Effects of Heating Treatment on the Physicochemical and Volatile Flavor Properties of Argentinian Shortfin Squid (Illex argentinus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Squid Samples and Treatments
2.3. Colorimetric Analysis
2.4. Sensory Evaluation
2.5. Free Amino Acid Analysis
2.6. Texture Profile Analysis
2.7. Volatile Organic Compound Analysis
2.8. Electronic Nose Analysis
2.9. Statistical Analysis
3. Results and Discussion
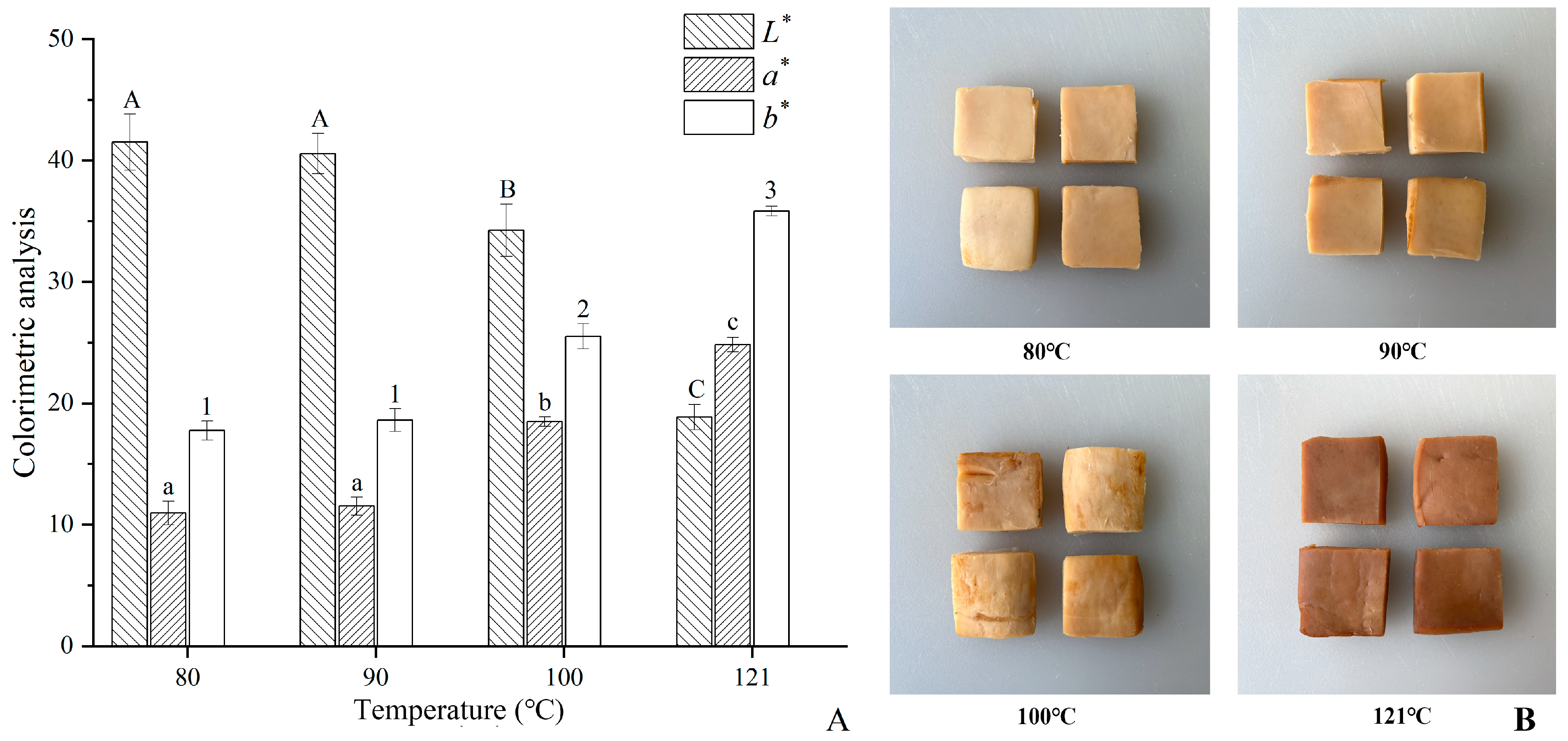
3.1. Colorimetric Analysis
3.2. Sensory Evaluation
3.3. Free Amino Acid Analysis
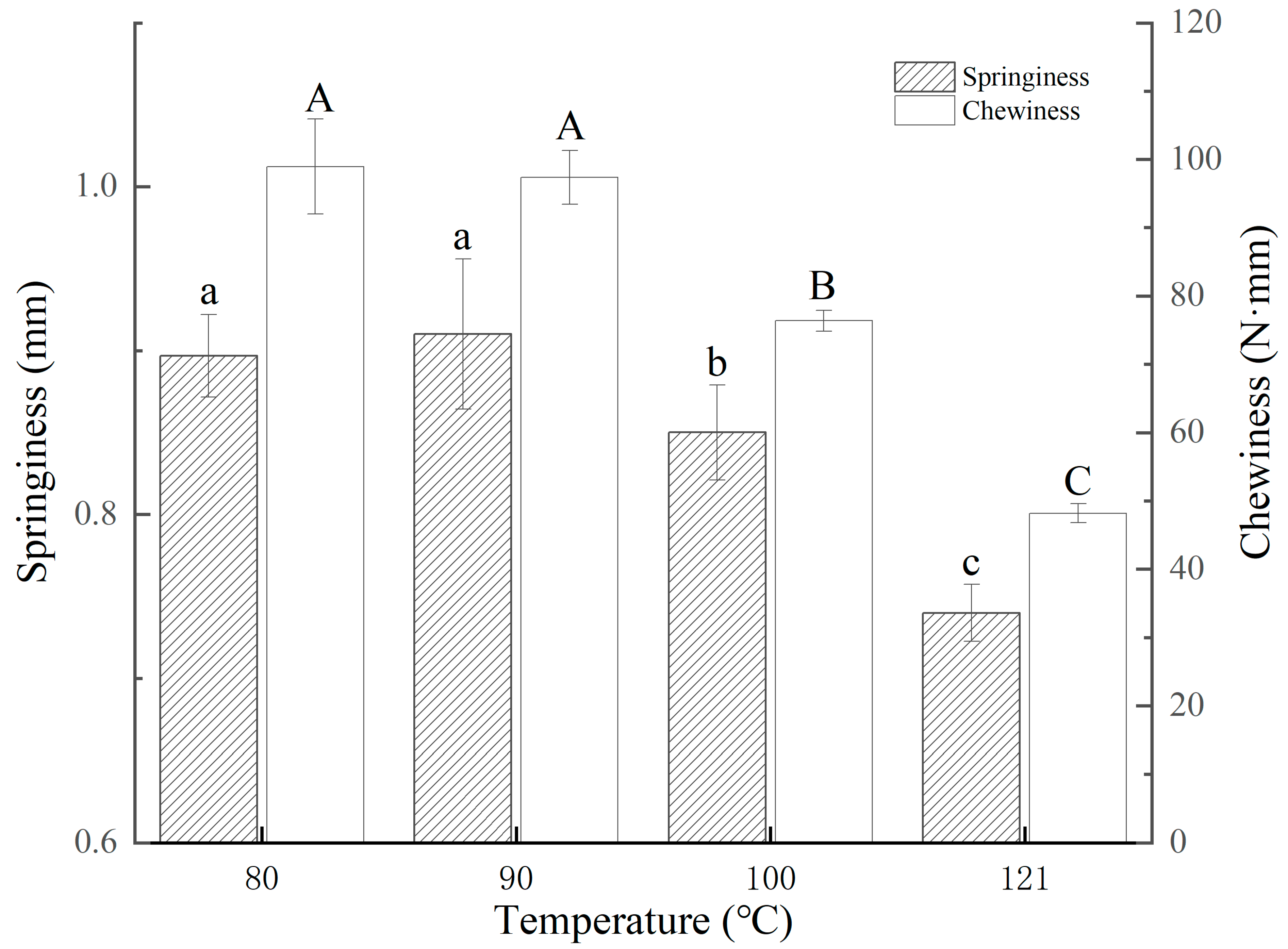
3.4. Texture Analysis
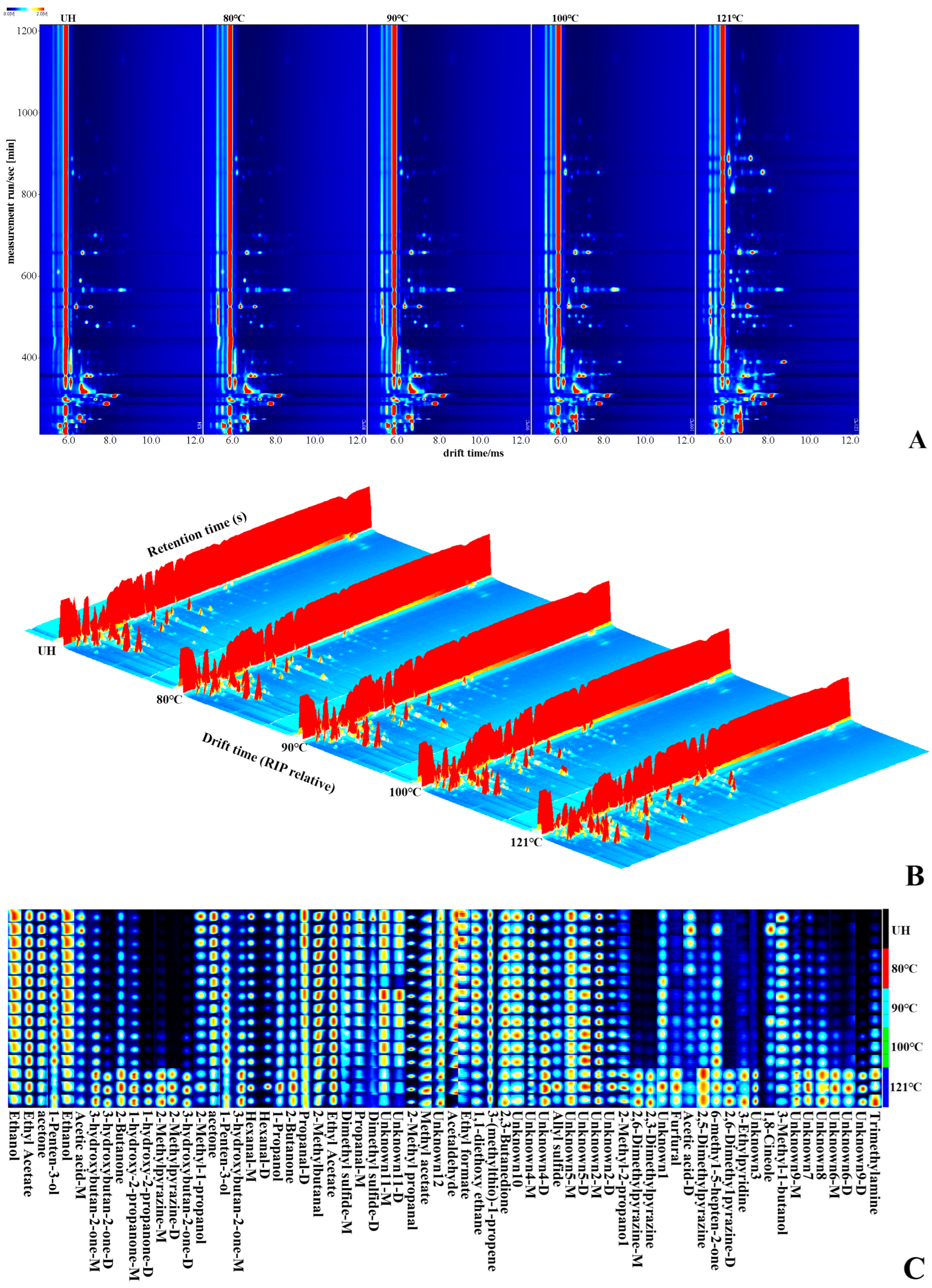
3.5. Analysis of HS-GC-IMS
3.5.1. Topographic Plots
3.5.2. Fingerprints
3.5.3. Flavor Characterization Analysis
Alcohols
Aldehydes
Ketones
Pyrazines, Sulfides, Esters, and Acids
3.5.4. Electronic Nose
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shui, S.-S.; Yao, H.; Jiang, Z.-D.; Benjakul, S.; Aubourg, S.P.; Zhang, B. The differences of muscle proteins between neon flying squid (Ommastrephes bartramii) and jumbo squid (Dosidicus gigas) mantles via physicochemical and proteomic analyses. Food Chem. 2021, 364, 130374. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.; Troncoso, O.; Rivas, E.; Gomez, C.; Lopez, D. Reversible stress softening of collagen based networks from the jumbo squid mantle (Dosidicus gigas). Mater. Sci. Eng. C 2014, 37, 9–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Omura, Y.; Yamazawa, M.; Yamashita, Y.; Okazaki, E.; Watabe, S. Relationship between postmortem changes and browning of boiled, dried, and seasoned product made from japanese common squid (Tedarodes pacificus) mantle muscle. J. Food Sci. 2007, 72, C044–C049. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Luo, Y.; Wang, Y.; Zhao, Y. Effect of different drying methods on the myosin structure, amino acid composition, protein digestibility and volatile profile of squid fillets. Food Chem. 2015, 171, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhao, M.; Su, G.; Sun, W. Binding of aroma compounds with myofibrillar proteins modified by a hydroxyl-radical-induced oxidative system. J. Agric. Food Chem. 2014, 62, 9544–9552. [Google Scholar] [CrossRef] [PubMed]
- Paarup, T.; Sanchez, J.; Moral, A.; Christensen, H.; Bisgaard, M.; Gram, L. Sensory, chemical and bacteriological changes during storage of iced squid (Todaropsis eblanae). J. Appl. Microbiol. 2002, 92, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Pulgar, J.S.D.; Gazquez, A.; Ruiz-Carrascal, J. Physico-chemical, textural and structural characteristics of sous-vide cooked pork cheeks as affected by vacuum, cooking temperature, and cooking time. Meat Sci. 2012, 90, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Zielbauer, B.I.; Franz, J.; Viezens, B.; Vilgis, T.A. Physical aspects of meat cooking: Time dependent thermal protein denaturation and water loss. Food Biophys. 2016, 11, 34–42. [Google Scholar] [CrossRef]
- Del Pulgar, J.S.; Roldan, M.; Ruiz-Carrascal, J. Volatile compounds profile of sous-vide cooked pork cheeks as affected by cooking conditions (vacuum packaging, temperature and time). Molecules 2013, 18, 12538–12547. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Purslow, P.P.; Larsen, L.M. The effect of cooking temperature on mechanical properties of whole meat, single muscle fibres and perimysial connective tissue. Meat Sci. 2000, 55, 301–307. [Google Scholar] [CrossRef]
