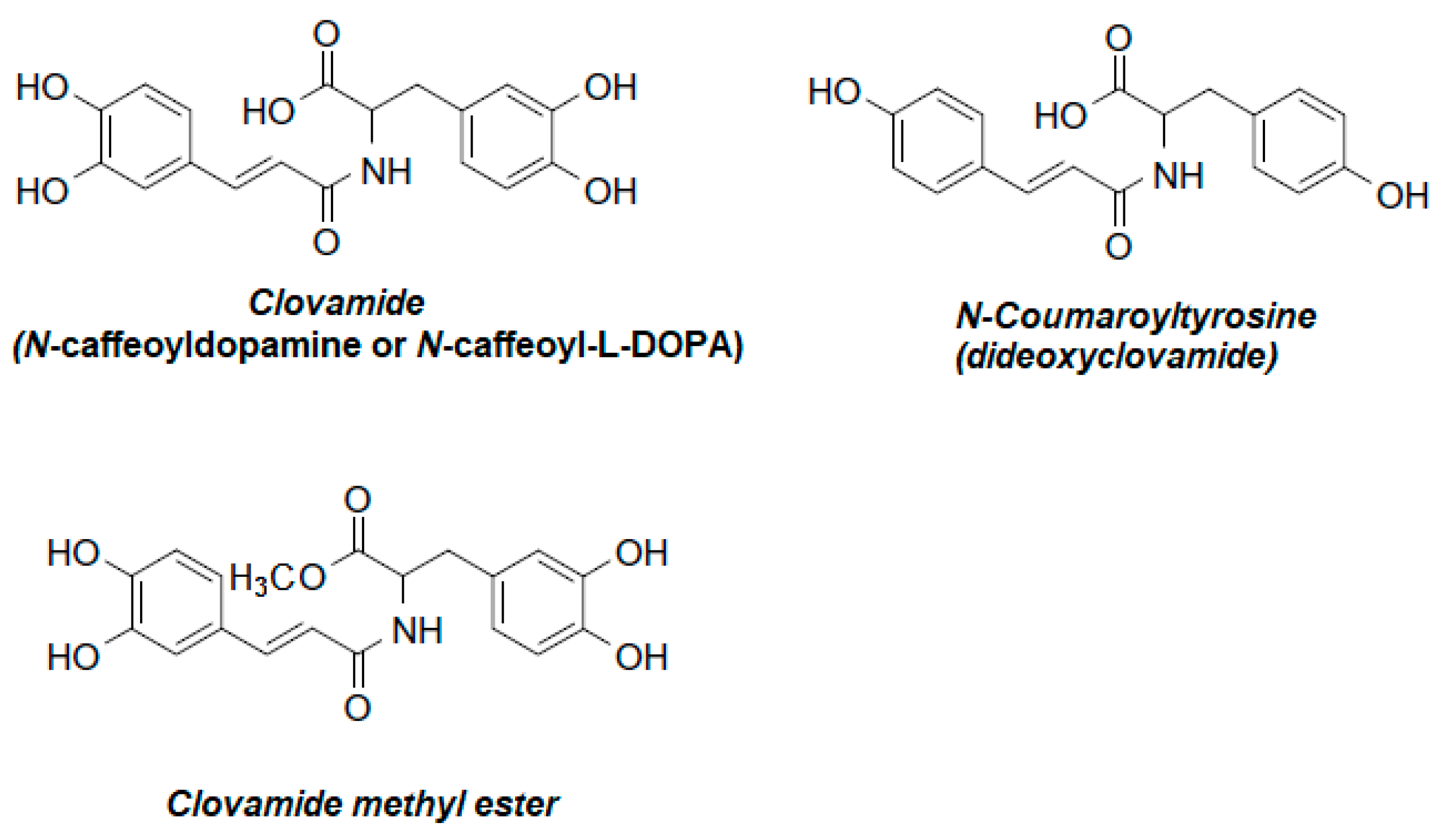
Clovamide and Its Derivatives—Bioactive Components of Theobroma cacao and Other Plants in the Context of Human Health
Abstract
1. Introduction
2. Plant Sources of Clovamides
3. Bioavailability and Metabolism of Clovamide-Type Compounds
4. Biological Activity of Clovamides
4.1. Antioxidant Action
| Experimental Model | EC50 or IC50 Values Established for Clovamide | EC50 or IC50 Values Established for Reference Compounds | References |
|---|---|---|---|
| DPPH• scavenging | Clovamide: 2.65 μg/mL | Caffeic acid: 2.93 μg/mL Epicatechin: 3.11 μg/mL Gallic acid: 1.03 μg/mL Rosmarinic acid: 2.49 μg/mL Myricetin: 1.95 μg/mL Quercetin: 1.99 μg/mL Kaempferol: 4.26 μg/mL BHA: 8.18 μg/mL Trolox: 3.32 μg/mL Octyl gallate: 1.65 μg/mL | [56] |
| Clovamide: 4.9 μg/mL | Caffeic acid: 4.2 μg/mL Chlorogenic acid: 8.3 μg/mL Trolox: 5.7 μg/mL | [30] | |
| Clovamide: 0.05 mol/mol | Rosmarinic acid: 0.58 mol/mol α-Tocopherol: 0.025 mol/mol Ascorbic acid: 0.025 mol/mol L-dopamine: 0.095 mol/mol | [59] | |
| ONOO− scavenging | Clovamide: 19.3 μg/mL | Caffeic acid: 15.0 μg/mL Chlorogenic acid: 27.4 μg/mL Trolox: <5 μg/mL | [30] |
| Superoxide anion scavenging | Clovamide: 60 nmol/L | Rosmarinic acid: 95 nmol/L α-Tocopherol: >10 000 nmol/L Ascorbic acid: 700 nmol/L L-dopamine: 200 nmol/L | [59] |
| β-carotene bleaching | Clovamide: 0.02 mmol/L | α-Tocopherol: 0.08 mmol/L Ascorbic acid: >0.09 L-dopamine: >1.1 mmol/L | [59] |
4.2. Anti-Inflammatory Effects
4.3. Neuroprotective Effects
4.4. Anti-Platelet Action
4.5. Anticancer Properties
4.6. Antiviral, Antibacterial and Anti-Trypanosomal Activities
4.7. Estrogenic Activity
5. Concluding Remarks
Funding
Data Availability Statement
Conflicts of Interest
References
- Lippi, D. Sin and Pleasure: The History of Chocolate in Medicine. J. Agric. Food Chem. 2015, 63, 9936–9941. [Google Scholar] [CrossRef] [PubMed]
- Montagna, M.T.; Diella, G.; Triggiano, F.; Caponio, G.R.; De Giglio, O.; Caggiano, G.R.; Di Ciaula, A.; Portincasa, P. Chocolate, “Food of the Gods”: History, Science, and Human Health. Int. J. Environ. Res. Public Health 2019, 16, 4960. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and Dark Chocolate Polyphenols: From Biology to Clinical Applications. Front. Immunol. 2017, 8, 677. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.Y.C.; Lim, X.Y.; Yeo, J.H.H.; Lee, S.W.H.; Lai, N.M. The Health Effects of Chocolate and Cocoa: A Systematic Review. Nutrients 2021, 13, 2909. [Google Scholar] [CrossRef] [PubMed]
- Roumani, M.; Duval, R.E.; Ropars, A.; Risler, A.; Robin, C.; Larbat, R. Phenolamides: Plant Specialized Metabolites with a Wide Range of Promising Pharmacological and Health-Promoting Interests. Biomed. Pharmacother. 2020, 131, 110762. [Google Scholar] [CrossRef] [PubMed]
