functionality of dietary fibre in okara by-product from soybean. Innov. Food Sci. Emerg. Technol. 2010, 11, 445–450. [Google Scholar] [CrossRef]
- De la Peña-Armada, R.; Villanueva-Suárez, M.J.; Rupérez, P.; Mateos-Aparicio, I. High hydrostatic pressure assisted by celluclast® releases oligosaccharides from apple by-product. Foods 2020, 9, 1058. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Medina, M.B. Simple and rapid method for the analysis of phenolic compounds in beverages and grains. J. Agric. Food Chem. 2011, 59, 1565–1571. [Google Scholar] [CrossRef]
- Chen, Z.; Bertin, R.; Froldi, G. EC50 estimation of antioxidant activity in DPPH assay using several statistical programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.T.; Matias, A.A.; Almeida, A.P.; Bronze, M.; Alves, P.M.; de Sousa, H.C.; Duarte, C.M. Processing cherries (Prunus avium) using supercritical fluid technology. Part 2. Evaluation of SCF extracts as promising natural chemotherapeutical agents. J. Supercrit. Fluids 2011, 55, 1007–1013. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Godswill, C.; Somtochukwu, V.; Kate, C. The functional properties of foods and flours. Int. J. Adv. Acad. Res. 2019, 5, 2488–9849. [Google Scholar]
- Yousf, N.; Nazir, F.; Salim, R.; Ahsan, H.; Sirwal, A. Water solubility index and water absorption index of extruded product from rice and carrot blend. J. Pharmacogn. Phytochem. 2017, 6, 2165–2168. [Google Scholar]
- Laaroussi, H.; Ferreira-Santos, P.; Genisheva, Z.; Bakour, M.; Ousaaid, D.; El Ghouizi, A.; Teixeira, J.A.; Lyoussi, B. Unveiling the techno-functional and bioactive properties of bee pollen as an added-value food ingredient. Food Chem. 2023, 405, 134958. [Google Scholar] [CrossRef] [PubMed]
- Teye, E.; Agbemafle, R.; Lamptey, F.P. Development and examination of sweet potato flour fortified with indigenous underutilized seasonal vegetables. Beverages 2018, 4, 5. [Google Scholar] [CrossRef]
- Jose, M.; Himashree, P.; Sengar, A.S.; Sunil, C. Valorization of food industry by-product (Pineapple Pomace): A study to evaluate its effect on physicochemical and textural properties of developed cookies. Meas. Food 2022, 6, 100031. [Google Scholar] [CrossRef]
- Lorente-Mento, J.M.; Lucas-González, R.; Sayas-Barbera, E.; Pérez-Álvarez, J.Á.; Fernández-López, J.; Viuda-Martos, M. Turrón coproducts as source of bioactive compounds: Assessment of chemical, physico-chemical, techno-functional and antioxidant properties. Foods 2020, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martí, J.; Panušková, K.; Larrea, V.; Bleha, R.; Quiles, A.; Hernando, I. Using different physical treatments to modify the structure and improve the technofunctional properties of clementine by-products. Food Struct. 2023, 38, 100346. [Google Scholar] [CrossRef]
- Megías-Pérez, R.; Ferreira-Lazarte, A.; Villamiel, M. Valorization of Grape Pomace as a Renewable Source of Techno-Functional and Antioxidant Pectins. Antioxidants 2023, 12, 957. [Google Scholar] [CrossRef]