- Oz, F.; Zikirov, E. The effects of sous-vide cooking method on the formation of heterocyclic aromatic amines in beef chops. LWT-Food Sci. Technol. 2015, 64, 120–125. [Google Scholar] [CrossRef]
- FDA. Fish and Fishery Products Hazards and Controls Guidance; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition: Washington, DC, USA, 2022; p. 553.
- Solo-De-Zaldívar, B.; Tovar, C.; Borderías, A.; Herranz, B. Pasteurization and chilled storage of restructured fish muscle products based on glucomannan gelation. Food Hydrocoll. 2015, 43, 418–426. [Google Scholar] [CrossRef]
- Harekrishna, J.; Mrinmay, G.; Keshab, M.; Bikas, P.; Abhijit, M. Study on the effect of low temperature pasteurization and storage temperature on the microbial dynamics in fresh water prawn. Sch. Acad. J. Biosci. 2015, 3, 576–582. Available online: https://www.researchgate.net/publication/279535096 (accessed on 15 October 2023).
- Ning, H.; Qiu, H.; Miao, J.; Qu, Y.; Lai, K. Effects of frying and baking processing conditions changes on biogenic amines and volatile components in Jumbo squid (Dosidicus gigas). Appl. Food Res. 2022, 2, 100114. [Google Scholar] [CrossRef]
- Tang, J.; Hong, Y.-K.; Inanoglu, S.; Liu, F. Microwave pasteurization for ready-to-eat meals. Curr. Opin. Food Sci. 2018, 23, 133–141. [Google Scholar] [CrossRef]
- Carrascon, V.; Escudero, A.; Ferreira, V.; Lopez, R. Characterisation of the key odorants in a squid broth (Illex argentinus). LWT-Food Sci. Technol. 2014, 57, 656–662. [Google Scholar] [CrossRef]
- Cui, Z.; Yan, H.; Manoli, T.; Mo, H.; Li, H.; Zhang, H. Changes in the volatile components of squid (Illex argentinus) for different cooking methods via headspace–gas chromatography–ion mobility spectrometry. Food Sci. Nutr. 2020, 8, 5748–5762. [Google Scholar] [CrossRef]
- Charlotte, V.S.; Lorenz, P.; Peter, L.F.; Karsten, O.; Ole, G.M.; Michael, B.F. Physicochemical characterization of sous vide cooked squid (Loligo forbesii and Loligo vulgaris) and the relationship to selected sensory properties and hedonic response. Int. J. Gastron. Food Sci. 2021, 23, 100298. [Google Scholar] [CrossRef]
- Sanz, T.; Salvador, A.; Fiszman, S. Effect of concentration and temperature on properties of methylcellulose-added batters Application to battered, fried seafood. Food Hydrocoll. 2004, 18, 127–131. [Google Scholar] [CrossRef]
- Afsin, C.; Nalan, G. Improving the physicochemical and textural properties of squid (Loligo vulgaris) muscle by sous-vide cooking in different time-temperature combinations. J. Aquat. Food Prod. Technol. 2022, 31, 872–881. [Google Scholar] [CrossRef]
- Tan, M.; Wang, J.; Li, P.; Xie, J. Storage time prediction of glazed frozen squids during frozen storage at different temperatures based on neural network. Int. J. Food Prop. 2020, 23, 1663–1677. [Google Scholar] [CrossRef]
