The Biotransformation and Influence on the Functional Activities of Metabolites during the Fermentation of Elaeagnus moorcroftii Wall.ex Schlecht. Juice by Bifidobacterium animalis subsp. lactis HN-3
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Bacterial Strains
2.1.1. Preparation of EWSJ
2.1.2. Activation of Bifidobacterium
2.2. Fermentation and Determination of Viable Counts
2.3. Measurement of Soluble Solids and pH
2.4. Total Flavonoid Content (TFC)
2.5. Determination of the Antioxidant Capacity of EWSJ
2.5.1. ABTS+• Radical-Scavenging Ability
2.5.2. DPPH• Radical-Scavenging Ability
2.6. Non-Targeted Metabolomic Analysis
2.6.1. Metabolite Extraction
2.6.2. UHPLC-MS/MS Analysis
2.6.3. Data Processing and Metabolite Identification
2.7. Statistical Analysis
3. Results
3.1. Viable Counts and Physicochemical Properties during B.an3 Fermentation
3.2. Metabolite Analysis of EWSJ Fermented by B. animalis subsp. lactis HN-3
3.2.1. Metabolic Responses to the Fermentation of EWSJ
3.2.2. Overview of Differential Metabolites after the Fermentation of EWSJ
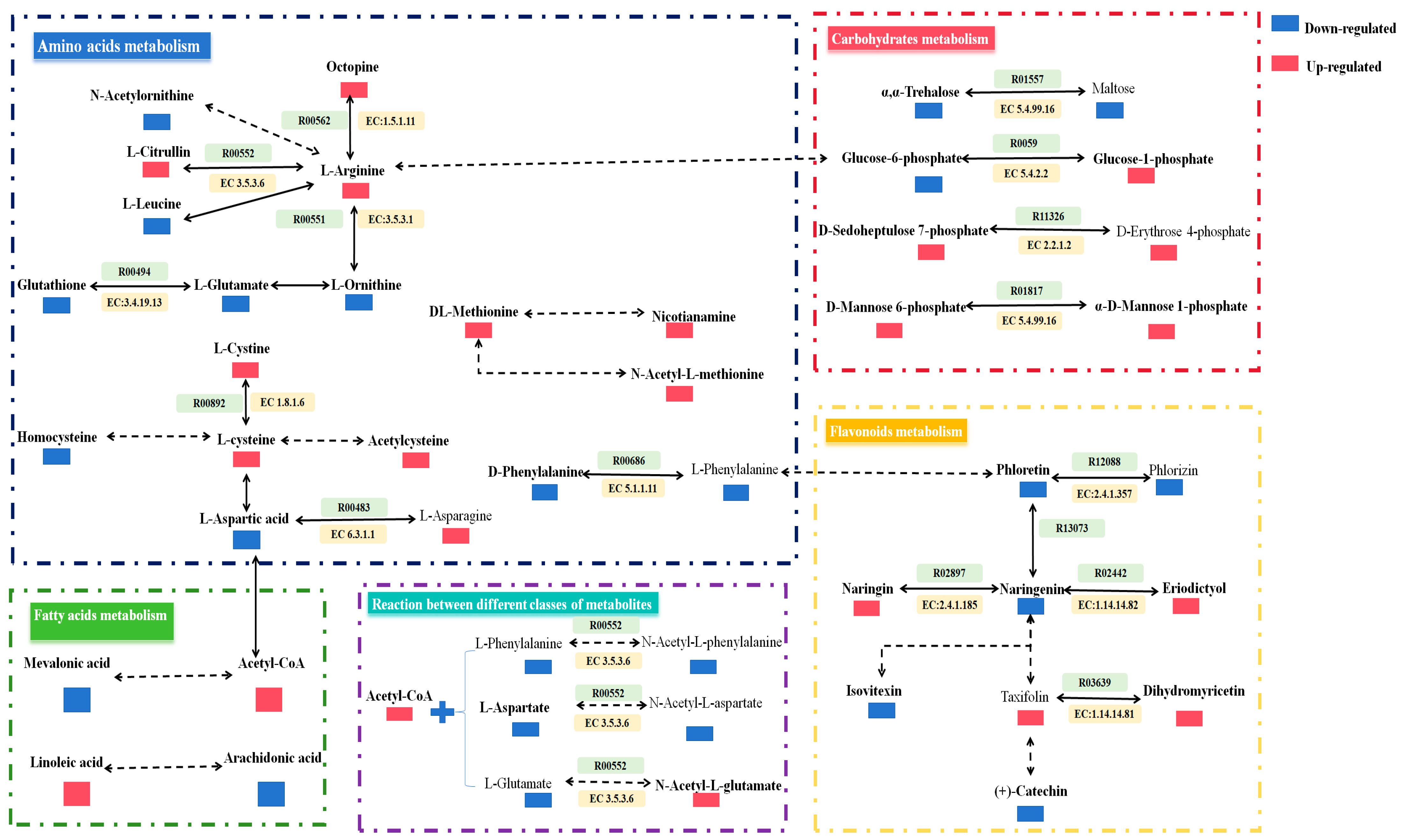
3.3. Major Metabolism during the Fermentation of EWSJ
4. Discussion
4.1. Effects on the Functional Activity during the Fermentation of EWSJ
4.1.1. Impact of B.an3 Growth
4.1.2. Antioxidant
Free Radical Scavenging Capacity
Metal-Chelating Ability
4.2. Major Transformation during the Fermentation of EWSJ
4.2.1. Reduction
4.2.2. Hydrolysis
4.2.3. Isomerization
4.2.4. Deglycosidation
4.2.5. Others
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lillo-Pérez, S.; Guerra-Valle, M.; Orellana-Palma, P.; Petzold, G. Probiotics in fruit and vegetable matrices: Opportunities for nondairy consumers. LWT 2021, 151, 112106. [Google Scholar] [CrossRef]
