Polygonatum sibiricum Saponin Prevents Immune Dysfunction and Strengthens Intestinal Mucosal Barrier Function in Cyclophosphamide-Induced Immunosuppressed BALB/c Mice
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Preparation of Polygonatum sibiricum Saponin
2.3. Animals and Experiments Design
2.4. Organ Index
2.5. Xylene-Induced Inflammatory Response
2.6. Serum Hemolysis Value (HC50) Assay
2.7. Hematological Analysis
2.8. Histological Analysis
2.9. Measurement of Immune-Related Cytokines in the Serum
2.10. Determination of Antioxidant Levels in Liver Tissue
2.11. Splenocyte Proliferation Assay
2.12. Lymphocyte Proliferation
2.13. Flow Cytometric Analysis
2.14. Western Bolt Analysis
2.15. Quantitative RT-PCR Analysis
2.16. Statistical Analysis
3. Results
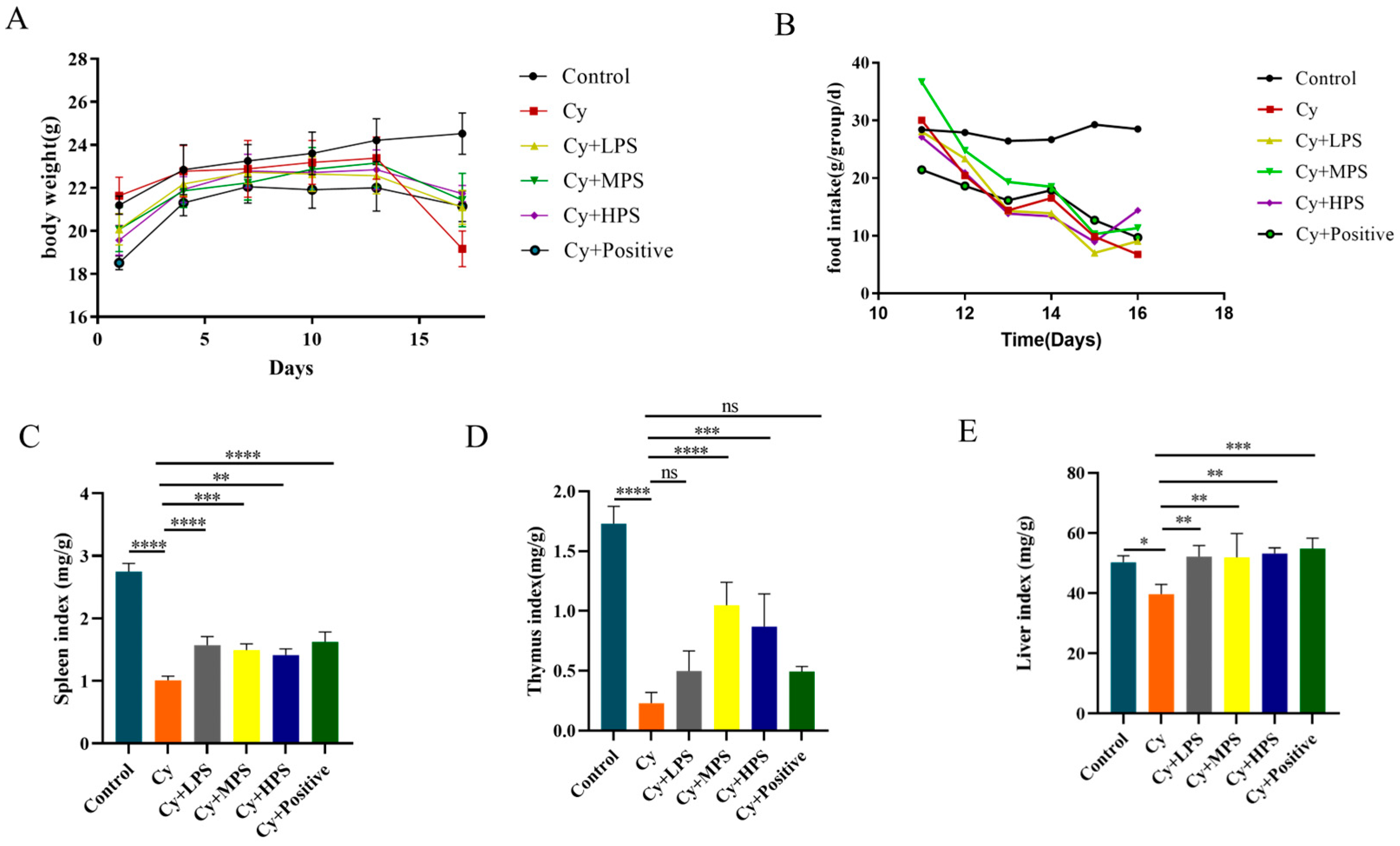
3.1. Body Weight and Organ Index Change of Mice
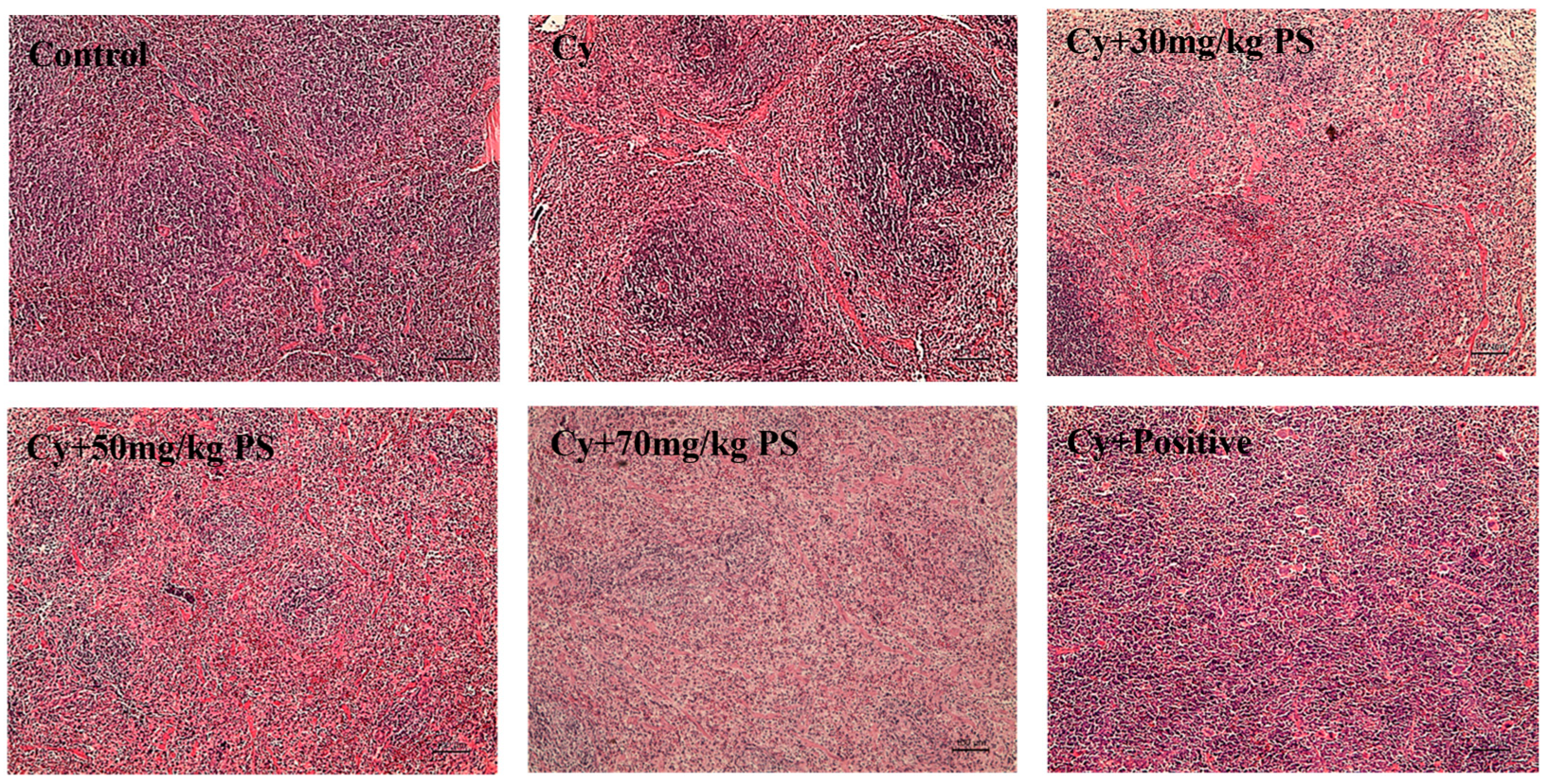
3.2. PS Supplementation Improved Histopathological Alterations in Cyclophosphamide-Induced Immunosuppressed Mice
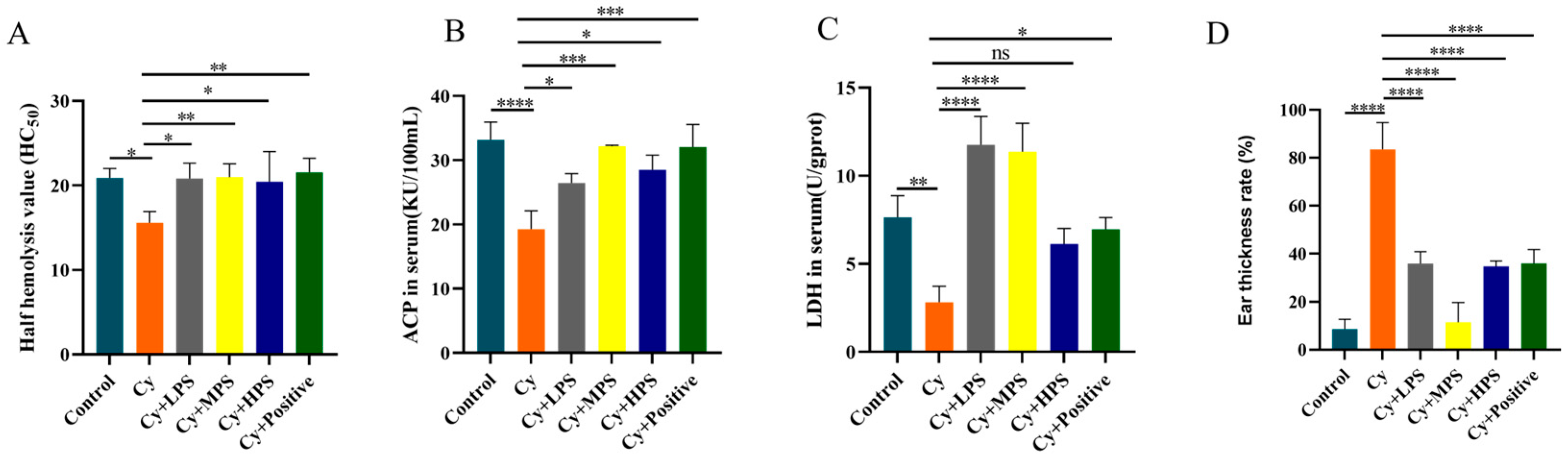
3.3. PS Modulated Hemolysin Levels in Cyclophosphamide-Induced Immunosuppressed Mice
3.4. PS Regulated Lactate Dehydrogenase and Acid Phosphatase Levels in Cyclophosphamide-Induced Immunosuppressed Mice
3.5. PS Alleviated Xylene-Induced Inflammation in Cyclophosphamide-Induced Immunosuppressed Mice
3.6. Recovery Effect of PS on Blood Counts in Cyclophosphamide-Induced Immunosuppressed Mice
3.7. PS Supplementation Improved Splenic Lymphocyte Proliferation in Cyclophosphamide-Induced Immunosuppressed Mice
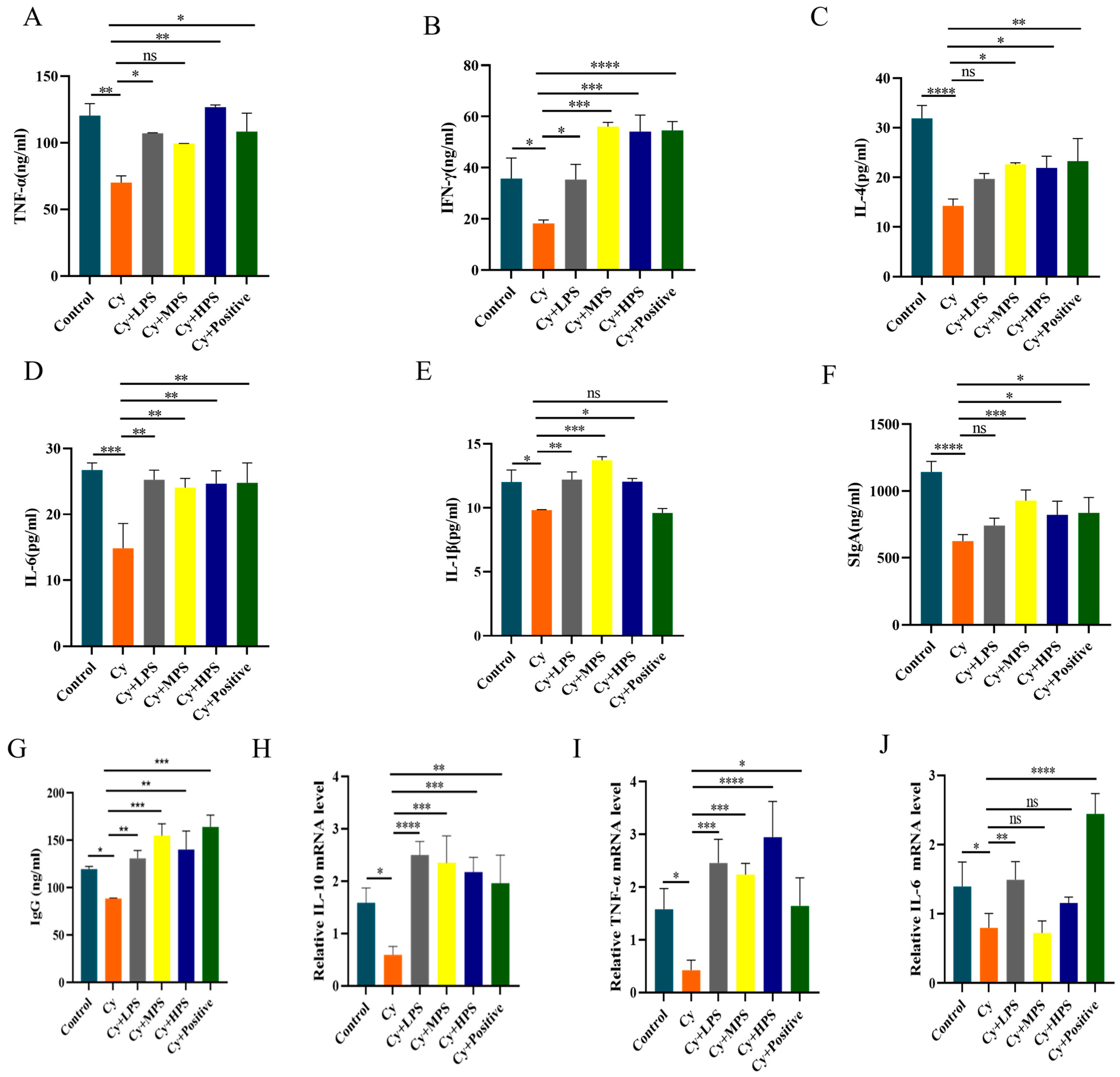
3.8. PS Modulated Inflammatory Factors and Immunoglobulin in Cyclophosphamide-Induced Immunosuppressed Mice
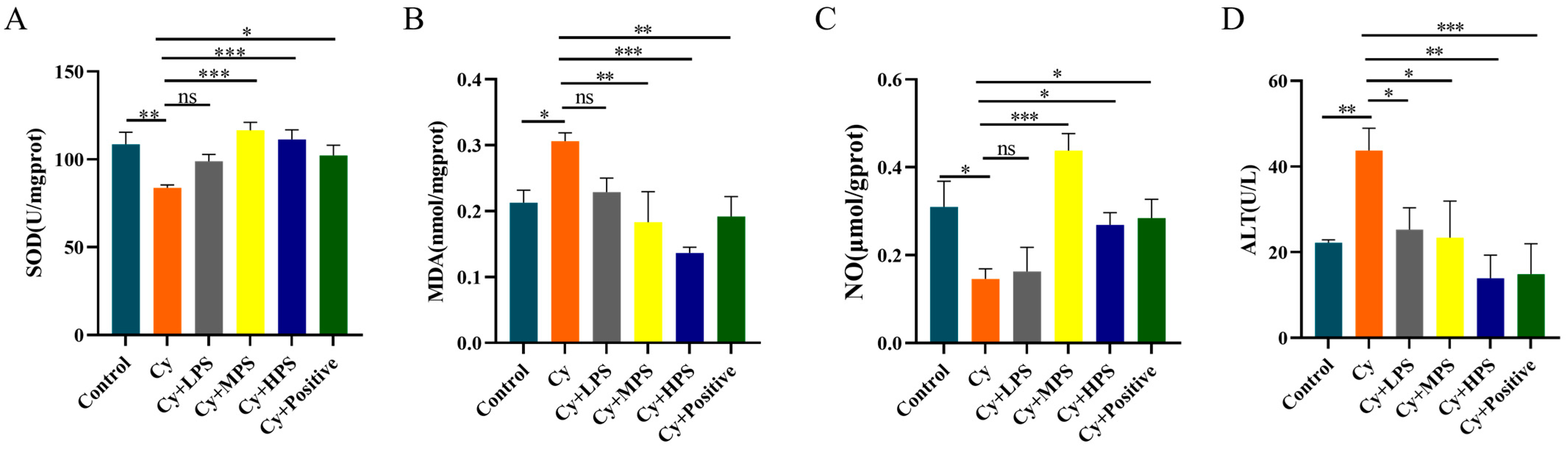
3.9. PS Modulated Oxidative Stress in Cyclophosphamide-Induced Immunosuppressed Mice
3.10. PS Enhanced the Intestinal Barrier in Cyclophosphamide-Induced Immunosuppressed Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| Cy | Cyclophosphamide |
| PS | Polygonatum sibiricum saponins |
| LPS | Low-dose Polygonatum sibiricum saponins |
| MPS | Middle-dose Polygonatum sibiricum saponins |
| HPS | High-dose Polygonatum sibiricum saponins |
| HC50 | Serum hemolysis value |
| CRBC | Chicken red blood cell |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1β |
| IL-10 | Interleukin-10 |
| IL-4 | Interleukin-4 |
| TNF-α | Tumor necrosis factor-α |
| IFN-γ | Interferon-γ |
| SIgA | Secretory immunoglobulin A |
| IgG | Immunoglobulin G |
| ACP | Acid phosphatase |
| LDH | Lactate Dehydrogenase |