- Oh, M.; Kim, E.-K.; Jeon, B.-T.; Tang, Y.; Kim, M.S.; Seong, H.-J.; Moon, S.-H. Chemical compositions, free amino acid contents and antioxidant activities of Hanwoo (Bos taurus coreanae) beef by cut. Meat Sci. 2016, 119, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Chu, Y.; Lv, Y.; Xie, J. Quality of frozen mackerel during storage as processed by different freezing methods. Int. J. Food Prop. 2022, 25, 593–607. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Zhu, Z.; Lei, Y.; Huang, S.; Huang, M. Effect of ageing time on the flavour compounds in Nanjing water-boiled salted duck detected by HS-GC-IMS. LWT-Food Sci. Technol. 2022, 155, 112870. [Google Scholar] [CrossRef]
- Li, X.; Cheng, X.; Yang, J.; Wang, X.; Lü, X. Unraveling the difference in physicochemical properties, sensory, and volatile profiles of dry chili sauce and traditional fresh dry chili sauce fermented by Lactobacillus plantarum PC8 using electronic nose and HS-SPME-GC-MS. Food Biosci. 2022, 50, 102057. [Google Scholar] [CrossRef]
- Nakamura, M.; Mao, W.; Fukuoka, M.; Sakai, N. Analysis of the color change in fish during the grilling process. Food Sci. Technol. Res. 2011, 17, 471–478. [Google Scholar] [CrossRef]
- Hamoen, J.; Vollebregt, H.; van der Sman, R. Prediction of the time evolution of pH in meat. Food Chem. 2016, 141, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.-T.; Takahashi, K.; Kaido, T.; Kasukawa, M.; Okazaki, E.; Osako, K. Relationship among pH, generation of free amino acids, and Maillard browning of dried Japanese common squid Todarodes pacificus meat. Food Chem. 2019, 283, 324–330. [Google Scholar] [CrossRef]
- Li, D.-Y.; Yuan, Z.; Liu, Z.-Q.; Yu, M.-M.; Guo, Y.; Liu, X.-Y.; Zhang, M.; Liu, H.-L.; Zhou, D.-Y. Effect of oxidation and maillard reaction on color deterioration of ready-to-eat shrimps during storage. LWT-Food Sci. Technol. 2020, 131, 109696. [Google Scholar] [CrossRef]
- Geng, J.-T.; Kaido, T.; Kasukawa, M.; Zhong, C.; Sun, L.-C.; Okazaki, E.; Osako, K. Mechanism study of high browning degree of mantle muscle meat from Japanese common squid Todarodes pacificus during air-drying. Food Chem. 2015, 176, 158–166. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, R.; Yang, X.; Gao, Z.; Yuan, Y.; Yue, T. Changes in aroma components and potential Maillard reaction products during the stir-frying of pork slices. Food Control 2021, 123, 107855. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Tanaka, M.; Ishizaki, S.; Taluengphol, A.; Chichanan, U. Physicochemical and textural properties of dried squid as affected by alkaline treatments. J. Sci. Food Agric. 2000, 80, 2142–2148. [Google Scholar] [CrossRef]
- Ji, L.; Muhammad, N.M.; Zhang, H.; Zhou, G.H.; Zhang, J.H. Effects of partial NaCl substitution with high-temperature ripening on proteolysis and volatile compounds during process of Chinese dry-cured lamb ham. Food Res. Int. 2021, 140, 110001. [Google Scholar] [CrossRef]
- Xu, H.J.; Cao, H.J.; Zhang, B.; Yao, H. The mechanistic effect of bromelain and papain on tenderization in jumbo squid (Dosidicus gigas) muscle. Food Res. Int. 2020, 131, 108991. [Google Scholar] [CrossRef]
- Torres-Arreola, W.; Ocaño-Higuera, V.M.; Ezquerra-Brauer, J.M.; López-Corona, B.E.; Rodríguez-Felix, F.; Castro-Longoria, R.; Ramírez-Guerra, H.E. Effect of cooking on physicochemical and structural properties of jumbo squid (Dosidicus gigas) muscle. J. Food Process. Preserv. 2017, 42, e13528. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, C. Calculated taste activity values and umami equivalences explain why dried sha-chong (Sipunculus nudus) is a valuable condiment. J. Aquat. Food Prod. Technol. 2016, 25, 177–184. [Google Scholar] [CrossRef]