- Ranadheera, C.; Vidanarachchi, J.; Rocha, R.; Cruz, A.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Fu, X.; Wu, J.; Ma, X.; Li, K.; Zhang, H.; Wu, S.; Sun, K. The Chromosome-Level Genome of Elaeagnus moorcroftii Wall., an Economically and Ecologically Important Tree Species in Drylands. Diversity 2022, 14, 468. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Li, X.; Wang, C.; Li, Q.; Xu, M.; Guan, X.; Lan, Z.; Ni, Y.; Zhang, Y. Widely targeted metabolomics analysis of enriched secondary metabolites and determination of their corresponding antioxidant activities in Elaeagnus angustifolia var. orientalis (L.) Kuntze fruit juice enhanced by Bifidobacterium animalis subsp. lactis HN-3 fermentation. Food Chem. 2022, 374, 131568. [Google Scholar] [CrossRef]
- Russell, D.A.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Metabolic activities and probiotic potential of bifidobacteria. Int. J. Food Microbiol. 2011, 149, 88–105. [Google Scholar] [CrossRef]
- Xue, Y.S.; Liu, Y.P.; Xie, Y.X.; Cong, C.X.; Wang, G.R.; An, L.; Teng, Y.X.; Chen, M.H.; Zhang, L. Antioxidant activity and mechanism of dihydrochalcone C-glycosides: Effects of C-glycosylation and hydroxyl groups. Phytochemistry 2020, 179, 112393. [Google Scholar] [CrossRef] [PubMed]
- Grellet Bournonville, C.; Filippone, M.P.; Di Peto, P.d.l.Á.; Trejo, M.F.; Couto, A.S.; Mamaní de Marchese, A.; Díaz Ricci, J.C.; Welin, B.; Castagnaro, A.P. Strawberry fatty acyl glycosides enhance disease protection, have antibiotic activity and stimulate plant growth. Sci. Rep. 2020, 10, 8196. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Ozawa, H.; Miyazawa, T.; Burdeos, G.C.; Miyazawa, T. Biological Functions of Antioxidant Dipeptides. J. Nutr. Sci. Vitaminol. 2022, 68, 162–171. [Google Scholar] [CrossRef]
- Wu, C.; Li, T.; Qi, J.; Jiang, T.; Xu, H.; Lei, H. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. LWT 2020, 122, 109064. [Google Scholar] [CrossRef]