| ALT | Alanine aminotransferase |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| NO | Nitric oxide |
| Mucin-2 | Mucoprotein-2 |
| ZO-1 | Zonula occludens protein 1 |
| TLR4 | Toll Like Receptor 4 |
| MyD88 | Myeloiddifferentiationfactor88 |
| WBC | White blood cell |
| RBC | Red blood cell |
| HGB | Hemoglobin |
| HCT | Hematocrit |
| PLT | Platelet |
| LYM | Lymphocyte |
| GRAN | Granulocyte |
| JC-1 | 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine |
| CCK-8 | Cell Counting Kit-8 |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef]
- Zhao, S.; Peng, X.; Zhou, Q.Y.; Huang, Y.Y.; Rao, X.; Tu, J.L.; Xiao, H.Y.; Liu, D.M. Bacillus coagulans 13002 and fructo-oligosaccharides improve the immunity of mice with immunosuppression induced by cyclophosphamide through modulating intestinal-derived and fecal microbiota. Food Res. Int. 2021, 140, 109793. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, R.; Heeba, G.H.; Hassanin, S.O.; Waz, S.; Amin, A. TLRs-JNK/ NF-κB Pathway Underlies the Protective Effect of the Sulfide Salt against Liver Toxicity. Front. Pharmacol. 2022, 13, 850066. [Google Scholar] [CrossRef]
- Awad, B.; Hamza, A.A.; Al-Maktoum, A.; Al-Salam, S.; Amin, A. Combining Crocin and Sorafenib Improves Their Tumor-Inhibiting Effects in a Rat Model of Diethylnitrosamine-Induced Cirrhotic-Hepatocellular Carcinoma. Cancers 2023, 15, 4063. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Jin, M.L.; Morris, G.A.; Zha, X.Q.; Chen, H.Q.; Yi, Y.; Li, J.E.; Wang, Z.J.; Gao, J.; Nie, S.P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. 1), S60–S84. [Google Scholar] [CrossRef]
- Murali, C.; Mudgil, P.; Gan, C.Y.; Tarazi, H.; El-Awady, R.; Abdalla, Y.; Amin, A.; Maqsood, S. Camel whey protein hydrolysates induced G2/M cellcycle arrest in human colorectal carcinoma. Sci. Rep. 2021, 11, 7062. [Google Scholar] [CrossRef]
- Bouabdallah, S.; Al-Maktoum, A.; Amin, A. Steroidal Saponins: Naturally Occurring Compounds as Inhibitors of the Hallmarks of Cancer. Cancers 2023, 15, 3900. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Li, C.; Shao, Y.; Chen, A. Polysaccharides from Spores of Cordyceps cicadae Protect against Cyclophosphamide-Induced Immunosuppression and Oxidative Stress in Mice. Foods 2022, 11, 515. [Google Scholar] [CrossRef]
- Qian, Y.; Huang, R.; Li, S.; Xie, R.; Qian, B.; Zhang, Z.; Li, L.; Wang, B.; Tian, C.; Yang, J.; et al. Ginsenoside Rh2 reverses cyclophosphamide-induced immune deficiency by regulating fatty acid metabolism. J. Leukoc. Biol. 2019, 106, 1089–1100. [Google Scholar] [CrossRef]