- Boye, J.; Wijesinha-Bettoni, R.; Burlingame, B. Protein quality evaluation twenty years after the introduction of the protein digestibility corrected amino acid score method. Br. J. Nutr. 2012, 108, S183–S211. [Google Scholar] [CrossRef]
- Shabbir, M.A.; Raza, A.; Anjum, F.M.; Khan, M.R.; Suleria, H.A.R. Effect of thermal treatment on meat proteins with special reference to heterocyclic aromatic amines (HAAs). Crit. Rev. Food Sci. Nutr. 2015, 55, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-H.; Qi, L.-B.; Fu, B.-S.; Chen, Z.-H.; Zhang, Y.-Y.; Du, M.; Dong, X.-P.; Zhu, B.-W.; Qin, L. Flavor formation in different production steps during the processing of cold-smoked Spanish mackerel. Food Chem. 2019, 286, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Barekat, S.; Soltanizadeh, N. Improvement of meat tenderness by simultaneous application of high-intensity ultrasonic radiation and papain treatment. Innov. Food Sci. Emerg. Technol. 2017, 39, 223–229. [Google Scholar] [CrossRef]
- Shi, T.; Xiong, Z.; Jin, W.; Yuan, L.; Sun, Q.; Zhang, Y.; Li, X.; Gao, R. Suppression mechanism of l-arginine in the heat-induced aggregation of bighead carp (Aristichthys nobilis) myosin: The significance of ionic linkage effects and hydrogen bond effects. Food Hydrocoll. 2020, 102, 105596. [Google Scholar] [CrossRef]
- Smit, B.A.; Engels, W.J.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, J.; Jia, J. Effects of thermal processing and various chemical substances on formaldehyde and dimethylamine formation in squid Dosidicus gigas. J. Sci. Food Agric. 2012, 92, 2436–2442. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, J.; Wang, X.; Wang, R.; Ren, F.; Zhang, Q.; Shan, Y.; Ding, S. Characterization of volatile component changes in jujube fruits during cold storage by using headspace-gas chromatography-ion mobility spectrometry. Molecules 2019, 24, 3904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Wang, Y.; Kong, B.; Chen, Q. Evaluation of the flavour properties of cooked chicken drumsticks as affected by sugar smoking times using an electronic nose, electronic tongue, and HS-SPME/GC-MS. LWT-Food Sci. Technol. 2021, 140, 110764. [Google Scholar] [CrossRef]
- Liu, J.; Han, L.; Han, W.; Gui, L.; Yuan, Z.; Hou, S.; Wang, Z.; Yang, B.; Raza, S.H.A.; Alowais, A.F.S.; et al. Effect of different heat treatments on the quality and flavor compounds of black tibetan sheep meat by hs-gc-ims coupled with multivariate analysis. Molecules 2023, 28, 165. [Google Scholar] [CrossRef] [PubMed]
- Mariutti, L.R.; Bragagnolo, N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Res. Int. 2017, 94, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Bai, L.; Feng, X.; Chen, Y.P.; Zhang, D.; Yao, W.; Zhang, H.; Chen, G.; Liu, Y. Characterization of Jinhua ham aroma profiles in specific to aging time by gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2020, 168, 108178. [Google Scholar] [CrossRef] [PubMed]