- Alvarez-Ossorio, C.; Orive, M.; Sanmartín, E.; Alvarez-Sabatel, S.; Labidi, J.; Zufia, J.; Bald, C. Composition and Techno-functional Properties of Grape Seed Flour Protein Extracts. ACS Food Sci. Technol. 2022, 2, 125–135. [Google Scholar] [CrossRef]
- Grasso, N.; Lynch, N.L.; Arendt, E.K.; O’Mahony, J.A. Chickpea protein ingredients: A review of composition, functionality, and applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Chukwu, M.; Kabuo, N.; Nwokocha, N. Effects of fermentation time on the functional properties of ogiri-ahuekere (Arachis hypogaea Linn) seed condiment. Int. J. Biotechnol. Food Sci. 2018, 6, 77–85. [Google Scholar] [CrossRef]
- Oladele, A.; Aina, J. Chemical composition and properties of flour produced from two varieties of tigernut (Cyperns esculentus). Afr. J. Biotechnol. 2009, 6, 2473–2476. [Google Scholar] [CrossRef]
- Aguilera, Y.; Benítez, V.; Mollá, E.; Esteban, R.M.; Martín-Cabrejas, M.A. Influence of dehydration process in castellano chickpea: Changes in bioactive carbohydrates and functional properties. Plant Foods Hum. Nutr. 2011, 66, 391–400. [Google Scholar] [CrossRef]
- Hashim, I.; Khalil, A. Composition and functional properties of the date fruit residue a byproduct of date syrup/Debis production. Nutr. Food Tech 2015, 1, 1–5. [Google Scholar]
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Heredia-Olea, E.; Serna-Saldívar, S.O.; Welti-Chanes, J. Differences in the dietary fiber content of fruits and their by-products quantified by conventional and integrated AOAC official methodologies. J. Food Compos. Anal. 2018, 67, 77–85. [Google Scholar] [CrossRef]
- Ma, J.; Adler, L.; Srzednicki, G.; Arcot, J. Quantitative determination of non-starch polysaccharides in foods using Gas Chromatography with flame ionization detection. Food Chem. 2017, 220, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.E.B. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 2014, 93, 2380–2393. [Google Scholar] [CrossRef] [PubMed]
- Noorbakhsh, H.; Khorasgani, M.R. Date (Phoenix dactylifera L.) polysaccharides: A review on Chemical structure and nutritional properties. J. Food Meas. Charact. 2022, 16, 3240–3250. [Google Scholar] [CrossRef]
- Birkett, A.; Cho, S. Cereal fiber and health: Current knowledge. Cereal Food World 2013, 58, 309–313. [Google Scholar] [CrossRef]
- Mrabet, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Hamza, H.; Rodríguez-Arcos, R.; Guillén-Bejarano, R.; Sindic, M.; Jiménez-Araujo, A. Enzymatic conversion of date fruit fiber concentrates into a new product enriched in antioxidant soluble fiber. LWT 2017, 75, 727–734. [Google Scholar] [CrossRef]
- Bano, Y.; Rakha, A.; Khan, M.I.; Asgher, M. Chemical composition and antioxidant activity of date (Phoenix dactylifera L.) varieties at various maturity stages. Food Sci. Technol. 2022, 42, e29022. [Google Scholar] [CrossRef]