- Wen, X.; Peng, H.; Zhang, H.; He, Y.; Guo, F.; Bi, X.; Liu, J.; Sun, Y. Wheat Bran Polyphenols Ameliorate DSS-Induced Ulcerative Colitis in Mice by Suppressing MAPK/NF-κB Inflammasome Pathways and Regulating Intestinal Microbiota. Foods 2024, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Luo, J.; Bao, Y. Effects of Polygonatum sibiricum saponin on hyperglycemia, gut microbiota composition and metabolic profiles in type 2 diabetes mice. Biomed. Pharmacother. 2021, 143, 112155. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.A.; Mohamed, M.G.; Fawzy, M.; Lashin, F.M.; Amin, A. Dandelion prevents liver fibrosis, inflammatory response, and oxidative stress in rats. J. Basic Appl. Zool. 2020, 81, 43. [Google Scholar] [CrossRef]
- Koboziev, I.; Scoggin, S.; Gong, X.; Mirzaei, P.; Zabet-Moghaddam, M.; Yosofvand, M.; Moussa, H.; Jones-Hall, Y.; Moustaid-Moussa, N. Effects of Curcumin in a Mouse Model of Very High Fat Diet-Induced Obesity. Biomolecules 2020, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.G.; Huang, X.J.; Chen, J. Separation and purification of saponins from Semen Ziziphus jujuba and their sedative and hypnotic effects. J. Pharm. Pharmacol. 2007, 59, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, X.; Lin, L.; Ding, G.; Yu, F. Immunomodulatory Activity of Low Molecular-Weight Peptides from Nibea japonica in RAW264.7 Cells via NF-κB Pathway. Mar. Drugs 2019, 17, 404. [Google Scholar] [CrossRef]
- Meng, M.; Sun, Y.; Bai, Y.; Xu, J.; Sun, J.; Han, L.; Sun, H.; Han, R. A polysaccharide from Pleurotus citrinopileatus mycelia enhances the immune response in cyclophosphamide-induced immunosuppressed mice via p62/Keap1/Nrf2 signal transduction pathway. Int. J. Biol. Macromol. 2023, 228, 165–177. [Google Scholar] [CrossRef]
- Tiwari, R.; Latheef, S.K.; Ahmed, I.; Iqbal, H.M.N.; Bule, M.H.; Dhama, K.; Samad, H.A.; Karthik, K.; Alagawany, M.; El-Hack, M.E.A.; et al. Herbal Immunomodulators—A Remedial Panacea for Designing and Developing Effective Drugs and Medicines: Current Scenario and Future Prospects. Curr. Drug Metab. 2018, 19, 264–301. [Google Scholar] [CrossRef]
- Guan, Q.Y.; Lin, Y.R.; Li, L.Y.; Tang, Z.M.; Zhao, X.H.; Shi, J. In Vitro Immunomodulation of the Polysaccharides from Yam (Dioscorea opposita Thunb.) in Response to a Selenylation of Lower Extent. Foods 2021, 10, 2788. [Google Scholar] [CrossRef]
- Elango, C.; Devaraj, S.N. Immunomodulatory effect of Hawthorn extract in an experimental stroke model. J. Neuroinflamm. 2010, 7, 97. [Google Scholar] [CrossRef]