- Munro, I.C.; Ford, R.A.; Kennepohl, E.; Sprenger, J.G. Correlation of structural class with no-observed-effect levels: A proposal for establishing a threshold of concern. Food Chem. Toxicol. 1996, 34, 829–867. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wu, G.C.; Zhang, H.; Jin, Q.Z.; Wang, X.G. Deep-fried flavor: Characteristics, formation mechanisms, and influencing factors. Crit. Rev. Food Sci. Nutr. 2019, 60, 1496–1514. [Google Scholar] [CrossRef]
- Pugliese, C.; Sirtori, F.; Calamai, L.; Franci, O. The evolution of volatile compounds profile of “Toscano” dry-cured ham during ripening as revealed by SPME-GC-MS approach. J. Mass Spectrom. 2010, 45, 1056–1064. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Zhou, L.; Zhang, R. Study on the influences of ultrasound on the flavor profile of unsmoked bacon and its underlying metabolic mechanism by using HS-GC-IMS. Ultrason. Sonochem. 2021, 80, 105807. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Lv, Y.; Wen, R.; Wang, Y.; Chen, Q.; Kong, B. Characterization of selected Harbin red sausages on the basis of their flavour profiles using HS-SPME-GC/MS combined with electronic nose and electronic tongue. Meat Sci. 2021, 172, 108345. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Liu, C.; Lu, X.; Fang, D.; Hu, Q.; Zhang, Y.; Zhao, L. Characterization of flavor frame in shiitake mushrooms (Lentinula edodes) detected by HS-GC-IMS coupled with electronic tongue and sensory analysis: Influence of drying techniques. LWT-Food Sci. Technol. 2021, 146, 111402. [Google Scholar] [CrossRef]
- Carvalho, M.; Ruiz-Carrascal, J. Improving crunchiness and crispness of fried squid rings through innovative tempura coatings: Addition of alcohol and CO2 incubation. J. Food Sci. Technol. 2018, 55, 2068–2078. [Google Scholar] [CrossRef]
- Dong, W.J.; Hu, R.S.; Hu, R.S.; Long, Y.Z.; Li, H.H.; Zhang, Y.J.; Zhu, K.X.; Chu, Z. Comparative evaluation of the volatile profiles and taste properties of roasted coffee beans as affected by drying method and detected by electronic nose, electronic tongue, and HS-SPME-GC-MS. Food Chem. 2019, 272, 723–731. [Google Scholar] [CrossRef]

| Temperature (°C) | Odor | Taste | Tenderness | Juiciness | Total Score |
|---|---|---|---|---|---|
| 80 | 6.4 ± 0.7 a | 6.0 ± 0.9 a | 5.6 ± 0.4 a | 5.8 ± 0.6 a | 6.0 ± 0.6 a |
| 90 | 6.6 ± 0.5 a | 6.0 ± 0.5 a | 5.4 ± 0.5 a | 5.8 ± 0.5 a | 6.1 ± 0.4 a |
| 100 | 5.4 ± 0.8 b | 5.2 ± 0.4 b | 4.2 ± 0.4 b | 4.8 ± 0.7 b | 5.1 ± 0.3 b |
| 121 | 3.5 ± 0.5 c | 1.9 ± 0.7 c | 4.4 ± 0.5 b | 3.4 ± 0.5 b | 2.8 ± 0.5 c |
| FAA | Threshold | Taste Characteristics | UH | 80 °C | 90 °C | 100 °C | 121 °C | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | TAV | Content | TAV | Content | TAV | Content | TAV | Content | TAV | |||
| Asp | 100 | fresh | 23.0 ± 0.8 ab | 0.23 | 21.7 ± 0.5 b | 0.22 | 17.0 ± 0.0 c | 0.17 | 23.7 ± 1.3 a | 0.24 | 17.3 ± 0.5 c | 0.17 |
| * Thr | 260 | sweet | 20.3 ± 0.9 b | 0.08 | 22.0 ± 0.0 a | 0.08 | 19.3 ± 0.5 b | 0.07 | 19.0 ± 0.8 b | 0.07 | 14.3 ± 0.5 c | 0.06 |
| Ser | 150 | sweet | 16.3 ± 0.9 ab | 0.11 | 17.0 ± 0.0 a | 0.11 | 15.3 ± 0.5 b | 0.10 | 16.0 ± 0.8 ab | 0.11 | 12.3 ± 0.5 c | 0.08 |
| Glu | 30 | fresh | 246.7 ± 12.5 ab | 8.22 | 226.7 ± 4.7 b | 7.56 | 190.0 ± 0.0 c | 6.33 | 260.0 ± 16.3 a | 8.67 | 143.3 ± 4.7 d | 4.78 |
