Flavor Chemical Research on Different Bee Pollen Varieties Using Fast E-Nose and E-Tongue Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Reagent Acquisition
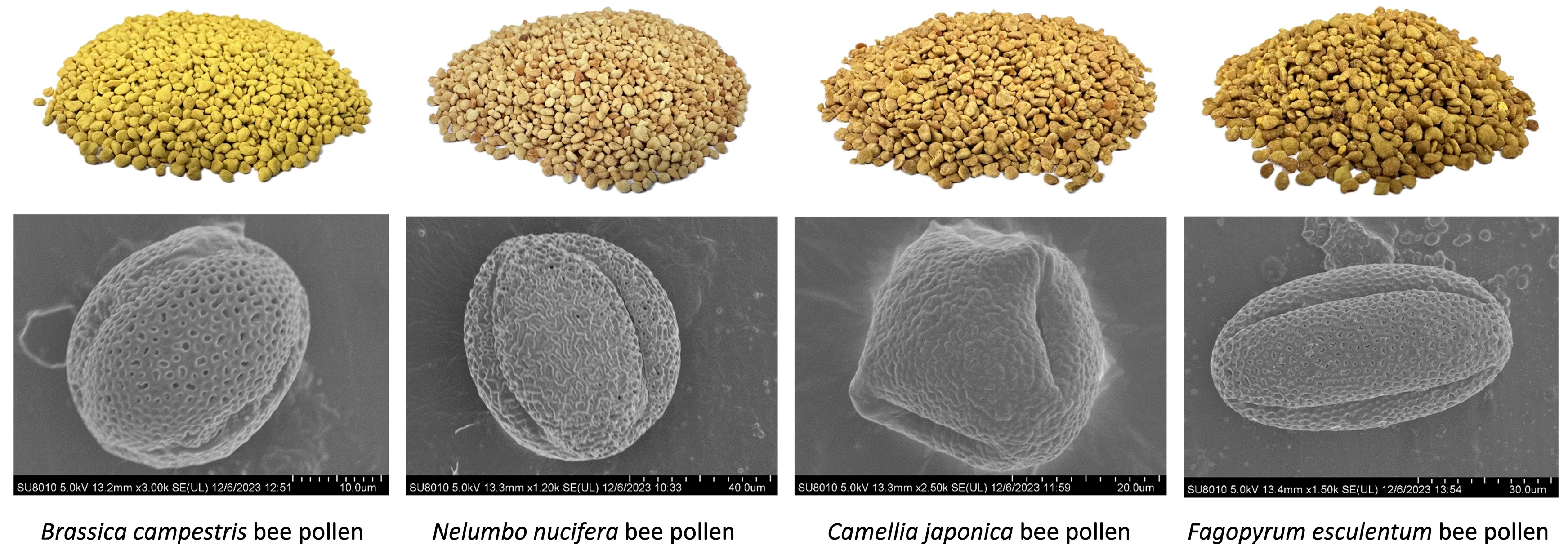
2.2. Scanning Electron Microscopy Examination on Bee Pollen Samples
2.3. Preparation of Bee Pollen Samples for Analysis
2.4. E-Nose Analysis
2.5. E-Tongue Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Bee Pollen Morphology Analysis
3.2. E-Nose Analysis of Bee Pollen
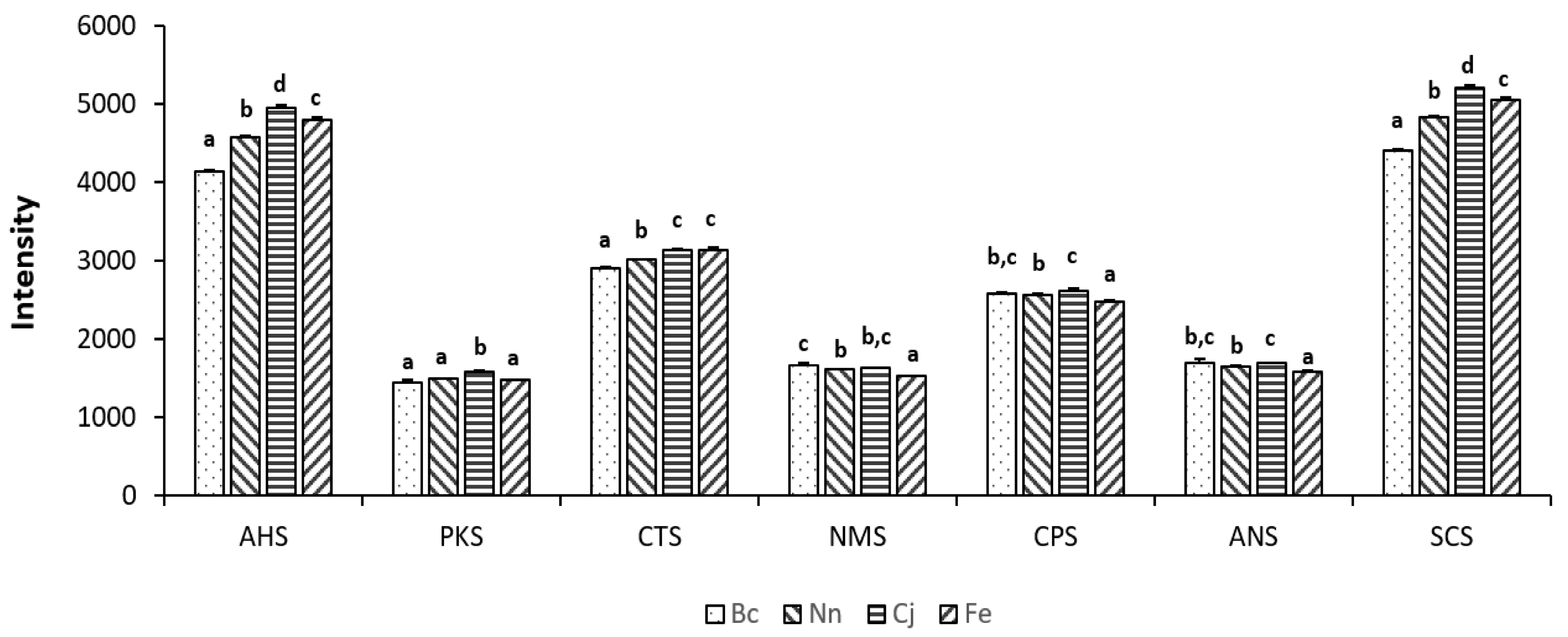
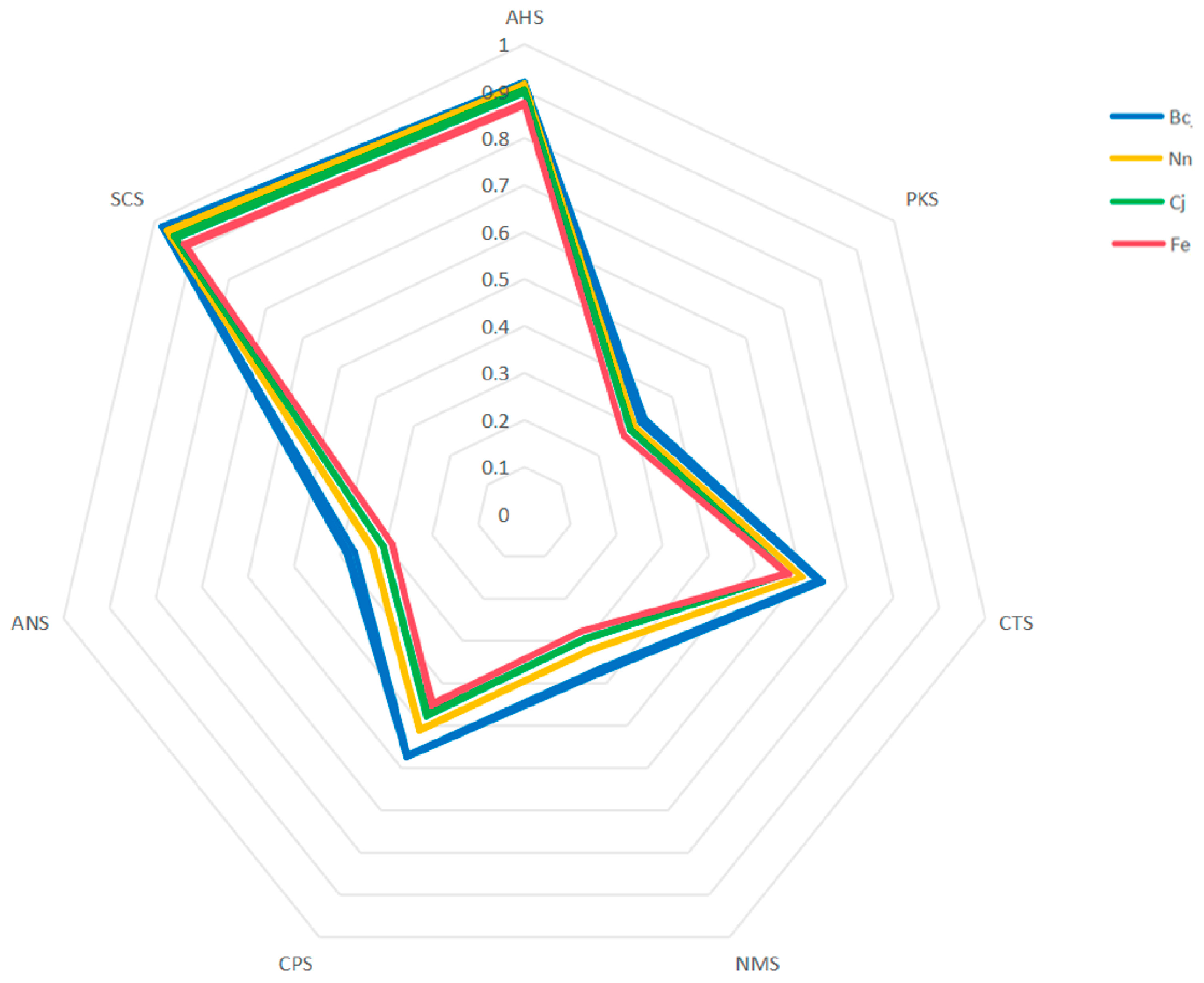
3.3. E-Tongue Analysis of Bee Pollen
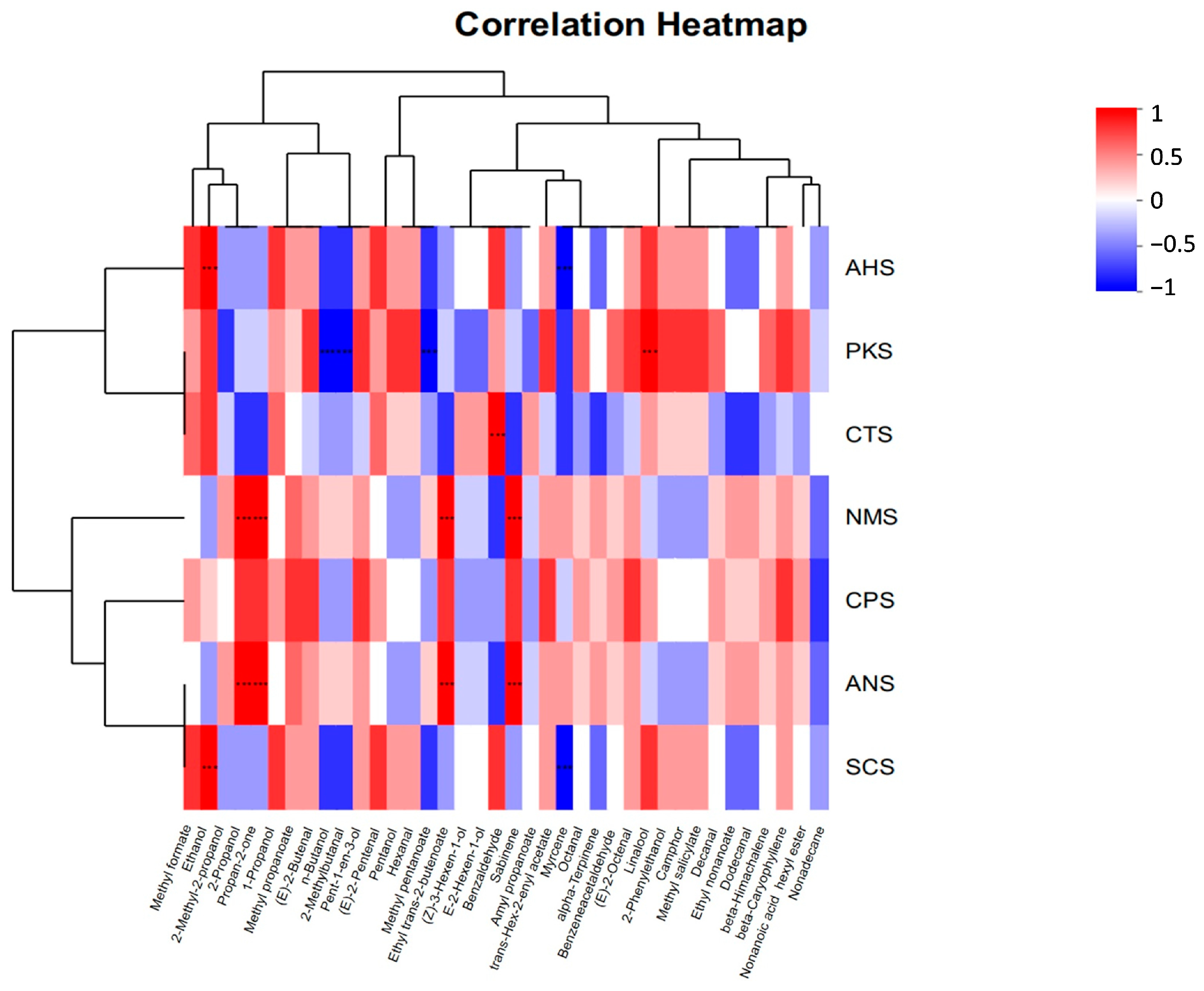
3.4. Correlation Analysis between E-Nose and E-Tongue Datasets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Forbes-Hernández, T.Y.; Orantes-Bermejo, F.J.; Alvarez-Suarez, J.M.; Battino, M. Bee products: An emblematic example of underutilized sources of bioactive compounds. J. Agric. Food Chem. 2022, 70, 6833–6848. [Google Scholar] [CrossRef]
- Wang, S.; Bi, Y.; Zhou, Z.; Peng, W.; Tian, W.; Wang, H.; Fang, X. Effects of pulsed vacuum drying temperature on drying kinetics, physicochemical properties and microstructure of bee pollen. LWT-Food Sci. Technol. 2022, 169, 113966. [Google Scholar] [CrossRef]
- Chen, M.; Yang, X.; Ji, Z.; Zhao, H.; Cheng, N.; Cao, W. Combined treatment of drying, ethanol, and cold plasma for bee pollen: Effects on microbial inactivation and quality attributes. Food Biosci. 2024, 57, 103542. [Google Scholar] [CrossRef]