- Ötles, S.; Ozgoz, S. Health effects of dietary fiber. Acta Sci. Pol. Technol. Aliment. 2014, 13, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Djaoudene, O.; Bey, M.B.; Louaileche, H. Physicochemical Characteristics and Nutritional Compositions of Some Date (Phoenix dactylifera L.) Fruit Cultivars. Acta Univ. Cibiniensis. Ser. E Food Technol. 2019, 23, 129–138. [Google Scholar] [CrossRef]
- Hariri, A.; Ouis, N.; Bouhadi, D. Effect of substitution of sugars by date powders variety H’lowa on the quality of the soft drinks. J. Appl. Biotechnol. Bioeng. 2017, 3, 450–457. [Google Scholar]
- Assirey, E.A.R. Nutritional composition of fruit of 10 date palm (Phoenix dactylifera L.) cultivars grown in Saudi Arabia. J. Taibah Univ. Sci. 2015, 9, 75–79. [Google Scholar] [CrossRef]
- Aslam, A.; Leghari, S.; Asrar, M.; Saeed, S.; Shafi, M.; Siddiqi, M.; Sumalani, M.; Maham, F.; Merri, A. Physico-Chemical Diversity and Microbial Burden in Four Dates Palm (Phoenix Dactylifera L.) Fruit Varieties Grown in Agro-Climatic Condition of Turbat, Balochistan-Pakistan. Appl. Ecol. Environ. Res. 2019, 17, 6625–6642. [Google Scholar] [CrossRef]
- Taha, R.; Sihem, B.M.; Marianne, S.; Ali, S.; Ahmed, N.; Mars, M. Variability of physicochemical properties of ‘Deglet Nour’date fruits collected from different oases in Djerid Region, Tunisia. J. Hortic. Postharvest Res. 2020, 3, 85–100. [Google Scholar]
- Hussain, I.; Ahmad, S.; Amjad, M.; Ahmed, R. Execution of strands thinning improves the phytochemicals and sugars profiling in date palm (Phoenix dactylifera L.) fruit. Pak. J. Pharm. Sci. 2016, 29, 1209–1215. [Google Scholar]
- Haider, M.S.; Khan, I.A.; Naqvi, S.A.; Jaskani, M.; Khan, R.W.; Nafees, M.; Pasha, I. Fruit developmental stages effects on biochemical attributes in date palm. Pak. J. Agric. Sci. 2013, 50, 577–583. [Google Scholar]
- Airouyuwa, J.O.; Mostafa, H.; Riaz, A.; Stathopoulos, C.; Maqsood, S. Natural Deep Eutectic Solvents and Microwave-Assisted Green Extraction for Efficient Recovery of Bioactive Compounds from By-Products of Date Fruit (Phoenix dactylifera L.) Processing: Modeling, Optimization, and Phenolic Characterization. Food Bioprocess Technol. 2023, 16, 824–843. [Google Scholar] [CrossRef]
- Bouhlali, E.d.T.; Hmidani, A.; Bourkhis, B.; Khouya, T.; Ramchoun, M.; Filali-Zegzouti, Y.; Alem, C. Phenolic profile and anti-inflammatory activity of four Moroccan date (Phoenix dactylifera L.) seed varieties. Heliyon 2020, 6, e03436. [Google Scholar] [CrossRef]
- Benmeddour, Z.; Mehinagic, E.; Le Meurlay, D.; Louaileche, H. Phenolic composition and antioxidant capacities of ten Algerian date (Phoenix dactylifera L.) cultivars: A comparative study. J. Funct. Foods 2013, 5, 346–354. [Google Scholar] [CrossRef]
- Khallouki, F.; Ricarte, I.; Breuer, A.; Owen, R.W. Characterization of phenolic compounds in mature Moroccan Medjool date palm fruits (Phoenix dactylifera) by HPLC-DAD-ESI-MS. J. Food Compos. Anal. 2018, 70, 63–71. [Google Scholar] [CrossRef]