| Gly | 130 | sweet | 21.0 ± 0.8 a | 0.16 | 22.0 ± 0.0 a | 0.17 | 22.3 ± 0.5 a | 0.17 | 19.0 ± 0.8 b | 0.15 | 15.3 ± 0.5 c | 0.12 |
| Ala | 60 | sweet | 90.7 ± 3.4 b | 1.51 | 100.0 ± 0.0 a | 1.67 | 100.0 ± 0.0 a | 1.67 | 88.7 ± 4.2 bc | 1.48 | 83.3 ± 2.6 c | 1.39 |
| Cys | - | tasteless | 1.8 ± 0.1 b | - | 2.0 ± 0.1 a | - | 2.0 ± 0.1 a | - | 1.6 ± 0.1 c | - | 1.5 ± 0.1 d | - |
| * Val | 40 | bitter | 16.3 ± 0.9 ab | 0.41 | 17.3 ± 0.5 a | 0.43 | 15.3 ± 0.5 b | 0.38 | 16.3 ± 0.9 ab | 0.41 | 13.3 ± 0.5 c | 0.33 |
| * Met | 30 | bitter | 20.3 ± 0.9 a | 0.68 | 21.0 ± 1.4 a | 0.70 | 21.7 ± 0.5 a | 0.72 | 16.3 ± 0.9 b | 0.54 | 13.7 ± 0.5 c | 0.46 |
| Iso | 90 | bitter | 9.5 ± 0.8 a | 0.11 | 8.4 ± 1.3 a | 0.09 | 8.9 ± 0.3 a | 0.10 | 10.1 ± 1.3 a | 0.11 | 7.9 ± 0.4 a | 0.09 |
| * Leu | 190 | bitter | 24.3 ± 2.4 ab | 0.13 | 20.3 ± 2.9 b | 0.11 | 22.0 ± 0.8 ab | 0.12 | 27.3 ± 3.1 a | 0.14 | 23.0 ± 1.4 ab | 0.12 |
| Tyr | - | bitter | 10.8 ± 1.1 ab | - | 8.1 ± 1.4 c | - | 9.0 ± 0.6 bc | - | 11.3 ± 0.9 a | - | 7.6 ± 0.3 c | - |
| * Phe | 90 | bitter | 2.8 ± 0.3 b | 0.03 | 1.8 ± 0.1 c | 0.02 | 2.2 ± 0.4 bc | 0.02 | 3.6 ± 0.3 a | 0.04 | 1.7 ± 0.1 c | 0.02 |
| * Lys | 50 | tasteless | 18.3 ± 0.9 a | 0.37 | 18.0 ± 0.0 a | 0.36 | 15.0 ± 0.0 b | 0.30 | 16.7 ± 1.3 ab | 0.33 | 12.7 ± 0.5 c | 0.25 |
| His | 20 | bitter | 13.3 ± 0.5 b | 0.67 | 14.0 ± 0.0 a | 0.70 | 12.0 ± 0.0 c | 0.60 | 6.6 ± 0.3 d | 0.33 | 2.5 ± 0.1 e | 0.13 |
| Arg | 50 | bitter | 120.0 ± 0.0 a | 2.40 | 113.3 ± 4.7 a | 2.27 | 91.0 ± 0.8 b | 1.82 | 95.0 ± 4.6 b | 1.90 | 58.0 ± 1.6 c | 1.16 |
| Pro | 300 | tasteless | 106.7 ± 4.7 b | 0.36 | 110.0 ± 0.0 b | 0.37 | 120.0 ± 0.0 a | 0.40 | 106.0 ± 5.7 b | 0.35 | 93.3 ± 2.6 c | 0.31 |
| TAA | 762.2 ± 31.2 a | 743.7 ± 1.9 a | 683.1 ± 5.0 b | 737.3 ± 42.5 ab | 521.3 ± 15.4 c |
| Volatiles | No. | Compound | CAS | Retention | Retention | Drift Time | Intensity (V) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | Times (s) | (ms) | UH | 80 °C | 90 °C | 100 °C | 121 °C | ||||
| Alcohols | 1 | Ethanol | C64175 | 922.4 | 319.1 | 1.1 | 4738.1 ± 224.6 c | 5924.7 ± 92.7 b | 6540.8 ± 148.6 a | 6523.4 ± 106.1 a | 6568.1 ± 108.7 a |
| 2 | 1-Propanol | C71238 | 1026 | 416.8 | 1.1 | 885.6 ± 194.9 a | 798.1 ± 28.4 a | 780.5 ± 40.6 a | 807.0 ± 73.5 a | 829.9 ± 47.2 a | |
| 3 | 2-Methyl-2-propanol | C75650 | 902.8 | 306.1 | 1.3 | 673.1 ± 52.9 a | 469.3 ± 16.1 b | 386.1 ± 31.5 c | 373.3 ± 26.6 c | 331.5 ± 23.0 c | |
| 4 | 2-Methyl-1-propanol | C78831 | 1073.5 | 487.4 | 1.2 | 620.1 ± 201.4 b | 566.1 ± 39. 4 b | 644.0 ± 42.7 b | 611.8 ± 135.1 b | 996.7 ± 95.8 a | |
| 5 | 3-Methyl-1-butanol | C123513 | 1179.1 | 702.5 | 1.2 | 434.9 ± 64.8 c | 573.9 ± 28.6 b | 593.1 ± 36.3 b | 624.3 ± 75.1 a | 677.8 ± 71.1 a | |
| 6 | 1-Penten-3-ol | C616251 | 1136.4 | 613.8 | 0.9 | 1104.4 ± 138.4 ab | 1236.5 ± 39.6 a | 973.4 ± 41.6 bc | 1146.4 ± 20.7 a | 827.9 ± 20.4 c | |
| 7 | 1,8-Cineole | C470826 | 1174.2 | 693.1 | 1.3 | 346.9 ± 65.3 b | 538.1 ± 122.9 a | 396.3 ± 79.2 b | 575.1 ± 226.2 a | 597.5 ± 331.3 a | |
| Total | 8803.22 a | 10,106.8 b | 10,314.02 b | 10,661.9 b | 10,829.5 c | ||||||
| Aldehydes | 8 | Acetaldehyde | C75070 | 753.8 | 223.4 | 0.9 | 6764.6 ± 179.9 a | 5978.5 ± 255.7 ab | 5985.9 ± 220.6 ab | 4694.1 ± 212.8 b | 2269.1 ± 1357.6 c |
