Encapsulation of Indicaxanthin-Rich Opuntia Green Extracts by Double Emulsions for Improved Stability and Bioaccessibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Solvents, and Standards
2.2. Plant Material and Physicochemical Analysis
2.3. Obtention of Green Extracts Rich in Bioactive Compounds
2.4. Analysis of Betalains and Phenolic Compounds by HPLC-DAD-MS
2.5. In Vitro Antioxidant Capacity of Opuntia ficus-indica var. Colorada Pulp Green Extracts
2.6. Elaboration of Double Emulsion Systems W1/O/W2
2.6.1. Tween 20-Based W1/O/W2 Double Emulsion Systems (TW)
2.6.2. Sodium Caseinate-Based W1/O/W2 Double Emulsion Systems (SC)
2.7. In Vitro Gastro-Intestinal Digestion Assay
2.8. Characterization of Double Emulsions (W1/O/W2)
2.8.1. Encapsulation Efficiency
2.8.2. Optical and Confocal Microscopy Study of TW and SC Double Emulsions
2.8.3. Physical Stability of Double Emulsions during Cold Storage
2.8.4. Particle Size and Zeta Potential
2.9. Statistical Analysis
3. Results and Discussion
3.1. Composition in Main Bioactives of O. ficus-indica var. Colorada Pulps Extracts and TW and SC Double Emulsions
3.2. Antioxidant Capacity of O. ficus-indica var. Colorada Pulps Extracts
3.3. Characterization of the Double Emulsion Systems (W1/O/W2)
3.3.1. Encapsulation Efficiency of the Main Bioactives Encapsulated by Double Emulsions (W1/O/W2)
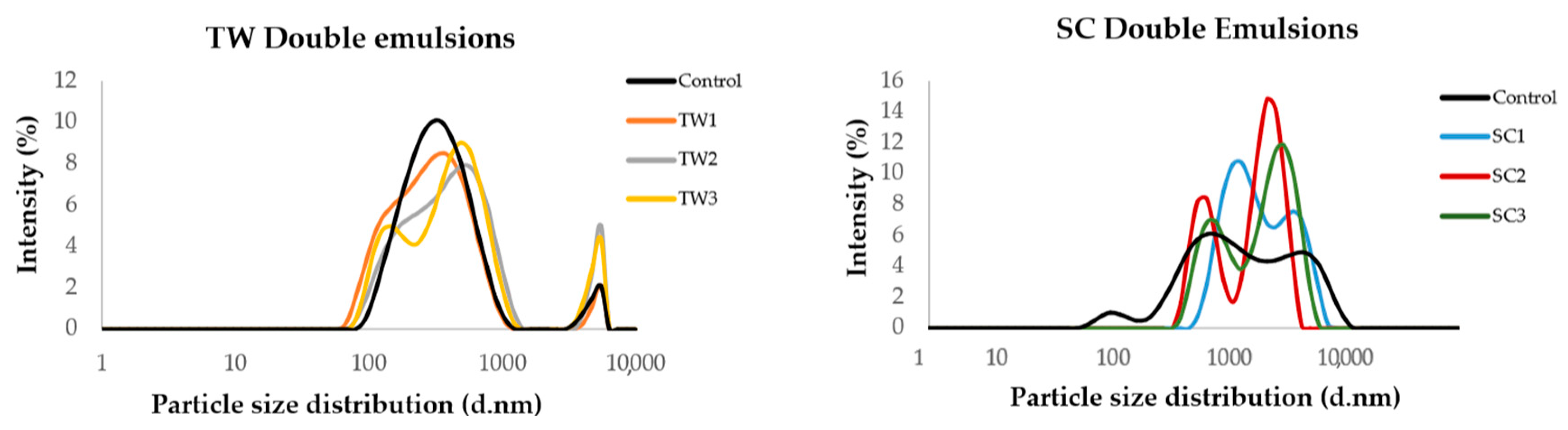
3.3.2. Morphology of Double Emulsion Systems (W1/O/W2)
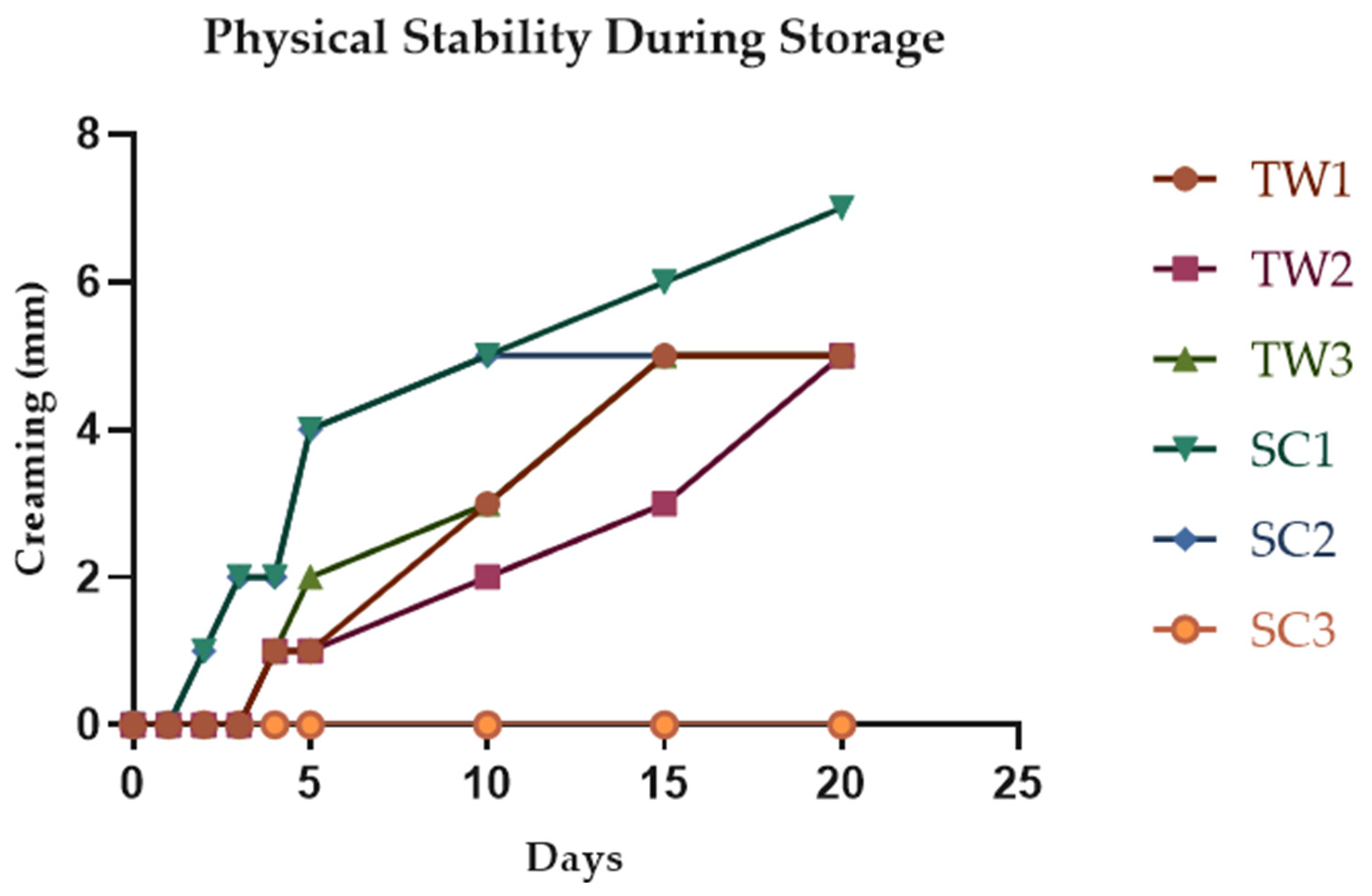
3.3.3. Physical Stability of Double Emulsions
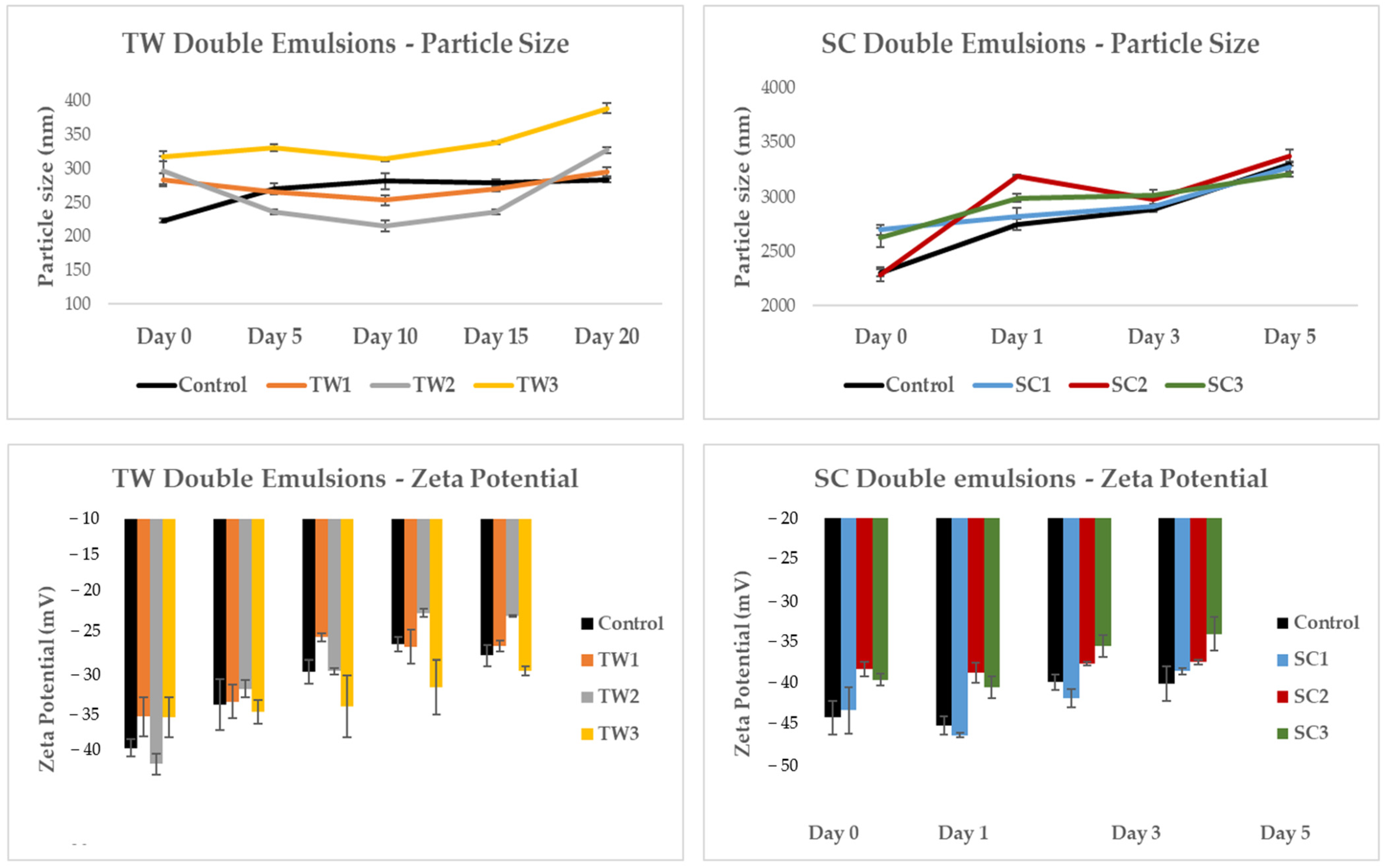
3.3.4. Particle Size and Zeta Potential
3.4. In Vitro Gastro-Intestinal Digestion Assay
3.4.1. Confocal Laser Microscopy Analysis of Gastro-Intestinal Digestion Phases
3.4.2. Particle Size and Zeta Potential of Double Emulsions during Gastro-Intestinal Digestion
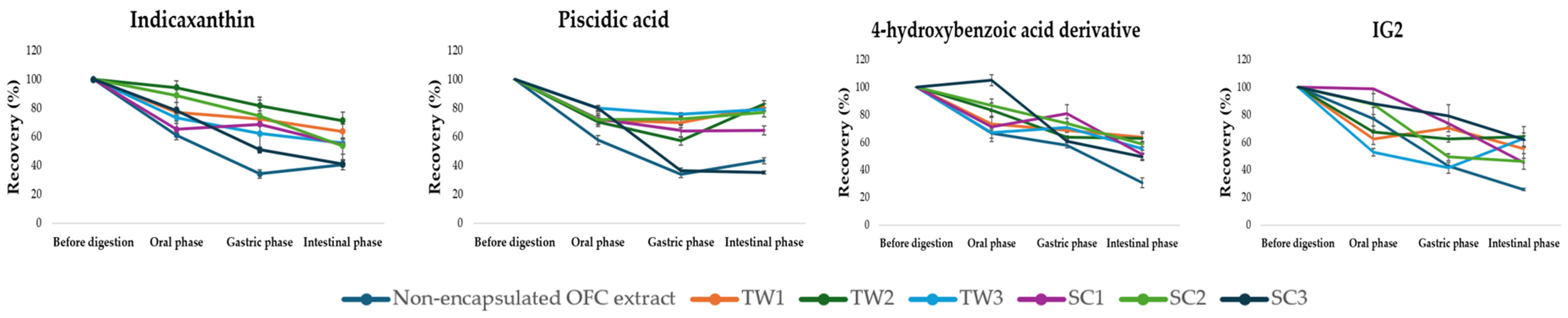
3.4.3. Digestive Stability, Recovery, and Bioaccessibility of Encapsulated OFC Bioactives by Double Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boudjouan, F.; Zeghbib, W.; Carneiro, J.; Silva, R.; Morais, J.; Vasconcelos, V.; Lopes, G. Comparison study on wild and cultivated Opuntia sp.: Chemical, taxonomic, and antioxidant evaluations. Agriculture 2022, 12, 1755. [Google Scholar] [CrossRef]
- Paiva, P.; Souza, I.; Costa, M.; Santos, A.; Coelho, L. Opuntia sp. cactus: Biological characteristics, cultivation and applications. Adv. Res. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; Antunes-Ricardo, M.; Welti-Chanes, J.Y.; Cano, M.P. Digestive Stability and Bioaccessibility of Antioxidants in Prickly Pear Fruits from the Canary Islands: Healthy Foods and Ingredients. Antioxidants 2020, 9, 164. [Google Scholar] [CrossRef]