- Maftei, N.-M.; Bogdan, R.E.G.; Boev, M.; Marin, D.B.; Ramos-Villarroel, A.Y.; Iancu, A.-V. Innovative Fermented Soy Drink with the Sea Buckthorn Syrup and the Probiotics Co-Culture of Lactobacillus Paracasei ssp. Paracasei (L. Casei® 431) and Bifidobacterium Animalis ssp. Lactis (Bb-12®). Fermentation 2023, 9, 806. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, S.; Wu, T.; Zhang, J.; Kwok, L.-Y.; Sun, Z.; Zhang, H.; Wang, J. Untargeted mass spectrometry-based metabolomics approach unveils biochemical changes in compound probiotic fermented milk during fermentation. NPJ Sci. Food 2023, 7, 21. [Google Scholar] [CrossRef]
- Reche, J.; Hernández, F.; Almansa, M.S.; Carbonell-Barrachina, Á.A.; Legua, P.; Amorós, A. Physicochemical and nutritional composition, volatile profile and antioxidant activity differences in Spanish jujube fruits. LWT 2018, 98, 1–8. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J. Sci. Food Agric. 2018, 99, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.M.; Nguyen, T.B.; Nguyen, V.D.; Bujna, E.; Dam, M.S.; Nguyen, Q.D. Changes in bitterness, antioxidant activity and total phenolic content of grapefruit juice fermented by Lactobacillus and Bifidobacterium strains. Acta Aliment. 2020, 49, 103–110. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2012, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yin, Q.; Kang, M.; Ma, N.; Li, X.; Yang, Z.; Jin, H.; Lin, M.; Zhuang, P.; Zhang, Y. Untargeted metabolomics reveals novel serum biomarker of renal damage in rheumatoid arthritis. J. Pharm. Biomed. Anal. 2020, 180, 113068. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef]