- Duan, H.; Dong, Z.; Li, H.; Li, W.; Shi, S.; Wang, Q.; Cao, W.; Fang, X.; Fang, A.; Zhai, K. Quality evaluation of bee pollens by chromatographic fingerprint and simultaneous determination of its major bioactive components. Food Chem. Toxicol. 2019, 134, 110831. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, Y.; Mi, J.; Zhang, H.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. Simulated digestion and fermentation in vitro by human gut microbiota of polysaccharides from bee collected pollen of Chinese wolfberry. J. Agric. Food Chem. 2018, 66, 898–907. [Google Scholar] [CrossRef]
- Breda, L.S.; de Melo Nascimento, J.E.; Alves, V.; de Toledo, V.D.A.A.; de Lima, V.A.; Felsner, M.L. Green and fast prediction of crude protein contents in bee pollen based on digital images combined with Random Forest algorithm. Food Res. Int. 2024, 179, 113958. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hu, Z.; Hu, X.; Li, D.; Tian, S. Electronic tongue and electronic nose for food quality and safety. Food Res. Int. 2022, 162, 112214. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Chen, W.; Hu, D.; Miao, A.; Qiao, X.; Qiu, G.; Liang, J.; Guo, W.; Ma, C. Rapid discrimination of quality grade of black tea based on near-infrared spectroscopy (NIRS), electronic nose (E-nose) and data fusion. Food Chem. 2024, 440, 138242. [Google Scholar] [CrossRef]
- Estivi, L.; Buratti, S.; Fusi, D.; Benedetti, S.; Rodríguez, G.; Brandolini, A.; Hidalgo, A. Alkaloid content and taste profile assessed by electronic tongue of Lupinus albus seeds debittered by different methods. J. Food Compos. Anal. 2022, 114, 104810. [Google Scholar] [CrossRef]
- Roy, R.B.; Tudu, B.; Shaw, L.; Jana, A.; Bhattacharyya, N.; Bandyopadhyay, R. Instrumental testing of tea by combining the responses of electronic nose and tongue. J. Food Eng. 2012, 110, 356–363. [Google Scholar]