- Attanzio, A.; Restivo, I.; Tutone, M.; Tesoriere, L.; Allegra, M.; Livrea, M.A. Redox Properties, Bioactivity and Health Effects of Indicaxanthin, a Bioavailable Phytochemical from Opuntia ficus indica, L.: A Critical Review of Accumulated Evidence and Perspectives. Antioxidants 2022, 11, 2364. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Escribano, J.y.; García-Carmona, F. Biological Activities of Plant Pigments Betalains. Crit. Rev. Food Sci. Nutr. 2016, 56, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Maqueo, A.; Soccio, M.Y.; Cano, M.P. In Vitro Antioxidant Capacity of Opuntia spp. Fruits Measured by the LOX-FL Method and its High Sensitivity Towards Betalains. Plant Foods Hum. Nutr. 2021, 76, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.Y.; Bartosz, G. Biological Properties and Applications of Betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Gentile, C.; Angileri, F.; Attanzio, A.; Tutone, M.; Allegra, M.Y.; Livrea, M.A. Trans-epithelial transport of the betalain pigments indicaxanthin and betanin across Caco-2 cell monolayers and influence of food matrix. Eur. J. Nutr. 2013, 52, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Allegra, M.; Tutone, M.; Tesoriere, L.; Almerico, A.M.; Culletta, G.; Livrea, M.A.; Attanzio, A. Indicaxanthin, a multi-target natural compound from Opuntia ficus-indica fruit: From its poly-pharmacological effects to biochemical mechanisms and molecular modelling studies. Eur. J. Med. Chem. 2019, 179, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Sánchez, M.; García-Cayuela, T.; Gómez-Maqueo, A.; García, H.S.y.; Cano, M.P. In vitro gastrointestinal stability, bioaccessibility and potential activities of betalains and phenolic compounds in cactus berry fruits (Myrtillocactus geometrizans). Food Chem. 2021, 342, 128087. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft AJ, A.B. A brief review on bioactive compounds in plants. Bioact. Compd. Plants-Benefits Risks Man Anim. 2010, 50, 11–17. [Google Scholar]
- Heidari, F.; Jafari, S.M.; Ziaiifar AM y Malekjani, N. Stability and release mechanisms of double emulsions loaded with bioactive compounds: A critical review. Adv. Colloid Interface Sci. 2021, 299, 102567. [Google Scholar] [CrossRef]
- Kanouni, M.; Rosano, H.L.; Naouli, N. Preparation of a stable double emulsion (W1/O/W2): Role of the interfacial films on the stability of the system. Adv. Colloid Interface Sci. 2002, 99, 229–254. [Google Scholar] [CrossRef]
- Ghasemi, H.; Darjani, S.; Mazloomi, H.; Mozaffari, S. Preparation of stable multiple emulsions using food-grade emulsifiers: Evaluating the effects of emulsifier concentration, W/O phase ratio, and emulsification process. SN Appl. Sci. 2020, 2, 2002. [Google Scholar] [CrossRef]