- Tang, Y.; Tang, W.; Wang, M.; Zhang, Z.; Chen, Y. A conservative distribution of tridomain NDP-heptose synthetases in actinobacteria. Sci. China Life Sci. 2021, 65, 1014–1023. [Google Scholar] [CrossRef]
- Nihira, T.; Suzuki, E.; Kitaoka, M.; Nishimoto, M.; Ohtsubo, K.I.; Nakai, H. Discovery of β-1,4-d-Mannosyl-N-acetyl-d-glucosamine Phosphorylase Involved in the Metabolism of N-Glycans. J. Biol. Chem. 2013, 288, 27366–27374. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jiang, T.; Liu, N.; Wu, C.; Xu, H.; Lei, H. Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chem. 2021, 339, 127859. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.G.G.; da Silva Campelo Borges, G.; dos Santos Lima, M.; Martín-Belloso, O.; Magnani, M. Effects of probiotics on the content and bioaccessibility of phenolic compounds in red pitaya pulp. Food Res. Int. 2019, 126, 108681. [Google Scholar] [CrossRef] [PubMed]
- Dasila, K.; Singh, M. Bioactive compounds and biological activities of L.: An underutilized fruit of North-East Himalaya, India. S. Afr. J. Bot. 2022, 145, 177–185. [Google Scholar] [CrossRef]
- Cho, J.; Horikawa, Y.; Enya, M.; Takeda, J.; Imai, Y.; Imai, Y.; Handa, H.; Imai, T. L-Arginine prevents cereblon-mediated ubiquitination of glucokinase and stimulates glucose-6-phosphate production in pancreatic β-cells. Commun. Biol. 2020, 3, 497. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Jang, J.H.; Choi, I.H.; Lim, C.G.; Jeon, E.J.; Bae Bang, H.; Jeong, K.J. Production of trans-cinnamic acid by whole-cell bioconversion from l-phenylalanine in engineered Corynebacterium glutamicum. Microb. Cell Factories 2021, 20, 145. [Google Scholar] [CrossRef]
- Fox, A.R.; Soto, G.; Mozzicafreddo, M.; Garcia, A.N.; Cuccioloni, M.; Angeletti, M.; Salerno, J.C.; Ayub, N.D. Understanding the function of bacterial and eukaryotic thiolases II by integrating evolutionary and functional approaches. Gene 2014, 533, 5–10. [Google Scholar] [CrossRef]