- Kim, Y.; Lee, U.; Eo, H.J. Effect of NaCl pretreatment on the relationship between the color characteristics and taste of Cirsium setidens processed using a micro-oil-sprayed thermal air technique. Plants 2023, 12, 3193. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, M.; Wan, Z.; Liang, J.; Betti, M.; Hrynets, Y.; Xue, X.; Wu, L.; Wang, K. Bee pollen extracts modulate serum metabolism in lipopolysaccharide-induced acute lung injury mice with anti-inflammatory effects. J. Agric. Food Chem. 2019, 67, 7855–7868. [Google Scholar] [CrossRef]
- Végh, R.; Csóka, M.; Sörös, C.; Sipos, L. Food safety hazards of bee pollen—A review. Trends Food Sci. Technol. 2021, 114, 490–509. [Google Scholar] [CrossRef]
- Dong, J.; Gao, K.; Wang, K.; Xu, X.; Zhang, H. Cell wall disruption of rape bee pollen treated with combination of protamex hydrolysis and ultrasonication. Food Res. Int. 2015, 75, 123–130. [Google Scholar] [CrossRef]
- Jabeen, S.; Zafar, M.; Ahmad, M.; Ali, M.A.; Elshikh, M.S.; Makhkamov, T.; Mamarakhimov, O.; Yuldashev, A.; Khaydarov, K.; Gafforov, Y.; et al. Micrometer insights into Nepeta genus: Pollen micromorphology unveiled. Micron 2024, 177, 103574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, Z.; He, X.; Liu, K.; Debliquy, M.; Zhou, Y.; Zhang, C. Electronic nose based on metal oxide semiconductor sensors for medical diagnosis. Prog. Nat. Sci. Mater. Int. 2024, in press. [Google Scholar]
- Huang, G.; Liu, T.; Mao, X.; Quan, X.; Sui, S.; Ma, J.; Sun, L.; Li, H.; Shao, Q.; Wang, Y. Insights into the volatile flavor and quality profiles of loquat (Eriobotrya japonica Lindl.) during shelf-life via HS-GC-IMS, E-nose, and E-tongue. Food Chem. X 2023, 20, 100886. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Zhang, M.; Qi, J.; Zhao, W.; Gu, J.; Guo, W.; Li, Y. Screening of specific quantitative peptides of beef by LC–MS/MS coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gambacorta, G.; Tlais, A.Z.A.; Cantatore, V.; Gobbetti, M. Volatilome and bioaccessible phenolics profiles in lab-scale fermented bee pollen. Foods 2021, 10, 286. [Google Scholar] [CrossRef]
- Bi, Y.; Ni, J.; Xue, X.; Zhou, Z.; Tian, W.; Orsat, V.; Yan, S.; Peng, W.; Fang, X. Effect of different drying methods on the amino acids, α-dicarbonyls and volatile compounds of rape bee pollen. Food Sci. Hum. Well. 2024, 13, 517–527. [Google Scholar] [CrossRef]
- Ni, J.; Bi, Y.; Vidyarthi, S.K.; Xiao, H.; Han, L.; Wang, J.; Fang, X. Non-thermal electrohydrodynamic (EHD) drying improved the volatile organic compounds of lotus bee pollen via HS-GC-IMS and HS-SPME-GC-MS. LWT-Food Sci. Technol. 2023, 176, 114480. [Google Scholar] [CrossRef]
- Cai, Q. Analysis of Volatile Components and Identification of Main Aroma Components in Four Kinds of Bee Products; Guangdong Ocean University: Zhanjiang, China, 2023. (In Chinese) [Google Scholar]
- Nakib, R.; Rodríguez-Flores, M.S.; Escuredo, O.; Ouelhadj, A.; Coello, M.C.S. Retama sphaerocarpa, Atractylis serratuloides and Eruca sativa honeys from Algeria: Pollen dominance and volatile profiling (HS-SPME/GC–MS). Microchem. J. 2022, 174, 107088. [Google Scholar] [CrossRef]




| Parameters | Value |
|---|---|
| Headspace vial | 20 mL |
| Sample amount | 0.2 g |
| Incubation temperature | 80 °C |
| Incubation time | 20 min |
| Inlet volume | 5000 μL |
| Inlet speed | 125 μL/s |
| Inlet temperature | 200 °C |
| Inlet duration | 45 s |
| Initial trap temperature | 40 °C |
| Split mode | 10 mL/min |
| Injection duration | 50 s |
| Final trap temperature | 240 °C |