- Liu, L.; Dong, Q.; Dong, X.T.; Fang, J.N.; Ding, K. Structural investigation of two neutral polysaccharides isolated from rhizome of Polygonatum sibiricum. Carbohydr. Polym. 2007, 70, 304–309. [Google Scholar] [CrossRef]
- Tang, C.; Yu, Y.M.; Qi, Q.L.; Wu, X.D.; Wang, J.; Tang, S.A. Steroidal saponins from the rhizome of Polygonatum sibiricum. J. Asian Nat. Prod. Res. 2019, 21, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Wang, Y.; Zeng, L.; Dong, J.; Bi, Q.; Yang, X.; Che, Y.; He, S.; Yu, J. Polysaccharides from Polygonatum kingianum improve glucose and lipid metabolism in rats fed a high fat diet. Biomed. Pharmacother. 2020, 125, 109910. [Google Scholar] [CrossRef]
- Shen, F.; Xie, P.; Li, C.; Bian, Z.; Wang, X.; Peng, D.; Zhu, G. Polysaccharides from Polygonatum cyrtonema Hua Reduce Depression-Like Behavior in Mice by Inhibiting Oxidative Stress-Calpain-1-NLRP3 Signaling Axis. Oxidative Med. Cell. Longev. 2022, 2022, 2566917. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Wang, X.; Zhao, B.; Zhao, Y.; Liu, H.; Chang, Y.; Wang, Z.; Yang, W.; Zhang, X.; Yu, K. Metabolome and transcriptome signatures shed light on the anti-obesity effect of Polygonatum sibiricum. Front. Plant Sci. 2023, 14, 1181861. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, Z.; Yang, R.; Ye, Y.; Pei, L.; Xiong, S.; Wang, S.; Wang, L.; Liu, S. Polysaccharide-rich extract from Polygonatum sibiricum protects hematopoiesis in bone marrow suppressed by triple negative breast cancer. Biomed. Pharmacother. 2021, 137, 111338. [Google Scholar] [CrossRef]
- Yang, J.X.; Wu, S.; Huang, X.L.; Hu, X.Q.; Zhang, Y. Hypolipidemic Activity and Antiatherosclerotic Effect of Polysaccharide of Polygonatum sibiricum in Rabbit Model and Related Cellular Mechanisms. Evid.-Based Complement. Altern. Med. 2015, 2015, 391065. [Google Scholar] [CrossRef]
- Xie, Y.; Mu, C.; Kazybay, B.; Sun, Q.; Kutzhanova, A.; Nazarbek, G.; Xu, N.; Nurtay, L.; Wang, Q.; Amin, A.; et al. Network pharmacology and experimental investigation of Rhizoma polygonati extract targeted kinase with herbzyme activity for potent drug delivery. Drug Deliv. 2021, 28, 2187–2197. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, L.; Li, Z.; Shao, Z.; Qi, Y.; Gao, K.; Liu, S.; Sun, Y.; Li, P.; Liu, J. Immunomodulatory Effects of (24R)-Pseudo-Ginsenoside HQ and (24S)-Pseudo-Ginsenoside HQ on Cyclophosphamide-Induced Immunosuppression and Their Anti-Tumor Effects Study. Int. J. Mol. Sci. 2019, 20, 836. [Google Scholar] [CrossRef]
- Ma, Y.L.; Zhang, Y.S.; Zhang, F.; Zhang, Y.Y.; Thakur, K.; Zhang, J.G.; Wei, Z.J. Methyl protodioscin from Polygonatum sibiricum inhibits cervical cancer through cell cycle arrest and apoptosis induction. Food Chem. Toxicol. 2019, 132, 110655. [Google Scholar] [CrossRef]