| 9 | Propanal-M | C123386 | 801.8 | 247.3 | 1.0 | 1825.1 ± 76.9 a | 1631.5 ± 57.9 b | 1468.8 ± 39.6 c | 1175.3 ± 35.8 d | 741.4 ± 56.3 e | |
| 10 | Propanal-D | C123386 | 801.8 | 247.3 | 1.1 | 5113.2 ± 234.8 a | 4243.6 ± 262.3 b | 4618.8 ± 347.7 ab | 4314.7 ± 112.7 b | 4464.7 ± 54.8 b | |
| 11 | 2-Methyl propanal | C78842 | 808 | 250.6 | 1.3 | 532.9 ± 43.4 a | 532.2 ± 56.4 a | 538.9 ± 65.1 a | 571.2 ± 23.7 a | 559.2 ± 62.6 a | |
| 12 | 2-Methylbutanal | C96173 | 907.8 | 309.4 | 1.4 | 5984.0 ± 168.0 a | 6173.0 ± 214.2 a | 6168.4 ± 233.0 a | 6346.3 ± 30.1 a | 5559.6 ± 69.7 b | |
| 13 | Hexanal-M | C66251 | 1069.1 | 479.8 | 1.3 | 1502.3 ± 220.0 a | 1229.3 ± 82.7 a | 1273.6 ± 200.4 a | 1393.0 ± 91.7 a | 1557.5 ± 267.0 a | |
| 14 | Hexanal-D | C66251 | 1070 | 481.2 | 1.6 | 395.2 ± 114.6 a | 285.2 ± 38.6 a | 329.2 ± 83.1 a | 393.7 ± 62.9 a | 517.7 ± 186.0 a | |
| 15 | Furfural | C98011 | 1425.7 | 1383.4 | 1.1 | 133.7 ± 23.4 b | 140.1 ± 36.0 b | 184.5 ± 52.5 b | 203.0 ± 9.8 b | 533.7 ± 59.3 a | |
| Total | 22,200.9 a | 20,193.4 b | 20,558.1 b | 19,111.9 c | 16,203.0 d | ||||||
| Ketones | 16 | Acetone | C67641 | 819.4 | 256.7 | 1.1 | 7751.0 ± 294.0 a | 7328.2 ± 145.2 ab | 7072.2 ± 287.3 b | 7208.6 ± 116.2 ab | 6829.1 ± 368.3 b |
| 17 | 1-Hydroxy-2-propanone-M | C116096 | 1264.2 | 888.2 | 1.0 | 1417.5 ± 75.0 d | 1647.3 ± 72.8 c | 1786.2 ± 96.0 c | 2370.7 ± 122.6 b | 5050.6 ± 123.6 a | |
| 18 | 1-Hydroxy-2-propanone-D | C116096 | 1265.7 | 891. 9 | 1.2 | 103.1 ± 10.2 b | 153.4 ± 12.4 b | 184.1 ± 20.2 b | 363.6 ± 51.1 b | 3996.8 ± 489.2 a | |
| 19 | 2-Butanone | C78933 | 893.3 | 300.0 | 1.2 | 1380.2 ± 94.5 c | 1678.0 ± 85.7 c | 1710.2 ± 39.1 c | 2152.7 ± 100.6 b | 4160.4 ± 370.5 a | |
| 20 | 3-Hydroxybutan-2-one-M | C513860 | 1251.5 | 857.8 | 1.0 | 1499.1 ± 47.6 d | 1780.3 ± 103.5 c | 1874.3 ± 73.6 c | 2644.5 ± 86.7 b | 3770.0 ± 29.4 a | |
| 21 | 3-Hydroxybutan-2-one-D | C513860 | 1250.5 | 855.3 | 1.3 | 182.1 ± 7.0 c | 263.9 ± 23.5 c | 304.9 ± 14.4 c | 732.9 ± 63.2 b | 3583.7 ± 193.6 a | |
| 22 | 2,3-Butanedione | C431038 | 977.3 | 358.3 | 1.2 | 2399.2 ± 69.4 a | 2674.9 ± 163.9 a | 2819.1 ± 162.1 a | 2818.1 ± 25.1 a | 2528.1 ± 304.3 a | |
| 23 | 6-Methyl-5-hepten-2-one | C110930 | 1300.9 | 982.4 | 1.2 | 205.3 ± 101.1 a | 238.01 ± 54.7 a | 282.6 ± 118.4 a | 367.2 ± 57.6 a | 390.8 ± 49.4 a | |
| Total | 14,937.5 a | 15,764.1 b | 16,033.4 b | 18,658.4 c | 30,309.5 d | ||||||
| Pyrazines | 24 | 2-Methylpyrazine-M | C109080 | 1232.3 | 813.7 | 1.1 | 277.0 ± 11.1 c | 376.4 ± 34.6 c | 411.8 ± 49.4 c | 799.3 ± 14.9 b | 4773.7 ± 132.1 a |
| 25 | 2-Methylpyrazine-D | C109080 | 1231.1 | 810.9 | 1.4 | 40.5 ± 4.1 b | 38.1 ± 3.5 b | 36.2 ± 1.4 b | 45.9 ± 4.8 b | 1004.0 ± 74.0 a | |
| 26 | 2,3-Dimethylpyrazine | C5910894 | 1301.1 | 982.9 | 1.1 | 75.6 ± 5.5 c | 92.6 ± 9.1 bc | 103.4 ± 9.9 bc | 139.1 ± 0.9 b | 1003.6 ± 50.9 a | |
| 27 | 2,5-Dimethylpyrazine | C123320 | 1265.1 | 890.4 | 1.1 | 85.3 ± 18.9 d | 156.5 ± 6.9 c | 191.3 ± 14.7 c | 287.6 ± 16.0 b | 532.2 ± 34.8 a | |
| 28 | 2,6-Dimethylpyrazine-M | C108509 | 1286.8 | 944.9 | 1.1 | 69.9 ± 5.1 c | 99.9 ± 8.2 c | 104.1 ± 13.7 c | 192.9 ± 8.2 b | 1420.8 ± 60.2 a | |
| 29 | 2,6-Dimethylpyrazine-D | C108509 | 1285.8 | 942.4 | 1.5 | 69.9 ± 5.1 c | 99.9 ± 8.2 c | 104.1 ± 13.7 c | 192.9 ± 8.2 b | 1420.8 ± 60.2 a | |