| Initial column temperature | 40 °C (30 s) |
| RAMP | 0.5 °C/s −60 °C (0 s) 2.0 °C/s −250 °C (15 s) |
| Acquisition time | 180 s |
| Detector temperature | 260 °C |
| FID | 12 |
| Compounds | Retention Time -Column 5 (s) | Retention Time -Column 1701 (s) | CAS | Odor Description | Bc | Nn | Cj | Fe |
|---|---|---|---|---|---|---|---|---|
| Methyl formate | 17.73 | 17.30 | 107-31-3 | Agreeable; Fruity; Plum | 11,469 | 4321 | 39,886 | 15,056 |
| Ethanol | 19.90 | 21.35 | 64-17-5 | Alcoholic; Ethanol; Fragrant; Pleasant; Pungent; Sweet | 141,776 | 165,650 | 256,029 | 212,202 |
| 2-Methyl-2-propanol | 21.83 | 28.61 | 75-65-0 | Camphor | 123,605 | 19,961 | 30,873 | 51,927 |
| Propan-2-one | 24.01 | 23.46 | 67-64-1 | Apple; Characteristic; Fruity; Pear; Solvent; Sweet; Violet | 22,363 | 19,003 | 20,321 | 16,956 |
| 2-Propanol | 24.79 | 24.32 | 67-63-0 | Acetone; Alcoholic; Ethanol; Floral; Pleasant; Woody | 40,371 | 1391 | 3655 | 0 |
| 1-Propanol | 27.90 | 35.95 | 71-23-8 | Alcoholic; Ethanol; Fermented; Fruity; Fusel; Plastic; Pungent | 3874 | 2572 | 16,968 | 5417 |
| Methyl propanoate | 35.82 | 39.33 | 554-12-1 | Apple; Fresh; Fruity; Rum; Strawberry; Sweet | 3861 | 2370 | 10,038 | 3088 |
| (E)-2-Butenal | 43.42 | 57.49 | 123-73-9 | Floral; Plastic; Pungent | 1028 | 1046 | 8496 | 264 |
| n-Butanol | 44.30 | 63.51 | 71-36-3 | Alcoholic; Amyl alcohol; Banana; Cheese; Fermented; Fruity; Fusel; Harsh; Medicinal; Oil; Sweet | 740,380 | 7689 | 4109 | 26,918 |
| 2-Methylbutanal | 46.37 | 51.96 | 96-17-3 | Almond; Apple; Burnt; Cocoa; Coffee; Fermented; Fruity; Iodoform; Malty; Nutty; Sour | 6561 | 850 | 811 | 6399 |
| Pent-1-en-3-ol | 51.37 | 66.32 | 616-25-1 | Burnt; Butter; Fruity; Grassy; Horseradish; Meaty; Milky; Pungent; Vegetable | 4994 | 9368 | 10,060 | 2565 |