- Hilary, S.; Tomás-Barberán, F.A.; Martinez-Blazquez, J.A.; Kizhakkayil, J.; Souka, U.; Al-Hammadi, S.; Habib, H.; Ibrahim, W.; Platat, C. Polyphenol characterisation of Phoenix dactylifera L. (date) seeds using HPLC-mass spectrometry and its bioaccessibility using simulated in-vitro digestion/Caco-2 culture model. Food Chem. 2020, 311, 125969. [Google Scholar] [CrossRef]
- Bayrak, Ç.; Birinci, C.; Kemal, M.; Kolayli, S. The Phenolic Composition and Antioxidant Properties of Figs (Ficus carica L.) Grown in the Black Sea Region. Plant Foods Hum. Nutr. 2023, 78, 539–545. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Gullon, B.; Amarowicz, R.; Misihairabgwi, J.M.; Lorenzo, J.M. Phoenix dactylifera products in human health—A review. Trends Food Sci. Technol. 2020, 105, 238–250. [Google Scholar] [CrossRef]
- Maqsood, S.; Adiamo, O.; Ahmad, M.; Mudgil, P. Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients. Food Chem. 2020, 308, 125522. [Google Scholar] [CrossRef]
- Costa-Pérez, A.; Ferrer, M.A.; Calderón, A.A. Combined Effects of Cytokinin and UV-C Light on Phenolic Pattern in Ceratonia siliqua Shoot Cultures. Agronomy 2023, 13, 621. [Google Scholar] [CrossRef]
- Valerga, L.; González, R.E.; Pérez, M.B.; Concellón, A.; Cavagnaro, P.F. Differential and Cultivar-Dependent Antioxidant Response of Whole and Fresh-Cut Carrots of Different Root Colors to Postharvest UV-C Radiation. Plants 2023, 12, 1297. [Google Scholar] [CrossRef] [PubMed]
- Xuejiao, Z.; Xiaoyuan, Z.; Ye, H.; Ruirui, Y.; Qihui, W.; Di, G.; Yongcai, L.; Dov, P.; Yang, B. UV-C irradiation maintains cell membrane integrity at wounds of potato tubers during healing by regulating ROS homeostasis and increasing antioxidant activity. Postharvest Biol. Technol. 2023, 199, 112308. [Google Scholar]
- Harrison, K.; Were, L. Effect of gamma irradiation on total phenolic content yield and antioxidant capacity of almond skin extracts. Food Chem. 2007, 102, 932–937. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Gallo, C.R.; Shahidi, F. Gamma-irradiation induced changes in microbiological status, phenolic profile and antioxidant activity of peanut skin. J. Funct. Foods 2015, 12, 129–143. [Google Scholar] [CrossRef]
- Taheri, S.; Abdullah, T.L.; Karimi, E.; Oskoueian, E.; Ebrahimi, M. Antioxidant capacities and total phenolic contents enhancement with acute gamma irradiation in Curcuma alismatifolia (Zingiberaceae) leaves. Int. J. Mol. Sci. 2014, 15, 13077–13090. [Google Scholar] [CrossRef]
- Gerolis, L.G.L.; Lameiras, F.S.; Krambrock, K.; Neves, M.J. Effect of gamma radiation on antioxidant capacity of green tea, yerba mate, and chamomile tea as evaluated by different methods. Radiat. Phys. Chem. 2017, 130, 177–185. [Google Scholar] [CrossRef]