- Kaimainen, M.; Marze, S.; Järvenpää, E.; Anton, M.; Huopalahti, R. Encapsulation of betalain into w/o/w double emulsion and release during in vitro intestinal lipid digestion. LWT-Food Sci. Technol. 2015, 60, 899–904. [Google Scholar] [CrossRef]
- Tran, Q.H.; Chu, H.K.T.; Nguyen, P.T.; Nguyen, V.M.; Nguyen, Q.T.; Tran, C.D.; Nguyen, T.D. Double Nanoemulsion Loading Betalains Extract of Beetroot (Beta vulgaris L.): Ultrasound-Assisted Synthesis, Storage Stability, and Antioxidant Activity. ACS Food Sci. Tech. 2023, 3, 2229–2237. [Google Scholar] [CrossRef]
- Robert, P.; Vergara, C.; Silva-Weiss, A.; Osorio, F.A.; Santander, R.; Sáenz, C.; Giménez, B. Influence of gelation on the retention of purple cactus pear extract in microencapsulated double emulsions. PLoS ONE 2020, 15, e0227866. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Kaur, G.; Mehta, S.K. Developments of Polysorbate (Tween) based microemulsions: Preclinical drug delivery, toxicity and antimicrobial applications. Int. J. Pharm. 2017, 529, 134–160. [Google Scholar] [CrossRef]
- Lam, R.S.; Nickerson, M.T. Food proteins: A review on their emulsifying properties using a structure–function approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fuorescein as the fuorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Parralejo-Sanz, S.; Gómez-López, I.; González-Álvarez, E.; Montiel-Sánchez, M.y.; Cano, M.P. Oil-Based Double Emulsion Microcarriers for Enhanced Stability and Bioaccesibility of Betalains and Phenolic Compounds from Opuntia stricta var. dillenii Green Extracts. Foods 2023, 12, 2243. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Hong, I.K.; Kim, S.I.; Lee, S.B. Effects of HLB value on oil-in-water emulsions: Droplet size, rheological behavior, zeta-potential, and creaming index. J. Ind. Eng. Chem. 2018, 67, 123–131. [Google Scholar] [CrossRef]
- Tesoriere, L.; Fazzari, M.; Angileri, F.; Gentile, C.; Livrea, M.A. In vitro digestion of betalainic foods. Stability and bioaccessibility of betaxanthins and betacyanins and antioxidative potential of food digesta. J. Agric. Food Chem. 2008, 56, 10487–10492. [Google Scholar] [CrossRef]
- Fernández-Repetto, A.; Gómez-Maqueo, A.; García-Cayuela, T.; Guajardo-Flores, D.; Cano, M.P. Analysis of hydrocolloid excipients for controlled delivery of high-value microencapsulated prickly pear extracts. Food Hydrocoll. Health 2023, 3, 100–115. [Google Scholar] [CrossRef]
- Kuti, J.O. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004, 85, 527–533. [Google Scholar] [CrossRef]
- Carmona, J.C.; Robert, P.; Vergara, C.; Sáenz, C. Microparticles of yellow-orange cactus pear pulp (Opuntia ficus-indica) with cladode mucilage and maltodextrin as a food coloring in yogurt. LWT 2021, 138, 110672. [Google Scholar] [CrossRef]