- Knollenberg, B.J.; Li, G.-X.; Lambert, J.D.; Maximova, S.N.; Guiltinan, M.J. Clovamide, a Hydroxycinnamic Acid Amide, Is a Resistance Factor Against Phytophthora spp. in Theobroma cacao. Front. Plant Sci. 2020, 11, 617520. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. Quantitation of Clovamide-Type Phenylpropenoic Acid Amides in Cells and Plasma Using High-Performance Liquid Chromatography with a Coulometric Clectrochemical Detector. J. Agric. Food Chem. 2005, 53, 8135–8140. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, B.; Yu, K.; Shi, F.; Liu, T.; Xu, W. Caffeic Acid Derivatives: A New Type of Influenza Neuraminidase Inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 3556–3560. [Google Scholar] [CrossRef]
- Bouchez, P.; Teixeira Benites, V.; Baidoo, E.E.K.; Mortimer, J.C.; Sullivan, M.L.; Scheller, H.V.; Eudes, A. Production of Clovamide and Its Analogues in Saccharomyces cerevisiae and Lactococcus lactis. Lett. Appl. Microbiol. 2019, 69, 181–189. [Google Scholar]
- Yoshihara, T.; Yoshikawa, H.; Sakamura, S.; Sakuma, T. Clovamides: L-DOPA Conjugated with Trans- and Cis-Caffeic acids in Red Clover. Agric. Biol. Chem. 1974, 38, 1107–1109. [Google Scholar] [CrossRef]
- Sanbongi, C.; Osakabe, N.; Natsume, M.; Takizawa, T.; Gomi, S.; Osawa, T. Antioxidative Polyphenols Isolated from Theobroma cacao. J. Agric. Food Chem. 1998, 46, 454–457. [Google Scholar] [CrossRef] [PubMed]
- de Moraes Barros, H.R.; García-Villalba, R.; Tomás-Barberán, F.A.; Genovese, M.I. Evaluation of the Distribution and Metabolism of Polyphenols Derived from Cupuassu (Theobroma grandiflorum) in Mice Gastrointestinal Tract by UPLC-ESI-QTOF. J. Funct. Foods 2016, 22, 477–489. [Google Scholar] [CrossRef]
- Yoshihara, T.; Yoshikawa, H.; Kunimatsu, S.; Sakamura, S.; Sakuma, T. New Amino Acid Derivatives Conjugated with Caffeic Acid and DOPA from Red Clover (Trifolium pratense). Agric. Biol. Chem. 1977, 41, 1679–1684. [Google Scholar]
- Szajwaj, B.; Moldoch, J.; Masullo, M.; Piacente, S.; Oleszek, W.; Stochmal, A. Amides and Esters of Phenylpropenoic Acids from the Aerial Parts of Trifolium pallidum. Nat. Prod. Commun. 2011, 6, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Masike, K.; Khoza, B.S.; Steenkamp, P.A.; Smit, E.; Dubery, I.A.; Madala, N.E. A Metabolomics-Guided Exploration of the Phytochemical Constituents of Vernonia fastigiata with the Aid of Pressurized Hot Water Extraction and Liquid Chromatography-Mass Spectrometry. Molecules 2017, 22, 1200. [Google Scholar] [CrossRef] [PubMed]
- Van Heerden, F.R.; Brandt, E.V.; Roux, D.G. Isolation and synthesis of trans- and cis-(−)-clovamides and their deoxy analogues from the bark of Dalbergia melanoxylon. Phytochemistry 1980, 19, 2125–2129. [Google Scholar] [CrossRef]