| (E)-2-Pentenal | 69.10 | 80.49 | 1576-87-0 | Apple; Fruity; Green; Oily; Orange; Pungent; Soapy; Strawberry; Tomato | 1233 | 693 | 4055 | 2500 |
| Pentanol | 72.81 | 85.78 | 71-41-0 | Alcoholic; Anise; Balsamic; Fruity; Fusel; Oil; Pungent; Sweet; Waxy | 227 | 934 | 797 | 258 |
| Hexanal | 84.01 | 89.44 | 66-25-1 | Acorn; Aldehydic; Fatty; Fishy; Fresh; Fruity; Grassy; Herbaceous; Leafy; Sharp; Sweaty; Tallowy; Vinous | 2141 | 13,722 | 10,314 | 3073 |
| Ethyl trans-2-butenoate | 85.99 | 95.48 | 623-70-1 | Alliaceous; Chemical; Pungent; Rum; Sweet | 1260 | 602 | 596 | 846 |
| Methyl pentanoate | 88.45 | 90.92 | 624-24-8 | Apple; Fruity; Nutty; Pineapple; Sweet | 1844 | 1125 | 1563 | 700 |
| E-2-Hexen-1-ol | 94.74 | 102.21 | 928-95-0 | Banana; Butter (cooked); Fresh; Fruity; Leafy; Medicinal; Walnut | 2265 | 792 | 951 | 24,072 |
| (Z)-3-Hexen-1-ol | 96.11 | 98.92 | 928-97-2 | Earthy; Floral; Fresh; Fruity; Leafy; Mossy; Oily; Petal | 3033 | 999 | 1591 | 21,817 |
| Benzaldehyde | 104.01 | 121.48 | 100-52-7 | Almond; Bitter; Bitter almond; Burnt sugar; Cherry; Fruity; Malty; Oil; Pepper; Sharp; Sweet; Woody | 922 | 999 | 2759 | 4799 |
| Sabinene | 106.99 | 105.12 | 3387-41-5 | Citrus; Fresh; Pepper; Pine; Spicy; Sweet; Turpentine; Woody | 2592 | 2066 | 2318 | 1863 |
| Amyl propanoate | 109.21 | 112.56 | 624-54-4 | Apricot; Fruity; Pineapple; Sweet | 1036 | 968 | 989 | 8780 |
| trans-Hex-2-enyl acetate | 110.41 | 116.65 | 2497-18-9 | Apple; Banana; Fresh; Sweet; Waxy | 583 | 853 | 1075 | 0 |
| Octanal | 112.63 | 115.85 | 124-13-0 | Aldehydic; Citrus; Fatty; Floral; Fruity; Lemon; Meat (boiled); Orange; Orange peel; Pungent; Soapy; Stew; Waxy | 918 | 736 | 412 | 687 |