- de Kruif, C.K.; Bhatt, H.; Anema, S.G.; Coker, C. Rheology of caseinate fractions in relation to their water holding capacity. Food Hydrocoll. 2015, 51, 503–511. [Google Scholar] [CrossRef]
- Sanhueza, L.; García, P.; Giménez, B.; Benito, J.M.; Matos, M.; Gutiérrez, G. Encapsulation of pomegranate peel extract (Punica granatum L.) by double emulsions: Effect of the encapsulation method and oil phase. Foods 2022, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Rigi, A.; Abbasi, S.; Emmambux, M.N. Background, limitations, and future perspectives in food grade microemulsions and nanoemulsions. Food Rev. Int. 2023, 39, 5048–5086. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of physicochemical characterization of nanoparticles in pharmaceutical product development. In Basic Fundamentals of Drug Delivery; Academic Press: Cambridge, MA, USA, 2019; pp. 369–400. [Google Scholar] [CrossRef]
- Mohammed, A.N.; Ishwarya, S.P.; Nisha, P. Nanoemulsion versus microemulsion systems for the encapsulation of beetroot extract: Comparison of physicochemical characteristics and betalain stability. Food Bioprocess Technol. 2021, 14, 133–150. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Nazar, M.F.; Mujeed, A.; Siddique, M.Y.; Zafar, M.; Saleem, M.A.; Khan, A.M.; Ashfaq, M.; Sumrra, S.H.; Zubair, M.; Zafar, M.N. Structural dynamics of tween-based microemulsions for antimuscarinic drug mirabegron. Colloid Polym. Sci. 2020, 298, 263–271. [Google Scholar] [CrossRef]
- Ribeiro RC, D.A.; Barreto SM DA, G.; Ostrosky, E.A.; Rocha-Filho, P.A.; Veríssimo, L.M.; Ferrari, M. Production and characterization of cosmetic nanoemulsions containing Opuntia ficus-indica (L.) Mill extract as moisturizing agent. Molecules 2015, 20, 2492–2509. [Google Scholar] [CrossRef]
- Medina-Pérez, G.; Estefes-Duarte, J.A.; Afanador-Barajas, L.N.; Fernández-Luqueño, F.; Zepeda-Velázquez, A.P.; Franco-Fernández, M.J.; Peláez-Acero, A.; Campos-Montiel, R.G. Encapsulation preserves antioxidant and antidiabetic activities of cactus acid fruit bioactive compounds under simulated digestion conditions. Molecules 2020, 25, 5736. [Google Scholar] [CrossRef]
- Schuster, S.; Bernewitz, R.; Guthausen, G.; Zapp, J.; Greiner, A.M.; Köhler, K.; Schuchmann, H.P. Analysis of W1/O/W2 double emulsions with CLSM: Statistical image processing for droplet size distribution. Chem. Eng. Sci. 2012, 81, 84–90. [Google Scholar] [CrossRef]