- Nascimento, L.E.S.; Arriola, N.D.A.; da Silva, L.A.L.; Faqueti, L.G.; Sandjo, L.P.; de Araújo, C.E.S.; Biavatti, M.W.; Barcelos-Oliveira, J.L.; de Mello Castanho Amboni, R.D. Phytochemical profile of different anatomical parts of jambu (Acmella oleracea (L.) R.K. Jansen): A comparison between hydroponic and conventional cultivation using PCA and cluster analysis. Food Chem. 2020, 332, 127393. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.; Melzig, M.F.; Jenett-Siems, K. New Phenolic Compounds of Acmella ciliata. Planta Medica 2010, 76, 633–635. [Google Scholar] [CrossRef] [PubMed]
- El-Sharawy, R.T.; Elkhateeb, A.; Marzouk, M.M.; Abd El-Latif, R.R.; Abdelrazig, S.E.; El-Ansari, M.A. Antiviral and An-tiparasitic Activities of Clovamide: The Major Constituent of Dichrostachys cinerea (L.) Wight et Arn. J. Appl. Pharm. Sci. 2017, 7, 219–223. [Google Scholar]
- Abouelela, M.E.; Orabi, M.A.A.; Abdelhamid, R.A.; Abdelkader, M.S.; Madkor, H.R.; Darwish, F.M.M.; Hatano, T.; Elsadek, B.E.M. Ethyl Acetate Extract of Ceiba pentandra (L.) Gaertn. Reduces Methotrexate-Induced Renal Damage in Rats via Antioxidant, Anti-inflammatory, and Antiapoptotic Actions. J. Tradit. Complement. Med. 2020, 10, 478–486. [Google Scholar] [CrossRef]
- Burlec, A.F.; Pecio, Ł.; Mircea, C.; Cioancă, O.; Corciovă, A.; Nicolescu, A.; Oleszek, W.; Hăncianu, M. Chemical Profile and Antioxidant Activity of Zinnia elegans Jacq. Fractions. Molecules 2019, 24, 2934. [Google Scholar] [CrossRef]
- Francišković, M.; Gonzalez-Pérez, R.; Orčić, D.; Sánchez de Medina, F.; Martínez-Augustin, O.; Svirčev, E.; Simin, N.; Mimica-Dukić, N. Chemical Composition and Immuno-Modulatory Effects of Urtica dioica L. (Stinging Nettle) Extracts. Phytother. Res. 2017, 31, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Belli, S.; Caruso, F.; Roy, G.; Rossi, M. Antioxidant Studies by Hydrodynamic Voltammetry and DFT, Quantitative Analyses by HPLC-DAD of Clovamide, a Natural Phenolic Compound Found in Theobroma cacao L. beans. Food Chem. 2021, 341, 128260. [Google Scholar] [CrossRef]
- Arlorio, M.; Locatelli, M.; Travaglia, F.; Coïsson, J.-D.; Del Grosso, E.; Minassi, A.; Appendino, G.; Martelli, A. Roasting Impact on the Contents of Clovamide (N-caffeoyl-L-DOPA) and the Antioxidant Activity of Cocoa Beans (Theobroma cacao L.). Food Chem. 2008, 106, 967–975. [Google Scholar] [CrossRef]
- Lechtenberg, M.; Henschel, K.; Liefländer-Wulf, U.; Quandt, B.; Hensel, A. Fast Determination of N-Phenylpropenoyl-l-Amino acids (NPA) in Cocoa Samples from Different Origins by Ultra-Performance Liquid Chro-matography and Capillary Electrophoresis. Food Chem. 2012, 135, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Kellard, B.; Ah-Sing, E. Caffeoyltyrosine from Green Robusta Coffee Beans. Phytochemistry 1989, 28, 1989–1990. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J. Trifolium species—The Latest Findings on Chemical Profile, Ethnomedicinal Use and Pharmaco-logical Properties. J. Pharm. Pharmacol. 2016, 68, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Kanadys, W.; Barańska, A.; Błaszczuk, A.; Polz-Dacewicz, M.; Drop, B.; Kanecki, K.; Malm, M. Evaluation of Clinical Meaningfulness of Red Clover (Trifolium pratense L.) Extract to Relieve Hot Flushes and Menopausal Symptoms in Peri- and Post-Menopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 1258. [Google Scholar] [CrossRef]