- Cao, R.; Fang, X.; Li, Z.; Li, S.; Guo, Q.; Chai, Y. Effect of Polygonatum sibiricum saponins on gut microbiota of mice with ulcerative colitis. Fitoterapia 2024, 174, 105855. [Google Scholar] [CrossRef]
- Zhou, D.; Li, X.; Chang, W.; Han, Y.; Liu, B.; Chen, G.; Li, N. Antiproliferative steroidal glycosides from rhizomes of Polygonatum sibiricum. Phytochemistry 2019, 164, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Guo, A.; Jing, Y.; Lin, J.; Sun, Y.; Kong, L.; Zheng, H.; Deng, Y. Immunomodulatory activity of puerarin in RAW264.7 macrophages and cyclophosphamide-induced immunosuppression mice. Immunopharmacol. Immunotoxicol. 2021, 43, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Wu, L.; Zhu, N.; Liu, X.; Liu, R.; Li, Y. Naked Oat (Avena nuda L.) Oligopeptides:Immunomodulatory Effects on Innate and AdaptiveImmunity in Mice via Cytokine Secretion, AntibodyProduction, and Th Cells Stimulation. Nutrients 2019, 11, 927. [Google Scholar] [CrossRef] [PubMed]
- Bihl, F.; Germain, C.; Luci, C.; Braud, V.M. Mechanisms of NK cell activation: CD4(+) T cells enter the scene. Cell. Mol. Life Sci. 2011, 68, 3457–3467. [Google Scholar] [CrossRef] [PubMed]
- Ladics, G.S. Use of SRBC antibody responses for immunotoxicity testing. Methods 2007, 41, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, J.; Zhang, Y.; He, J.; Guo, X.; Tian, G.; Jin, L. Studies of macrophage immuno-modulating activity of polysaccharides isolated from Paecilomyces tenuipes. Int. J. Biol. Macromol. 2008, 43, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Ngulde, S.I.; Sandabe, U.K.; Abounader, R.; Zhang, Y.; Hussaini, I.M. Activities of Some Medicinal Plants on the Proliferation and Invasion of Brain Tumor Cell Lines. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 3626879. [Google Scholar] [CrossRef] [PubMed]
- Benassi, E.; Fan, H.; Sun, Q.; Dukenbayev, K.; Wang, Q.; Shaimoldina, A.; Tassanbiyeva, A.; Nurtay, L.; Nurkesh, A.; Kutzhanova, A.; et al. Generation of particle assemblies mimicking enzymatic activity by processing of herbal food: The case of rhizoma polygonati and other natural ingredients in traditional Chinese medicine. Nanoscale Adv. 2021, 3, 2222–2235. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Meng, M.; Sun, H.; Li, Y.; Ren, Y.Y.; Zhang, Y. Immunostimulatory activity of glycopeptides from Paecilomyces sinensis under normal and cyclophosphamide induced immunosuppressive conditions in mice models. Food Funct. 2016, 7, 3566–3576. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, T.; Meng, F.; Wang, H.; Tian, P.; Tang, X.; Wang, X.; Wang, X.; Xin, H.; Wei, H. Therapeutic effect of herb residue fermentation supernatant on spleen-deficient mice. Mol. Med. Rep. 2018, 17, 2764–2770. [Google Scholar] [CrossRef]