- Portmann, M.O.; Birch, G. Sweet taste and solution properties of α,α-trehalose. J. Sci. Food Agric. 2006, 69, 275–281. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, F.; Lian, Y.; Xiao, H.; Zheng, J. Biosynthesis of citrus flavonoids and their health effects. Crit. Rev. Food Sci. Nutr. 2018, 60, 566–583. [Google Scholar] [CrossRef]
- Shin, S.; Gombedza, F.C.; Bandyopadhyay, B.C. l-ornithine activates Ca2+ signaling to exert its protective function on human proximal tubular cells. Cell. Signal. 2020, 67, 109484. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, A.M.; Pacheco, A.R.; Henrick, B.M.; Taft, D.; Xu, G.; Huda, M.N.; Mishchuk, D.; Goodson, M.L.; Slupsky, C.; Barile, D.; et al. Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiol. 2020, 20, 357. [Google Scholar] [CrossRef]
- Langa, S.; Ruiz de la Bastida, A.; Peirotén, Á.; Curiel, J.A.; Landete, J.M. Development of the first fermented soy beverages enriched in equol and 5-hydroxy-equol. LWT 2022, 168, 113899. [Google Scholar] [CrossRef]
- Thakker, D.P.; Narayanan, R. Arginine deiminase produced by lactic acid bacteria as a potent anti-cancer drug. Med. Oncol. 2023, 40, 175. [Google Scholar] [CrossRef]
- Yamashita, T.; Ashiuchi, M.; Ohnishi, K.; Kato, S.I.; Nagata, S.; Misono, H. Molecular identification of monomeric aspartate racemase from Bifidobacterium bifidum. Eur. J. Biochem. 2004, 271, 4798–4803. [Google Scholar] [CrossRef]
- Mei, Y.; Li, X.; Yang, B.; Zhao, J.; Zhang, H.; Chen, H.; Chen, W. Heterologous expression of a novel linoleic acid isomerase BBI, and effect of fusion tags on its performance. Curr. Res. Food Sci. 2022, 5, 2053–2060. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Konishi, H.; Horitsu, H.; Sakurai, H.; Takamizawa, K.; Suzuki, T.; Kawai, K. Purification and Characterization of D-Xylose Isomerase from Bifidobacterium adolescentis. Biosci. Biotechnol. Biochem. 2014, 58, 691–694. [Google Scholar] [CrossRef][Green Version]