- Oleszek, W.; Stochmal, A.; Janda, B. Concentration of Isoflavones and Other Phenolics in the Aerial Parts of Trifolium Species. J. Agric. Food Chem. 2007, 55, 8095–8100. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Krzyżanowska-Kowalczyk, J.; Sieradzka, M.; Nowak, P.; Stochmal, A. Clovamide and Clovamide-Rich Extracts of Three Trifolium Species as Antioxidants and Moderate Antiplatelet Agents In Vitro. Phytochemistry 2017, 143, 54–63. [Google Scholar] [CrossRef]
- Sullivan, M.L.; Zeller, W.E. Efficacy of Various Naturally Occurring Caffeic Acid Derivatives in Preventing Post-Harvest Protein Losses in Forages. J. Sci. Food Agric. 2013, 93, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Tava, A.; Pecio, Ł.; Stochmal, A.; Pecetti, L. Clovamide and Flavonoids from Leaves of Trifolium pratense and T. pratense subsp. nivale Grown in Italy. Nat. Prod. Commun. 2015, 10, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Stark, T.; Lang, R.; Keller, D.; Hense, A.; Hofmann, T. Absorption of N-Phenylpropenoyl-L-Amino Acids in Healthy Humans by Oral Administration of Cocoa (Theobroma cacao). Mol. Nutr. Food Res. 2008, 52, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 230S–242S. [Google Scholar] [CrossRef]
- Vitaglione, P.; Lumaga, R.B.; Ferracane, R.; Sellitto, S.; Morelló, J.R.; Miranda, J.R.; Shimoni, E.; Fogliano, V. Human Bioavailability of Flavanols and Phenolic acids from Cocoa-Nut Creams Enriched with Free or Microencapsulated Cocoa Polyphenols. Br. J. Nutr. 2013, 109, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Konishi, Y.; Kobayashi, S. Transepithelial Transport of Rosmarinic Acid in Intestinal Caco-2 Cell Monolayers. Biosci. Biotechnol. Biochem. 2005, 69, 583–591. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baba, S.; Osakabe, N.; Natsume, M.; Yasuda, A.; Muto, Y.; Hiyoshi, T.; Takano, H.; Yoshikawa, T.; Terao, J. Absorption, Metabolism, Degradation and Urinary Excretion of Rosmarinic Acid After Intake of Perilla frutescens extract in Humans. Eur. J. Nutr. 2005, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.Y.; Gonthier, M.-P.; Rémésy, C.; Mila, I.; Lapierre, C.; Lazarus, S.A.; Williamson, G.; Scalbert, A. Chocolate Intake Increases Urinary Excretion of Polyphenol-Derived Phenolic Acids in Healthy Human Subjects. Am. J. Clin. Nutr. 2003, 77, 912–918. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Llorach, R.; Khan, N.; Monagas, M.; Rotches-Ribalta, M.; Lamuela-Raventos, R.; Estruch, R.; Tinahones, F.J.; Andres-Lacueva, C. Effect of Milk on the Urinary Excretion of Microbial Phenolic Acids After Cocoa Powder Consumption in Humans. J. Agric. Food Chem. 2010, 58, 4706–4711. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The Role of Oxidative Stress during Inflammatory Processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef] [PubMed]