- Hu, M.; Liu, G.; Zhang, W.; Du, X.; Qi, B.; Li, Y. Co-encapsulation of (–)-epigallocatechin-3-gallate and quercetin in double emulsion hydrogel beads: Microstructures, functional properties, and digestion behaviors. Food Chem. 2022, 373, 131427. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Acevedo-Fani, A.; González-Aguilar, G.A.; Martín-Belloso, O. Encapsulation and stability of a phenolic-rich extract from mango peel within water-in-oil-in-water emulsions. JFF 2019, 56, 65–73. [Google Scholar] [CrossRef]
- Han, L.; Lu, K.; Zhou, S.; Qi, B.; Li, Y. Co-delivery of insulin and quercetin in W/O/W double emulsions stabilized by different hydrophilic emulsifiers. Food Chem. 2022, 369, 130918. [Google Scholar] [CrossRef] [PubMed]



| Opuntia ficus-indica var. Colorada 1 | |
|---|---|
| Pulp colour | Orange |
| Weight (g) | 122 ± 20 |
| Apical caliber (cm) | 6.8 ± 0.9 |
| Equatorial caliber (cm) | 5.1 ± 0.2 |
| Moisture (%) | 82.5 ± 1.3 |
| pH | 6.5 ± 0.1 |
| Soluble solids (°Brix) | 14.8 ± 0.1 |
| Titratable acidity (%) | 0.01 ± 0.0 |
| Pulp colour (CIELAB) | |
| L* | 40.2 ± 3.9 |
| a* | 10.8 ± 2.5 |
| b* | 20.6 ± 2.4 |
| TW Double Emulsion | SC Double Emulsion |
|---|---|
| Primary water phase (W1) | |
|
|
| Oil phase (O) | |
|
|
| Secondary water phase (W2) | |
|
|
| Peak 2 | Compound | Rt (Min) | λ Max (nm) | [M + H]+ | [M + H]− | MS/MS (m/z) |
|---|---|---|---|---|---|---|
| 1 | Portulacaxanthin III | 3.28 | 471 | 269.11 | 225.14, 136.06 | |
| 2 | Vulgaxanthin III | 3.78 | 474 | 326.14 | 325.14, 307.13, 220.10 | |
| 3 | Vulgaxanthin I | 4.15 | 470 | 340.11 | 308.09, 116.07, 84.04, 76.02 | |
| 4 | Vulgaxanthin II | 5.48 | 474 | 341.10 | 292.20, 147.04, 72.08 | |
| 5 | Indicaxanthin | 8.67 | 478 | 309.11 | 263.10, 217.10, 70.06 | |
| 6 | Piscidic acid | 10.41 | 232, 275 | 257.07 | 191.07, 147.04, 119.05, 107.05 | |
| 7 | Betanin | 10.96 | 534 | 551.15 | 390.10, 389.10 | |
| 8 | 4-hydroxybenzoic acid derivative (4-HAD) | 33.76 | 270 | 299 | 137, 119, 93 | |
| 9 | Quercetin glycoside I (QG1) | 38.2 | 266, 351 | 426.24 | 303.05, 191.07, 120.08 | |
| 10 | Quercetin glycoside II (QG2) | 39.34 | 269, 350 | 653.28 | 303.05, 177.05 | |
| 11 | Isorhamnetin glucoxyl-rhamnosyl-rhamnoside (IG1) | 40.07 | 254, 354 | 771.23 | 625.18, 317.07, 85.03 | |
| 12 | Isorhamnetin glucosyl-rhamnosyl-pentoside (IG2) | 41.39 | 253, 354 | 757.22 | 317.07, 167.07, 86.10 | |
| 13 | Quercetin-3-rutinoside (Rutin) | 42.16 | 250, 343 | 611.23 | 303.05, 229.11, 137.07 |
| Compound | Content (µg of Compound/Emulsion) | ||||||
|---|---|---|---|---|---|---|---|
| OFC Pulp Extract | TW Double Emulsions 1 | SC Double Emulsions 2 | |||||
| TW1 3 | TW2 3 | TW3 3 | SC1 3 | SC2 3 | SC3 3 | ||
| Portulacaxanthin III (Bx-glycine) | 26.8 ± 1.3 b | 20.9 ± 4.8 b | 32.5 ± 6.1 b | 58.6 ± 1.6 c | 15.4 ± 0.7 a | 31.7 ± 4.3 b | 51.2 ± 5.5 c |