- Ju, J.-H.; Jeon, S.-G.; Heo, S.-Y.; Kim, J.-S.; Jo, M.-H.; Kim, M.-S.; Kim, C.-H.; Oh, B.-R. Synbiotics production using Lactobacillus reuteri EC01, a strain that produces alternan-type exopolysaccharide. LWT 2023, 182, 114814. [Google Scholar] [CrossRef]
- Braune, A.; Blaut, M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Shen, J.; Manickam, S.; Li, S.; Tao, Y.; Li, D.; Liu, D.; Han, Y. Investigation of blueberry juice fermentation by mixed probiotic strains: Regression modeling, machine learning optimization and comparison with fermentation by single strain in the phenolic and volatile profiles. Food Chem. 2023, 405, 134982. [Google Scholar] [CrossRef]
- Lin, G.; Liu, Q.; Wang, L.; Li, H.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. The Comparative Analysis of Genomic Diversity and Genes Involved in Carbohydrate Metabolism of Eighty-Eight Bifidobacterium pseudocatenulatum Isolates from Different Niches of China. Nutrients 2022, 14, 2347. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Surh, J.; Chung, H.Y.; Hahn, B.-S.; Kang, S.A.; Jang, K.-H. Characteristics for ornithine-production and ornithine-synthase from Bifidobacterium longum. Int. Food Res. J. 2017, 24, 2711–2715. [Google Scholar]
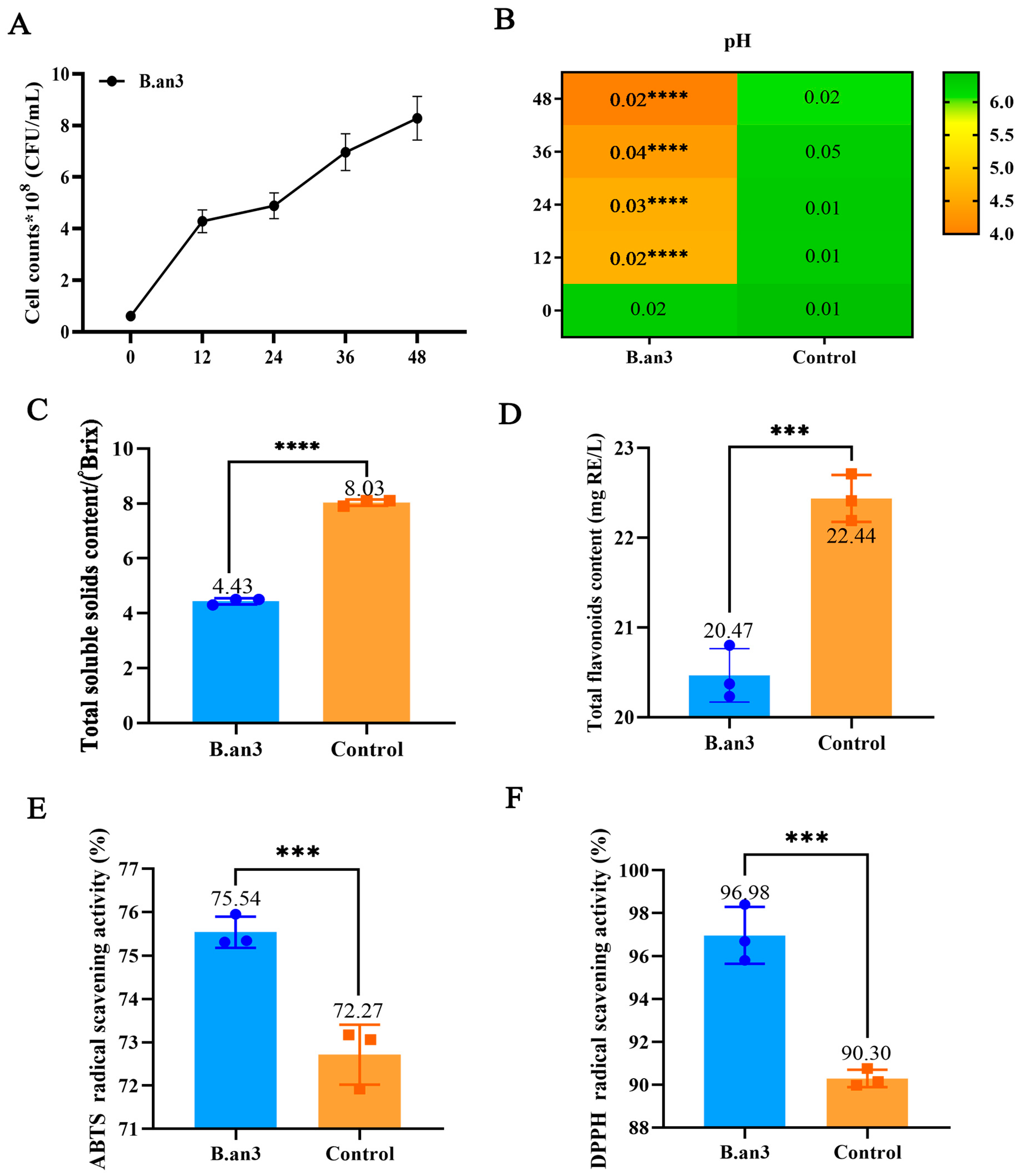
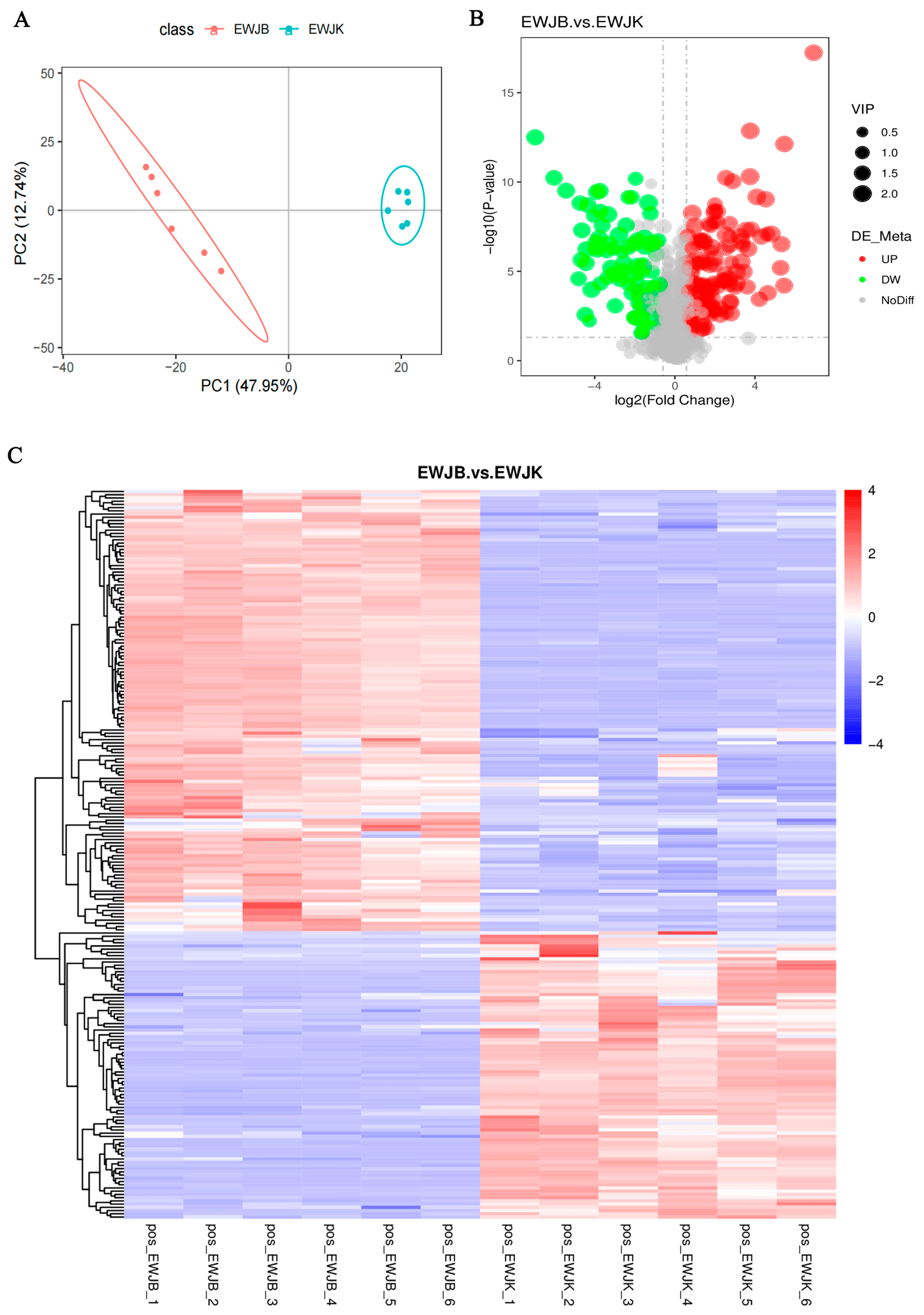

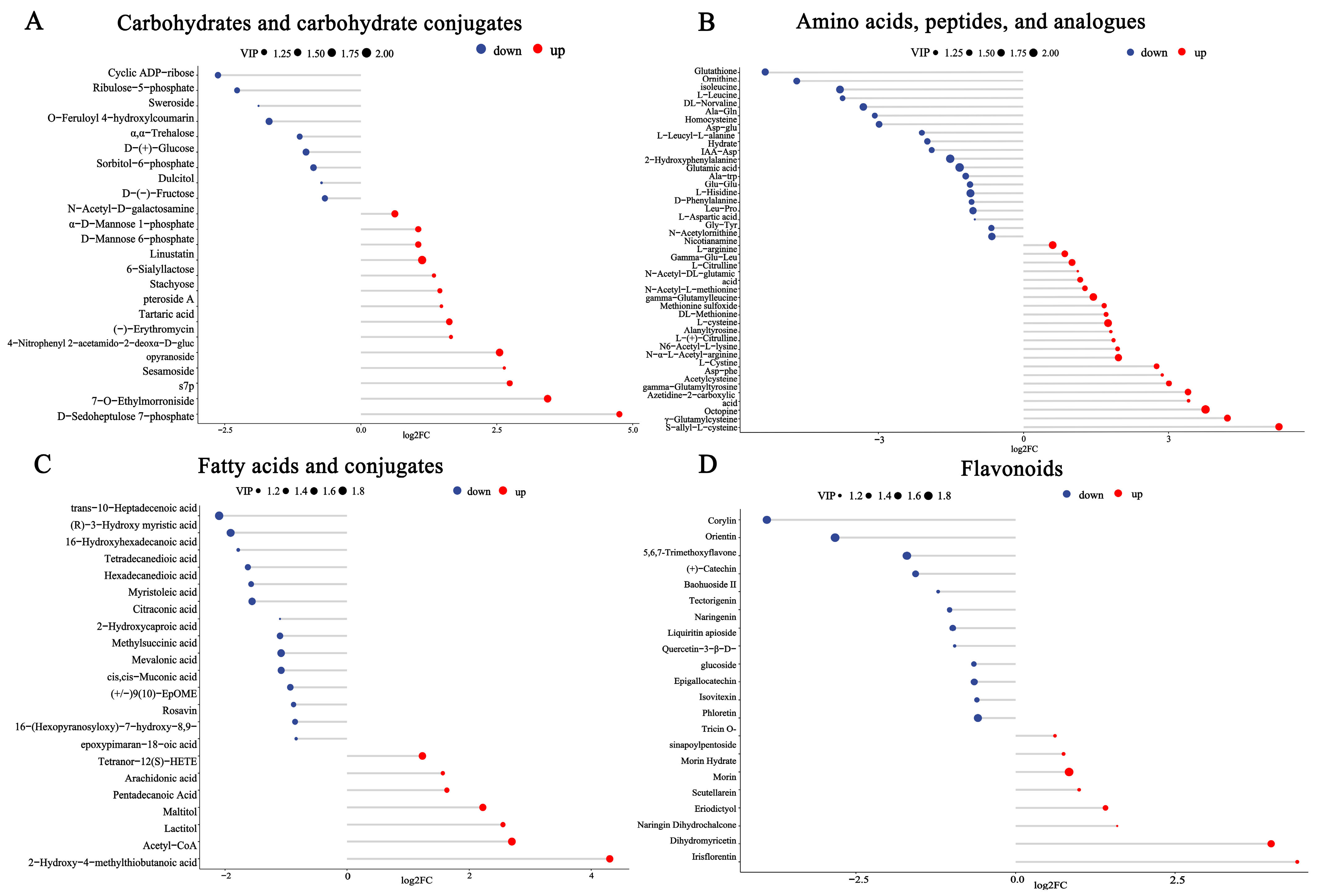
| Property | B.an3 Fermented EWSJ | Unfermented EWSJ |
|---|---|---|
| TSSC/(°Brix) | 4.43 ± 0.11 **** | 8.03 ± 0.12 |
| TFC/(mg RE/L) | 20.47 ± 0.03 *** | 22.44 ± 0.02 |
| ABTS/(%) | 75.54 ± 0.01 *** | 72.27 ± 0.01 |
| DPPH/(%) | 96.98 ± 0.01 *** | 90.30 ± 0.01 |