- Corrales, M.; de Souza, P.M.; Stahl, M.R.; Fernández, A. Effects of the decontamination of a fresh tiger nuts’ milk beverage (horchata) with short wave ultraviolet treatments (UV-C) on quality attributes. Innovative Food Sci. Emerg. Technol. 2012, 13, 163–168. [Google Scholar] [CrossRef]
- Crupi, P.; Pichierri, A.; Basile, T.; Antonacci, D. Postharvest stilbenes and flavonoids enrichment of table grape cv Redglobe (Vitis vinifera L.) as affected by interactive UV-C exposure and storage conditions. Food Chem. 2013, 141, 802–808. [Google Scholar] [CrossRef]
- Freitas, A.; Moldão-Martins, M.; Costa, H.S.; Albuquerque, T.G.; Valente, A.; Sanches-Silva, A. Effect of UV-C radiation on bioactive compounds of pineapple (Ananas comosus L. Merr.) by-products. J. Sci. Food Agric. 2015, 95, 44–52. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Villegas-Ochoa, M.A.; Martínez-Téllez, M.; Gardea, A.; Ayala-Zavala, J.F. Improving antioxidant capacity of fresh-cut mangoes treated with UV-C. J. Food Sci. 2007, 72, S197–S202. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Zhang, X.; Yong, H.; Wang, X.; Liu, Y.; Liu, J. Development and characterization of antioxidant active packaging and intelligent Al3+-sensing films based on carboxymethyl chitosan and quercetin. Int. J. Biol. Macromol. 2019, 126, 1074–1084. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Zhuang, X.-C.; Chen, G.-L.; Liu, Y.; Zhang, Y.-L.; Guo, M.-Q. New lignanamides with antioxidant and anti-inflammatory activities screened out and identified from Warburgia ugandensis combining affinity ultrafiltration LC-MS with SOD and XOD enzymes. Antioxidants 2021, 10, 370. [Google Scholar] [CrossRef]
- Tang, Y.; Zuo, C.; Li, J.; Li, L.; Zhang, H. Synergism of antioxidant and antibacterial activities between Hanzhongxianhao tea polyphenols and vitamin C. J. Food Sci. Technol. 2017, 35, 55–60. [Google Scholar]


| Property | Value |
|---|---|
| Swelling capacity (mL/g) | 3.94 ± 0.003 |
| Oil holding capacity (g/g) | 7.38 ± 0.30 |
| Water holding capacity (g/g) | 9.30 ± 0.25 |
| Bulk density (g/mL) | 1.81 ± 0.01 |
| Monomers | Date Fruit Powder (DFP) | ||
|---|---|---|---|
| IDF (g/100 g) | SDF (g/100 g) | TDF (g/100 g) | |
| Rhamnose | 0.60 ± 0.05 de | 0.71 ± 0.07 bc | 1.31 ± 0.03 d |
| Fucose | 0.55 ± 0.03 e | 0.67 ± 0.03 c | 1.22 ± 0.03 e |
| Arabinose | 4.28 ± 0.10 a | 0.79 ± 0.11 b | 5.07 ± 0.13 a |
| Xylose | 3.17 ± 0.14 b | 0.56 ± 0.10 d | 3.73 ± 0.13 b |
| Mannose | 0.64 ± 0.05 de | 0.51 ± 0.14 de | 1.15 ± 0.13 ab |
| Galactose | 1.03 ± 0.03 c | 0.36 ± 0.11 e | 1.38 ± 0.10 d |
| Glucose | 0.98 ± 0.06 cd | 0.17 ± 0.03 f | 1.02 ± 0.09 f |
| Uronic acids | 0.78 ± 0.05 d | 1.39 ± 0.09 a | 2.19 ± 0.10 c |
| Total | 11.89 ± 0.25 | 5.15 ± 0.33 | 17.06 ± 0.25 |
| Peak N° | Standards | Untreated (g/100 g) | 5 min (g/100 g) | 10 min (g/100 g) | 20 min (g/100 g) | 40 min (g/100 g) | |
|---|---|---|---|---|---|---|---|
| Mw (kDa) | RT (min) | ||||||
| Peak 1 | 100 | 11.90 | 0.38 ± 0.03 b | 0.22 ± 0.03 c | 0.28 ± 0.01 c | 0.53 ± 0.05 a | 0.53 ± 0.02 a |