- Wang, M.; Wang, H.; Tang, Y.; Kang, D.; Gao, Y.; Ke, M.; Dou, J.; Xi, T.; Zhou, C. Effective inhibition of a Strongylocentrotus nudus eggs polysaccharide against hepatocellular carcinoma is mediated via immunoregulation in vivo. Immunol. Lett. 2011, 141, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Fan, J.; Song, Z.; Du, X.; Chen, Y.; Wang, J.; Song, G. Characterization and immunoenhancement activities of Eucommia ulmoides polysaccharides. Carbohydr. Polym. 2016, 136, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Marits, P.; Wikström, A.C.; Popadic, D.; Winqvist, O.; Thunberg, S. Evaluation of T and B lymphocyte function in clinical practice using a flow cytometry based proliferation assay. Clin. Immunol. 2014, 153, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, X.; Wu, M. miRNAs reshape immunity and inflammatory responses in bacterial infection. Signal Transduct. Target. Ther. 2018, 3, 14. [Google Scholar] [CrossRef]
- Ahmad, I.; Valverde, A.; Naqvi, R.A.; Naqvi, A.R. Long Non-coding RNAs RN7SK and GAS5 Regulate Macrophage Polarization and Innate Immune Responses. Front. Immunol. 2020, 11, 604981. [Google Scholar] [CrossRef]
- Monmai, C.; You, S.; Park, W.J. Immune-enhancing effects of anionic macromolecules extracted from Codium fragile on cyclophosphamide-treated mice. PLoS ONE 2019, 14, e0211570. [Google Scholar] [CrossRef]
- Li, H.; Zhao, B.L.; Hou, J.W.; Xin, W.J. Two-peak kinetic curve of the chemiluminescence in phorbol-induced macrophage. Biochem. Biophys. Res. Commun. 1996, 223, 311–314. [Google Scholar] [CrossRef]
- Belska, N.V.; Guriev, A.M.; Danilets, M.G.; Trophimova, E.S.; Uchasova, E.G.; Ligatcheva, A.A.; Belousov, M.V.; Agaphonov, V.I.; Golovchenko, V.G.; Yusubov, M.S.; et al. Water-soluble polysaccharide obtained from Acorus calamus L. classically activates macrophages and stimulates Th1 response. Int. Immunopharmacol. 2010, 10, 933–942. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, W.; Li, C.; Zhang, M.; Jiang, Y.; Jiang, R.; Ba, H.; Li, C.; Wang, J.; Yin, B.; et al. Kupffer-cell-expressed transmembrane TNF-α is a major contributor to lipopolysaccharide and D-galactosamine-induced liver injury. Cell Tissue Res. 2016, 363, 371–383. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Miao, M.; Cheng, B.; Guo, L.; Shi, J. Effects of Fuzheng Paidu tablet on peripheral blood T lymphocytes, intestinal mucosa T lymphocytes, and immune organs in cyclophosphamide-induced immunosuppressed mice. Hum. Vaccines Immunother. 2015, 11, 2659–2663. [Google Scholar] [CrossRef]