| Class | B.an3 Fermented EWSJ | Unfermented EWSJ |
|---|---|---|
| Amino acids, peptides, and analogs | 10,872,660,962 ± 795,836,210.20 *** | 13,443,673,365 ± 1,362,572,160.00 |
| Carbohydrates and carbohydrate conjugates | 34,615,427,601 ± 4,615,419,307.00 **** | 54,506,204,164 ± 5,788,529,624.00 |
| Fatty acids and conjugates | 21,203,897,293 ± 558,396,702.60 **** | 39,033,293,181 ± 3,012,718,214.00 |
| Flavonoids | 664,407,561 ± 19,242,552.98 **** | 804,051,857 ± 48,092,759.05 |
| Total peak area | 1.24228 × 1012 ± 220,275,493.14 | 1.3371 × 1012 ± 112,031,813.99 |
| Type | Precursors | Products | Predictive Enzymes |
|---|---|---|---|
| Reduction (4) | L-Cystine (↑) | L-cysteine (↑) | Cystine reductase (EC 1.8.1.6) |
| Octopine (↑) | L-Arginine (↑) | D-octopine dehydrogenase (EC 1.5.1.11) | |
| Naringenin (↓) | Eriodictyol (↑) | Flavonoid 3’-monooxygenase (EC 1.14.14.82) | |
| Taxifolin (↑) | Dihydromyricetin (↑) | Flavanoid 3’,5’-hydroxylase (EC 1.14.14.81) | |
| Hydrolysis (3) | L-Arginine (↑) | L-Citrulline (↑) | Arginine deiminase (EC 3.5.3.6) |
| L-Ornithine (↓) | L-Arginine (↑) | Arginase (EC 3.5.3.1) | |
| Glutathione (↓) | L-Glutamic acid (↓) | Glutathione hydrolase (EC 3.4.19.13) | |
| Isomerization (4) | Glucose-6-phosphate (↓) | Glucose-1-phosphate (↑) | Phosphoglucomutase (EC 5.4.2.2) |
| L-Phenylalanine (↓) | D-Phenylalanine (↓) | Phenylalanine racemase (EC 5.1.1.11) | |
| α, α-Trehalose (↓) | Maltose (↓) | Maltose alpha-D-glucosyltransferase (EC 5.4.99.16) | |
| D-Mannose 6-phosphate (↑) | α-D-Mannose 1-phosphate (↑) | Phosphomannomutase (EC 5.4.2.8) | |
| Deglycosidation (2) | Phlorizin (↓) | Phloretin (↓) | Phloretin 2’-O-D-glucosyltransferase (EC 2.4.1.357) |
| Naringenin (↓) | Naringin (↑) | Naringenin 7-O-glucosyltransferase (EC 2.4.1.185) | |