| Myrcene | 113.77 | 108.06 | 123-35-3 | Balsamic; Fruity; Geranium; Lemon; Metallic; Plastic; Pleasant; Resinous; Soapy; Spicy; Sweet; Woody | 6442 | 13,005 | 6597 | 5918 |
| alpha-Terpinene | 115.47 | 114.86 | 99-86-5 | Citrus; Fruity; Gasoline; Lemon; Medicinal; Woody | 2390 | 3955 | 2350 | 1503 |
| Benzeneacetaldehyde | 118.39 | 121.52 | 122-78-1 | Cocoa; Floral; Grassy; Hawthorn; Honey; Hyacinth; Rose; Sweet | 976 | 8042 | 1403 | 959 |
| (E)-2-Octenal | 119.98 | 122.99 | 2548-87-0 | Burdock; Burnt; Fatty; Fruity; Mushroom; Nutty; Sour; Sweet; Tallowy; Waxy | 2179 | 2319 | 2561 | 1538 |
| Linalool | 121.78 | 127.27 | 78-70-6 | Anise; Bergamot; Citrus; Floral; Fragrant; Fresh; Fruity; Lavender; Lemon; Lily; Muscat; Oil; Parsley; Rose; Spicy; Sweet; Terpenic; Woody | 1076 | 2472 | 47,271 | 1884 |
| 2-Phenylethanol | 125.95 | 129.70 | 60-12-8 | Floral; Flower; Honey; Lilac; Perfumery; Rose; Spicy | 3241 | 14,188 | 11,858 | 4346 |
| Camphor | 129.99 | 132.94 | 76-22-2 | Aromatic; Camphor; Fragrant; Leafy | 1502 | 16,620 | 4886 | 1520 |
| Methyl salicylate | 132.73 | 134.34 | 119-36-8 | Berry; Minty; Peppermint; Sweet; Winey; Wintergreen | 464 | 12,074 | 866 | 586 |
| Decanal | 136.85 | 132.88 | 112-31-2 | Aldehydic; Burnt; Citrus; Fatty; Floral; Herbaceous; Lemon; Orange; Orange peel; Soapy; Stew; Sweet; Tallowy; Waxy | 2223 | 21,070 | 7094 | 1239 |
| Ethyl nonanoate | 139.29 | 137.78 | 123-29-5 | Fruity; Rose; Rum; Waxy | 1278 | 1283 | 1129 | 796 |
| Dodecanal | 146.23 | 144.73 | 112-54-9 | Aldehydic; Caprylic; Citrus; Fatty; Floral; Herbaceous; Lily; Oily; Soapy; Waxy | 783 | 2162 | 697 | 615 |
| beta-Himachalene | 150.69 | 152.62 | 1461-03-6 | - | 923 | 45,690 | 5719 | 459 |