- Rajasagi, N.K.; Rouse, B.T. IL-2 complex treatment amplifies CD8(+) T cell mediated immunity following herpes simplex virus-1 infection. Microbes Infect. 2016, 18, 735–746. [Google Scholar] [CrossRef]
- Simic, M.G.; Bergtold, D.S.; Karam, L.R. Generation of oxy radicals in biosystems. Mutat. Res. 1989, 214, 3–12. [Google Scholar] [CrossRef]
- Duggina, P.; Kalla, C.M.; Varikasuvu, S.R.; Bukke, S.; Tartte, V. Protective effect of centella triterpene saponins against cyclophosphamide-induced immune and hepatic system dysfunction in rats: Its possible mechanisms of action. J. Physiol. Biochem. 2015, 71, 435–454. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Mo, S.; Shen, M.; Chen, Y.; Yu, Q.; Li, Z.; Xie, J. Sulfated modification enhances the immunomodulatory effect of Cyclocarya paliurus polysaccharide on cyclophosphamide-induced immunosuppressed mice through MyD88-dependent MAPK/NF-κB and PI3K-Akt signaling pathways. Food Res. Int. 2021, 150, 110756. [Google Scholar] [CrossRef] [PubMed]
- Buettner, M.; Lochner, M. Development and Function of Secondary and Tertiary Lymphoid Organs in the Small Intestine and the Colon. Front. Immunol. 2016, 7, 342. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, L.; Xu, C.; Wang, Y.; Wang, Z.; Chen, M.; Jiang, Z.; Pan, J.; Yang, C.; Li, X.; et al. Cross-talk between the gut microbiota and monocyte-like macrophages mediates an inflammatory response to promote colitis-associated tumourigenesis. Gut 2020, 70, 1495–1506. [Google Scholar] [CrossRef]
- Mestecky, J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J. Clin. Immunol. 1987, 7, 265–276. [Google Scholar] [CrossRef]
- Kong, X.; Duan, W.; Li, D.; Tang, X.; Duan, Z. Effects of Polysaccharides from Auricularia auricula on the Immuno-Stimulatory Activity and Gut Microbiota in Immunosuppressed Mice Induced by Cyclophosphamide. Front. Immunol. 2020, 11, 595700. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Kuo, W.T.; Turner, J.R. Tight Junctions as Targets and Effectors of Mucosal Immune Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, Y.; Zhou, W.; Chen, D.; Cao, Y. Effects of polysaccharides from bee collected pollen of Chinese wolfberry on immune response and gut microbiota composition in cyclophosphamide-treated mice. J. Funct. Foods 2020, 72, 104057. [Google Scholar] [CrossRef]