| Vulgaxanthin III (Bx-asparagine) | 14.6 ± 3.4 a | 12.6 ± 2.4 a | 26.4 ± 3.8 b | 39.8 ± 6.6 c | 9.8 ± 0.3 a | 23.3 ± 5.5 b | 39.3 ± 3.1 c |
| Vulgaxanthin I (Bx-glutamine) | 13.7 ± 3 a | 11.6 ± 1.1 a | 25.2 ± 4.4 b | 36.3 ± 1.3 c | 11.5 ± 1.7 a | 23.4 ± 3.5 b | 35.6 ± 2.7 b |
| Vulgaxanthin II (Bx-glutamic acid) | 18.8 ± 2.7 a | 16.3 ± 3.1 a | 28.6 ± 0.7 b | 35.4 ± 3.5 c | 12.8 ± 0.6 a | 21.7 ± 8.8 b | 36.3 ± 6.4 c |
| Indicaxanthin (Bx-proline) | 508 ± 10 a | 243 ± 34 a | 850 ± 40 b | 1366 ± 16 c | 257 ± 20 a | 670 ± 71 b | 1444 ± 170 c |
| Piscidic acid | 2641 ± 92 a | 1694 ± 75 a | 4753 ± 210 b | 11,449 ± 156 d | 1614 ± 176 a | 6248 ± 356 c | 10,345 ± 436 d |
| 4-hydroxybenzoicacid derivative (4-HAD) | 117 ± 6 a | 62.4 ± 2 a | 295 ± 9 b | 471 ± 16 c | 62.9 ± 2 a | 214 ± 57 b | 285 ± 30 b |
| Quercetin glycoside 2 (QG2) | 18.1 ± 1.4 a | 14.8 ± 1.4 a | 35.5 ± 1.6 b | 43 ± 4.7 c | 12.3 ± 4 a | 27.1 ± 4.2 b | 43.6 ± 0.6 c |
| Isorhamnetin glucoxyl-rhamnosyl-pentoside (IG2) | 31.9 ± 1.4 a | 28.9 ± 4.2 a | 41.1 ± 1.4 a | 50.8 ± 7.1 a | 17.6 ± 1.5 a | 40.3 ± 2.8 a | 60.4 ± 1.2 b |
| Quercetin-3-rutinoside (Rutin) | 16.2 ± 0.1 a | 10.1 ± 0.1 a | 21.9 ± 1 b | 43.1 ± 2.4 c | 13 ± 1.3 a | 28.6 ± 2 b | 33.2 ± 2.4 b |
| Total Betalains | 382 ± 20.4 a | 304 ± 45 a | 962 ± 55 b | 1536 ± 26 c | 306 ± 23 a | 770 ± 93 b | 1606 ± 189 c |
| Total Phenolic Acids | 3758 ± 98 a | 2766 ± 96 a | 6048 ± 219 b | 12,920 ± 113 c | 2676 ± 178 a | 7462 ± 413 b | 12,630 ± 466 c |
| Total Flavonoids | 66.2 ± 2.9 a | 44.8 ± 5.7 a | 98.5 ± 4 b | 137 ± 14 c | 43 ± 6.8 a | 96 ± 9 b | 137 ± 4 c |
| Compound | Encapsulation Efficiency (%) | |||||
|---|---|---|---|---|---|---|
| TW Double Emulsions 1 | SC Double Emulsions 2 | |||||
| TW1 3 | TW2 3 | TW3 3 | SC1 3 | SC2 3 | SC3 3 | |
| Portulacaxanthin III | 78.2 ± 4.7 b | 67.9 ± 8.3 b | 48.6 ± 0.8 b | 57.6 ± 1 b | 44.5 ± 1.6 a | 50.7 ± 8.7 b |
| Vulgaxanthin III | 86.6 ± 6.5 b | 90.4 ± 9.8 b | 74.4 ± 1.3 b | 67.6 ± 1.5 b | 59.8 ± 2.9 a | 53.3 ± 2.1 a |
| Vulgaxanthin I | 85 ± 7 b | 96.8 ± 1.8 b | 95.3 ± 6.2 b | 84 ± 0.9 b | 79.2 ± 3 b | 55.0 ± 4.3 a |
| Vulgaxanthin II | 86.5 ± 2.9 a | 66.5 ± 2.3 a | 64.8 ± 9.7 a | 68.3 ± 3.1 a | 92.4 ± 9.6 a | 82.7 ± 0.5 a |
| Indicaxanthin | 78.9 ± 2.5 a | 97.2 ± 0.4 a | 86.6 ± 2.6 a | 83.5 ± 8.1 a | 84.7 ± 1.1 a | 77.7 ± 5.1 a |
| Piscidic acid | 74 ± 5.1 a | 97.3 ± 2.7 a | 95.5 ± 6.3 a | 71.8 ± 6.4 a | 87.6 ± 4.7 a | 88.9 ± 1.5 a |
| 4-hydroxybenzoic acid derivative (4-HAD) | 52.8 ± 2.4 b | 53.4 ± 2 b | 55.3 ± 3.4 b | 53.8 ± 1.6 b | 51.1 ± 4.9 a | 32.3 ± 2 a |
| Quercetin glycoside 2 (QG2) | 53 ± 8.6 a | 75.7 ± 2.9 a | 74.6 ± 7.1 a | 79.6 ± 4.3 a | 66.3 ± 5.0 a | 75.7 ± 10.3 b |
| Isorhamnetin glucoxyl- rhamnosyl-pentoside 2 (IG2) | 90.6 ± 1.3 b | 95.3 ± 0.9 b | 92.3 ± 8.3 b | 55.2 ± 4.2 a | 80.5 ± 5.2 b | 87.1 ± 1.6 a |