| Peak 2 | 100 | 14.55 | 4.43 ± 0.08 b | 4.52 ± 0.02 a | 4.52 ± 0.02 a | 4.56 ± 0.03 a | 4.48 ± 0.04 ab |
| Peak 3 | 20 | 15.20 | nd | 0.84 ± 0.03 c | 0.94 ± 0.03 b | 1.08 ± 0.03 a | nd |
| Peak 4 | 20 | 15.85 | nd | nd | nd | nd | 1.32 ± 0.01 b |
| Peak 5 | 0.50 | 29.00 | 0.17 ± 0.01 c | 0.42 ± 0.02 a | 0.37 ± 0.03 a | 0.33 ± 0.07 b | 0.16 ± 0.02 c |
| Peak 6 | 0.50 | 30.00 | nd | 0.23 ± 0.04 a | 0.16 ± 0.02 b | nd | nd |
| Peak 7 | 0.34 | 33.80 | nd | 0.05 ± 0.02 a | nd | nd | nd |
| Peak 8 | 0.34 | 34.30 | 0.06 ± 0.01 a | nd | 0.03 ± 0.01 b | 0.07 ± 0.03 a | 0.04 ± 0.005 b |
| Peak 9 | 0.34 | 35.10 | 0.17 ± 0.02 b | 0.25 ± 0.05 a | 0.21 ± 0.05 a | 0.21 ± 0.02 a | 0.23 ± 0.02 a |
| Peak 10 | 0.34 | 37.00 | 0.09 ± 0.01 b | 0.15 ± 0.03 a | 0.16 ± 0.01 a | 0.12 ± 0.01 b | 0.14 ± 0.02 a |
| Peak 11 | 0.18 | 41.90 | 34.90 ± 0.10 a | 33.58 ± 0.05 c | 33.59 ± 0.13 c | 33.84 ± 0.10 b | 33.29 ± 0.01 d |
| Peak 12 | 0.18 | 45.30 | 39.83 ± 0.14 b | 39.77 ± 0.03 b | 39.79 ± 0.02 b | 40.36 ± 0.02 a | 39.26 ± 0.10 c |
| Total | 80.02 ± 0.01 b | 80.03 ± 0.01 b | 80.05 ± 0.04 b | 81.10 ± 0.01 a | 78.45 ± 0.01 c | ||
| Sample | Fast-Blue (mg GAE/g dw) | Folin (mg GAE/g dw) | FRAP (mg TE/g dw) | ORAC (µmol TE/g dw) | DPPH EC50 (mg TE/mL) |
|---|---|---|---|---|---|
| Untreated | 106.17 ± 2.66 a | 16.12 ± 0.62 a | 146.54 ± 1.84 a | 75.60 ± 8.24 a | 21.30 ± 0.27 b |
| 5 min | 99.14 ± 0.52 b | 14.12 ± 0.08 b | 88.50 ± 0.12 b | 62.74 ± 12.55 ab | 58.24 ± 1.11 a |
| 10 min | 92.10 ± 1.40 c | 13.82 ± 1.03 bc | 86.35 ± 0.30 c | 61.65 ± 6.88 b | 58.27 ± 0.47 a |
| 20 min | 73.46 ± 0.17 d | 11.01 ± 2.03 c | 77.60 ± 0.24 d | 60.32 ± 2.05 b | 58.73 ± 0.97 a |
| 40 min | 104.81 ± 3.32 a | 15.28 ± 0.65 a | 77.70 ± 0.94 d | 64.57 ± 14.45 ab | 57.14 ± 0.40 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djaoud, K.; De la Peña-Armada, R.; García-Alonso, A.; Correcher, V.; Boulekbache-Makhlouf, L.; Mateos-Aparicio, I. UV-C Treatment Impact on the Availability of Water-Soluble Carbohydrates, Polyphenols, and Antioxidant Capacity of an Algerian Underutilized Date Fruit (Phoenix dactylifera L.). Foods 2024, 13, 893. https://doi.org/10.3390/foods13060893
Djaoud K, De la Peña-Armada R, García-Alonso A, Correcher V, Boulekbache-Makhlouf L, Mateos-Aparicio I. UV-C Treatment Impact on the Availability of Water-Soluble Carbohydrates, Polyphenols, and Antioxidant Capacity of an Algerian Underutilized Date Fruit (Phoenix dactylifera L.). Foods. 2024; 13(6):893. https://doi.org/10.3390/foods13060893
Chicago/Turabian StyleDjaoud, Kahina, Rocío De la Peña-Armada, Alejandra García-Alonso, Virgilio Correcher, Lila Boulekbache-Makhlouf, and Inmaculada Mateos-Aparicio. 2024. "UV-C Treatment Impact on the Availability of Water-Soluble Carbohydrates, Polyphenols, and Antioxidant Capacity of an Algerian Underutilized Date Fruit (Phoenix dactylifera L.)" Foods 13, no. 6: 893. https://doi.org/10.3390/foods13060893