- Zhang, H.M.; Rao, J.N.; Guo, X.; Liu, L.; Zou, T.; Turner, D.J.; Wang, J.Y. Akt kinase activation blocks apoptosis in intestinal epithelial cells by inhibiting caspase-3 after polyamine depletion. J. Biol. Chem. 2004, 279, 22539–22547. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| IL-10 | GGTTGCCAAGCCTTATCGGA | GAGAAATCGATGACAGCGCC |
| IL-6 | CTCAGCGCTGAGTTG | CCTGTAGCCCACGTCGTAGC |
| TNF-α | CGGGCAGGTCTACTTTGGAG | ACCCTGAGCCATAATCCCCT |
| Mucin-2 | CCGGATCTATGCCGTTGCTA | TCCAGGTGGGTATCGAGTGT |
| Zo-1 | ACCCGAAACTGATGCTGTGGATAG | AAATGGCCGGGCAGAACTTGTGTA |
| Occludin | TAGGGGCTCGGCAGGCTAT | CCGATCCATCTTTCTTCGGGT |
| GAPDH | TGTGTCCGTCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG |
| Hematological Parameter | Control | Cy | Cy + LPS | Cy + MPS | Cy + HPS | Cy + Positive |
|---|---|---|---|---|---|---|
| WBC (×109/L) | 9.55 ± 0.04 **** | 8.75 ± 0.08 | 9.23 ± 0.13 *** | 9.02 ± 0.07 * | 9.17 ± 0.08 *** | 9.11 ± 0.24 ** |
| RBC (×1012/L) | 13.05 ± 0.02 * | 12.90 ± 0.03 | 13.07 ± 0.13 * | 13.06 ± 0.04 * | 13.00 ± 0.01 ns | 13.01 ± 0.06 ns |
| HGB (g/dL) | 3.24 ± 0.01 **** | 3.08 ± 0.04 | 3.20 ± 0.01 *** | 3.22 ± 0.03 *** | 3.18 ± 0.01 ** | 3.15 ± 0.04 * |
| HCT (%) | 54.47 ± 2.89 *** | 38.65 ± 3.18 | 51.20 ± 1.22 * | 57.30 ± 5.33 **** | 49.33 ± 1.74 * | 48.00 ± 3.62 * |
| PLT (×109/L) | 12.04 ± 0.13 **** | 10.77 ± 0.03 | 11.35 ± 0.19 ** | 11.62 ± 0.14 **** | 11.56 ± 0.06 *** | 11.94 ± 0.20 **** |
| Lym (×109/L) | 9.43 ± 0.06 **** | 8.77 ± 0.07 | 9.04 ± 0.13 ** | 8.97 ± 0.06 * | 9.04 ± 0.05 ** | 9.19 ± 0.07 **** |
| Gran (×109/L) | 8.90 ± 0.09 ** | 8.45 ± 0.21 | 8.78 ± 0.15 ns | 8.48 ± 0 ns | 8.58 ± 0.16 ns | 8.67 ± 0.09 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Liu, H.; Yan, C.; Teng, Y.; Zou, Y.; Ren, X.; Xia, X. Polygonatum sibiricum Saponin Prevents Immune Dysfunction and Strengthens Intestinal Mucosal Barrier Function in Cyclophosphamide-Induced Immunosuppressed BALB/c Mice. Foods 2024, 13, 934. https://doi.org/10.3390/foods13060934
Zhao D, Liu H, Yan C, Teng Y, Zou Y, Ren X, Xia X. Polygonatum sibiricum Saponin Prevents Immune Dysfunction and Strengthens Intestinal Mucosal Barrier Function in Cyclophosphamide-Induced Immunosuppressed BALB/c Mice. Foods. 2024; 13(6):934. https://doi.org/10.3390/foods13060934
Chicago/Turabian StyleZhao, Dongyun, Huanhuan Liu, Chunhong Yan, Yue Teng, Yue Zou, Xiaomeng Ren, and Xiaodong Xia. 2024. "Polygonatum sibiricum Saponin Prevents Immune Dysfunction and Strengthens Intestinal Mucosal Barrier Function in Cyclophosphamide-Induced Immunosuppressed BALB/c Mice" Foods 13, no. 6: 934. https://doi.org/10.3390/foods13060934