| Quercetin-3-rutinoside (Rutin) | 68 ± 9.3 a | 94.5 ± 4.6 a | 89.8 ± 1.4 a | 86.2 ± 7.5 a | 89.5 ± 10.2 a | n.d. * |
| Total Betalains | 83 ± 4.7 b | 84.4 ± 4.5 b | 73.9 ± 4.1 b | 62.8 ± 4.2 a | 72.1 ± 3.6 a | 63.9 ± 4.1 a |
| Total Phenolic Acids | 63.4 ± 3.8 a | 75.4 ± 2.4 a | 65 ± 5.3 a | 62.8 ± 3 a | 75.6 ± 3.9 a | 61.7 ± 3.7 a |
| Total Flavonoids | 70.5 ± 6.4 a | 71.4 ± 3.3 a | 67.4 ± 5.2 a | 73.7 ± 5.3 a | 69.1 ± 6.2 a | 81.4 ± 5.6 a |
| Digestion Phase | OFC Pulp Extract Content * | Particle Size | Zeta Potential | ||
|---|---|---|---|---|---|
| TW 1 | SC 2 | TW 1 | SC 2 | ||
| Control | 1 | 252 ± 1 a | 2617 ± 168 b | −32.4 ± 2.1 b | −37.6 ± 5.1 a |
| 2 | 202 ± 2 a | 3331 ± 35 b | −34.7 ± 9.6 b | −37.2 ± 1.6 a | |
| 3 | 206 ± 4 a | 2768 ± 140 b | −31.9 ± 0.5 b | −31.8 ± 2.3 b | |
| Oral | 1 | 254 ± 4 a | 1832 ± 62 b | −31.6 ± 0.9 b | −24.1 ± 4.4 b |
| 2 | 217 ± 3 a | 1879 ± 25 b | −29.8 ± 0.8 b | −25.2 ± 5 b | |
| 3 | 222 ± 9 a | 1343 ± 165 b | −35.8 ± 1.2 b | −31.7 ± 3.4 b | |
| Gastric | 1 | 276 ± 6 a | 2697 ± 64 b | −42.1 ± 2.8 a | −24.2 ± 4.5 b |
| 2 | 225 ± 4 a | 2419 ± 450 b | −47.2 ± 2.1 a | −14.8 ± 2.9 c | |
| 3 | 241 ± 7 a | 2254 ± 197 b | −48.7 ± 0.6 a | −17.9 ± 0.7 c | |
| Intestinal | 1 | 1387 ± 84 b | 1660 ± 124 b | −12.1 ± 0.6 c | −15.8 ± 0.5 c |
| 2 | 1787 ± 91 b | 1623 ± 148 b | −13.2 ± 0.7 c | −17.9 ± 1.4 c | |
| 3 | 2374 ± 117 b | 1287 ± 176 b | −11.7 ± 0.5 c | −18.4 ± 0.3 c | |
| Compound | Digestive Stability (µg of Compound/Emulsion) | |||||||
|---|---|---|---|---|---|---|---|---|
| Digestion Phase | OFC Pulp Extract | TW Double Emulsions 2* | SC Double Emulsions 3* | |||||
| TW1 | TW2 | TW3 | SC1 | SC2 | SC3 | |||
| Indicaxanthin | Control 1 | 508 ± 10 a | 243 ± 34 a | 850 ± 40 b | 1366 ± 16 c | 257 ± 20 a | 670 ± 71 b | 1444 ± 70 c |
| Oral | 389 ± 3 a | 187 ± 9 a | 802 ± 52 c | 1203 ± 30 c | 168 ± 19 a | 596 ± 34 c | 1135 ± 131 d | |
| Gastric | 306 ± 9 a | 176 ± 8 a | 696 ± 42 c | 1001 ± 16 d | 177 ± 49 a | 499 ± 14 b | 739 ± 30 c | |
| Intestinal | 323 ± 11 a | 155 ± 13 a | 700 ± 26 c | 996 ± 18 d | 162 ± 10 a | 370 ± 38 b | 694 ± 18 c | |
| Piscidic acid | Control 1 | 2641 ± 92 a | 1694 ± 75 a | 4753 ± 210 b | 11,449 ± 156 d | 1614 ± 176 a | 6248 ± 356 c | 11,345 ± 436 d |
| Oral | 1112 ± 116 a | 930 ± 84 a | 3056 ± 290 b | 8443 ± 252 d | 914 ± 104 a | 4226 ± 222 c | 8876 ± 177 d | |
| Gastric | 247 ± 90 a | 891 ± 99 a | 2300 ± 296 b | 8434 ± 117 d | 701 ± 125 a | 4256 ± 326 c | 3493 ± 266 c | |
| Intestinal | 586 ± 79 a | 1174 ± 67 a | 3765 ± 198 b | 8853 ± 145 c | 707 ± 87 a | 4580 ± 226 b | 3345 ± 138 b | |
| 4-hydroxibenzoic acid derivative (4-HAD) | Control 1 | 117 ± 6 a | 85.4 ± 2 a | 295 ± 9 b | 471 ± 16 c | 62.9 ± 2 a | 214 ± 57 b | 285 ± 30 b |