| Others (5) | D-Sedoheptulose 7-phosphate (↑) | D-Erythrose 4-phosphate (↑) | Dihydroxyacetonetransferase (EC 2.2.1.2) |
| L-Aspartic acid (↓) | L-Asparagine (↑) | L-asparagine synthetase (EC 6.3.1.1) | |
| L-Phenylalanine | N-Acetyl-L-phenylalanine | Phenylalanine N-acetyltransferase (EC 2.3.1.53) | |
| L-Aspartate | N-Acetyl-L-aspartate | Aspartate N-acetyltransferase (EC 2.3.1.17) | |
| L-Glutamate | N-Acetyl-L-glutamate | Amino-acid N-acetyltransferase (EC 2.3.1.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, C.; Lan, Z.; Teng, Y.; Ni, Y.; Zhang, Y. The Biotransformation and Influence on the Functional Activities of Metabolites during the Fermentation of Elaeagnus moorcroftii Wall.ex Schlecht. Juice by Bifidobacterium animalis subsp. lactis HN-3. Foods 2024, 13, 926. https://doi.org/10.3390/foods13060926
Wang Y, Wang C, Lan Z, Teng Y, Ni Y, Zhang Y. The Biotransformation and Influence on the Functional Activities of Metabolites during the Fermentation of Elaeagnus moorcroftii Wall.ex Schlecht. Juice by Bifidobacterium animalis subsp. lactis HN-3. Foods. 2024; 13(6):926. https://doi.org/10.3390/foods13060926
Chicago/Turabian StyleWang, Yixuan, Chenxi Wang, Zhenghui Lan, Yingdi Teng, Yongqing Ni, and Yan Zhang. 2024. "The Biotransformation and Influence on the Functional Activities of Metabolites during the Fermentation of Elaeagnus moorcroftii Wall.ex Schlecht. Juice by Bifidobacterium animalis subsp. lactis HN-3" Foods 13, no. 6: 926. https://doi.org/10.3390/foods13060926
APA StyleWang, Y., Wang, C., Lan, Z., Teng, Y., Ni, Y., & Zhang, Y. (2024). The Biotransformation and Influence on the Functional Activities of Metabolites during the Fermentation of Elaeagnus moorcroftii Wall.ex Schlecht. Juice by Bifidobacterium animalis subsp. lactis HN-3. Foods, 13(6), 926. https://doi.org/10.3390/foods13060926