| beta-Caryophyllene | 153.05 | 146.61 | 87-44-5 | Fruity; Spicy; Sweet; Terpenic; Woody | 254 | 1329 | 2735 | 207 |
| Nonanoic acid hexyl ester | 163.75 | 158.78 | 6561-39-3 | Brandy; Floral; Fruity; Vegetable | 274 | 11,476 | 789 | 246 |
| Nonadecane | 176.73 | 170.74 | 629-92-5 | Alkane; Fuel; Fusel | 58 | 391 | 25 | 122 |
| Bc vs. Nn | Bc vs. Cj | Bc vs. Fe | Nn vs. Cj | Nn vs. Fe | Cj vs. Fe | |
|---|---|---|---|---|---|---|
| AHS | 0.001 * | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 |
| PKS | 0.099 | 0.021 | 0.149 | 0.003 | 0.012 | 0.002 |
| CTS | 0.020 | 0.005 | 0.000 | 0.000 | 0.016 | 0.988 |
| NMS | 0.047 | 0.150 | 0.008 | 0.069 | 0.000 | 0.003 |
| CPS | 0.365 | 0.216 | 0.044 | 0.013 | 0.003 | 0.001 |
| ANS | 0.142 | 0.757 | 0.032 | 0.023 | 0.001 | 0.002 |
| SCS | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Zhou, E.; Zhu, Y.; Li, Q.; Wu, L. Flavor Chemical Research on Different Bee Pollen Varieties Using Fast E-Nose and E-Tongue Technology. Foods 2024, 13, 1022. https://doi.org/10.3390/foods13071022
Liu C, Zhou E, Zhu Y, Li Q, Wu L. Flavor Chemical Research on Different Bee Pollen Varieties Using Fast E-Nose and E-Tongue Technology. Foods. 2024; 13(7):1022. https://doi.org/10.3390/foods13071022
Chicago/Turabian StyleLiu, Chenshuo, Enning Zhou, Yuying Zhu, Qiangqiang Li, and Liming Wu. 2024. "Flavor Chemical Research on Different Bee Pollen Varieties Using Fast E-Nose and E-Tongue Technology" Foods 13, no. 7: 1022. https://doi.org/10.3390/foods13071022
APA StyleLiu, C., Zhou, E., Zhu, Y., Li, Q., & Wu, L. (2024). Flavor Chemical Research on Different Bee Pollen Varieties Using Fast E-Nose and E-Tongue Technology. Foods, 13(7), 1022. https://doi.org/10.3390/foods13071022