| Oral | 78.1 ± 4 a | 62.8 ± 4 a | 246 ± 32 b | 316 ± 30 d | 44.8 ± 5 a | 186 ± 10 b | 300 ± 11 d | |
| Gastric | 67.9 ± 2.2 a | 58.7 ± 1.2 a | 188 ± 20 b | 334 ± 16 c | 51 ± 4 a | 158 ± 8 b | 174 ± 3 b | |
| Intestinal | 35.8 ± 4.1 a | 54.6 ± 3.3 a | 185 ± 14 b | 260 ± 18 c | 32.2 ± 2.1 a | 126 ± 4 b | 142 ± 8 b | |
| Isorhamnetin glucoxyl-rhamnosyl-pentoside 2 (IG2) | Control 1 | 31.9 ± 1.4 a | 28.9 ± 4.2 a | 41.4 ± 1.4 a | 50.8 ± 7.1 a | 17.6 ± 1.5 a | 40.3 ± 2.8 a | 60.4 ± 1.2 b |
| Oral | 24.6 ± 2.6 a | 18.1 ± 1.2 a | 28 ± 1.1 a | 26.9 ± 0.8 a | 17.4 ± 0.5 a | 35.4 ± 3 a | 53.3 ± 6.1 b | |
| Gastric | 13.7 ± 0.2 a | 20.4 ± 0.8 a | 25.9 ± 1.3 a | 21.2 ± 1.7 a | 13 ± 0.7 a | 19.9 ± 1 a | 48 ± 4.9 b | |
| Intestinal | 8.3 ± 0.3 a | 16 ± 1.9 b | 26.5 ± 2 b | 32 ± 3.3 c | 8.1 ± 0.3 a | 18.7 ± 2.3 b | 37.5 ± 3 c | |
| Compound | Bioaccessibility (%) | ||||||
|---|---|---|---|---|---|---|---|
| OFC Pulp Extract | TW 1* | SC 2* | |||||
| TW1 | TW2 | TW3 | SC1 | SC2 | SC3 | ||
| Indicaxanthin | 40.9 ± 3.6 a | 63.8 ± 5.3 a | 82.3 ± 6 b | 72.9 ± 1.3 b | 63 ± 3.4 a | 55.2 ± 5.7 a | 48.1 ± 1.2 a |
| Piscidic acid | 43.6 ± 2.2 a | 80.7 ± 2.5 c | 82.8 ± 1.5 c | 79.1 ± 1.2 c | 64.6 ± 3.3 b | 76.9 ± 3.1 c | 35.2 ± 1.1 a |
| 4-hydroxibenzoic acid derivative (4-HAD) | 30.6 ± 3.5 a | 63.9 ± 3.9 b | 62.8 ± 3.8 b | 55.3 ± 3.8 b | 51.2 ± 3.3 b | 58.9 ± 1.9 b | 49.7 ± 2.8 b |
| Isorhamnetin glucoxyl-rhamnosyl-pentoside 2 (IG2) | 25.9 ± 0.9 a | 55.2 ± 6.6 b | 64 ± 7 b | 63.1 ± 6.5 b | 45.5 ± 1.7 b | 46.3 ± 5.7 b | 62.1 ± 5 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parralejo-Sanz, S.; Quereda-Moraleda, I.; Requena, T.; Cano, M.P. Encapsulation of Indicaxanthin-Rich Opuntia Green Extracts by Double Emulsions for Improved Stability and Bioaccessibility. Foods 2024, 13, 1003. https://doi.org/10.3390/foods13071003
Parralejo-Sanz S, Quereda-Moraleda I, Requena T, Cano MP. Encapsulation of Indicaxanthin-Rich Opuntia Green Extracts by Double Emulsions for Improved Stability and Bioaccessibility. Foods. 2024; 13(7):1003. https://doi.org/10.3390/foods13071003
Chicago/Turabian StyleParralejo-Sanz, Sara, Isabel Quereda-Moraleda, Teresa Requena, and M. Pilar Cano. 2024. "Encapsulation of Indicaxanthin-Rich Opuntia Green Extracts by Double Emulsions for Improved Stability and Bioaccessibility" Foods 13, no. 7: 1003. https://doi.org/10.3390/foods13071003
APA StyleParralejo-Sanz, S., Quereda-Moraleda, I., Requena, T., & Cano, M. P. (2024). Encapsulation of Indicaxanthin-Rich Opuntia Green Extracts by Double Emulsions for Improved Stability and Bioaccessibility. Foods, 13(7), 1003. https://doi.org/10.3390/foods13071003