| Total | 593.8 a | 811.7 b | 893.0 b | 1509.7 c | 9021.6 d | ||||||
| Sulfides | 30 | Dimethyl sulfide-M | C75183 | 776.9 | 234.6 | 0.9 | 5115.3 ± 168.8 a | 5142.6 ± 306.2 a | 5565.6 ± 206.2 a | 5193.1 ± 178.1 a | 3653.0 ± 550.9 b |
| 31 | Dimethyl sulfide-D | C75183 | 772.7 | 232.5 | 1.1 | 1073.0 ± 178.8 a | 715.4 ± 93.9 b | 726.4 ± 103.2 b | 619.2 ± 25.6 b | 467.1 ± 123.1 b | |
| 32 | 3-(Methylthio)-1-propene | C10152768 | 958.1 | 344.1 | 1.0 | 4164.3 ± 99.1 a | 4127.5 ± 243.2 a | 4289.8 ± 233.4 a | 4161.0 ± 288.6 a | 3930.9 ± 158.9 a | |
| 33 | Allyl sulfide | C592881 | 1126.4 | 591.7 | 1.1 | 1127.4 ± 107.9 a | 1087.4 ± 155.5 a | 1210.9 ± 117.4 a | 1471.0 ± 386.1 a | 1602.564.4 a | |
| Total | 11,480.4 a | 11,072.8 a | 11,792.8 a | 11,444.4 a | 9653.8 b | ||||||
| Esters | 34 | Methyl acetate | C79209 | 810.7 | 251.9 | 1.2 | 452.8 ± 28.9 a | 457.4 ± 24.4 a | 506.28.0 a | 514.4 ± 8.8 a | 476.9 ± 31.4 a |
| 35 | Ethyl Acetate | C141786 | 875.3 | 288.9 | 1.3 | 7731.0 ± 84.2 a | 7677.3 ± 401.3 b | 7469.3 ± 306.1 b | 7761.5 ± 92.7 a | 6994.3 ± 434.0 b | |
| 36 | Ethyl formate | C109944 | 807.1 | 250.1 | 1.2 | 251.5 ± 19.2 a | 208.5 ± 17.5 ab | 207.7 ± 28.7 ab | 188.3 ± 4.1 bc | 160.3 ± 22.3 c | |
| Total | 8435.3 a | 8343.3 a | 8183.1 b | 8464.2 a | 7631.6 c | ||||||
| Acids | 37 | Acetic acid-M | C64197 | 1435.5 | 1420.9 | 1.0 | 6448.6 ± 347.6 a | 5129.2 ± 587.9 b | 4879.4 ± 74.1 b | 4610.7 ± 306.0 b | 4762.4 ± 309.1 b |
| 38 | Acetic acid-D | C64197 | 1432.9 | 1411.1 | 1.2 | 367.4 ± 38.5 a | 240.7 ± 64.2 b | 219.6 ± 15.8 b | 204.2 ± 39.8 b | 166.8 ± 34.8 b | |
| Total | 6816.0 a | 5369.9 b | 5099.0 c | 4814.9 d | 4929.2 d | ||||||
| Others | 39 | 1,1-Diethoxy ethane | C105577 | 885.8 | 295.3 | 1.0 | 1798.8 ± 160.3 a | 1794.4 ± 121.5 a | 1960.6 ± 31.1 a | 1740.8 ± 95.5 a | 1241.1 ± 39.9 b |
| 40 | Trimethylamine | C75503 | 765.4 | 228.9 | 1.1 | 1092.9 ± 303.5 c | 3104.1 ± 276.7 c | 3928.9 ± 430.0 bc | 7237.9 ± 592.4 b | 13,851.3 ± 3537.03 a | |
| 41 | 3-Ethylpyridine | C536787 | 1327.5 | 1056.6 | 1.1 | 81.2 ± 9.9 b | 130.4 ± 10.2 b | 126.3 ± 8.9 b | 153.6 ± 17.5 b | 344.3 ± 82.6 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, Z.; Deng, S.; Benjakul, S.; Zhang, B.; Huo, J. Effects of Heating Treatment on the Physicochemical and Volatile Flavor Properties of Argentinian Shortfin Squid (Illex argentinus). Foods 2024, 13, 1025. https://doi.org/10.3390/foods13071025
Li J, Li Z, Deng S, Benjakul S, Zhang B, Huo J. Effects of Heating Treatment on the Physicochemical and Volatile Flavor Properties of Argentinian Shortfin Squid (Illex argentinus). Foods. 2024; 13(7):1025. https://doi.org/10.3390/foods13071025
Chicago/Turabian StyleLi, Jiagen, Zhaoqi Li, Shanggui Deng, Soottawat Benjakul, Bin Zhang, and Jiancong Huo. 2024. "Effects of Heating Treatment on the Physicochemical and Volatile Flavor Properties of Argentinian Shortfin Squid (Illex argentinus)" Foods 13, no. 7: 1025. https://doi.org/10.3390/foods13071025
APA StyleLi, J., Li, Z., Deng, S., Benjakul, S., Zhang, B., & Huo, J. (2024). Effects of Heating Treatment on the Physicochemical and Volatile Flavor Properties of Argentinian Shortfin Squid (Illex argentinus). Foods, 13(7), 1025. https://doi.org/10.3390/foods13071025