- Cecerska-Heryć, E.; Polikowska, A.; Serwin, N.; Roszak, M.; Grygorcewicz, B.; Heryć, R.; Michalczyk, A.; Dołęgowska, B. Importance of Oxidative Stress in the Pathogenesis, Diagnosis, and Monitoring of Patients with Neuropsychiatric Disorders, a Review. Neurochem. Int. 2022, 153, 105269. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Aggarwal, B.B.; Singh, R.B.; Buttar, H.S.; Wilson, D.; De Meester, F. Food Antioxidants and Their Anti-Inflammatory Properties: A Potential Role in Cardiovascular Diseases and Cancer Prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Bin Dukhyil, A.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2022, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Madala, N.E.; Kabanda, M.M. LC-MS Based Validation and DFT Investigation on the Antioxidant Properties of Clovamide: •OH and •OOH Scavenging and Cu(II) Chelation Mechanisms. J. Mol. Struct. 2021, 15, 1236. [Google Scholar] [CrossRef]
- Locatelli, M.; Travaglia, F.; Giovannelli, L.; Coïsson, J.D.; Bordiga, M.; Pattarino, F.; Arlorio, M. Clovamide and Phenolics from Cocoa Beans (Theobroma cacao L.) Inhibit Lipid Peroxidation in Liposomal Systems. Food Res. Int. 2013, 50, 129–134. [Google Scholar] [CrossRef]
- Lotito, S.B.; Frei, B. Consumption of Flavonoid-Rich Foods and Increased Plasma Antioxidant Capacity in Humans: Cause, Consequence, or Epiphenomenon? Free. Radic. Biol. Med. 2006, 41, 1727–1746. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; German, J.B. Antioxidants in Foods and Health: Problems and Fallacies in the Field. J. Sci. Food Agric. 2006, 86, 1999–2001. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. Nutrients and Phytochemicals: From Bioavailability to Bioefficacy Beyond Antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The Biological Relevance of Direct Antioxidant Effects of Polyphenols for Cardiovascular Health in Humans Is Not Established. J. Nutr. 2011, 141, 989–1009. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Gindro, R.; Travaglia, F.; Coïsson, J.-D.; Rinaldi, M.; Arlorio, M. Study of the DPPH-Scavenging Activity: Development of a Free Software for the Correct Interpretation of Data. Food Chem. 2009, 114, 889–897. [Google Scholar] [CrossRef]
- Marinova, E.; Georgiev, L.; Totseva, I.; Seizova, K.; Milkova, T. Antioxidant Activity and Mechanism of Action of Some Synthesised Phenolic acid Amides of Aromatic Amines. Czech J. Food Sci. 2013, 31, 5–13. [Google Scholar] [CrossRef]
- Sarr, S.O.; Gassama, A.; Manga, F.; Grellepois, F.; Lavaud, C. Synthesis and Study of Antioxidant Activities of Trans-(-)-Clovamide Derivatives. Am. J. Chem. Appl. 2018, 5, 58–63. [Google Scholar]
- Ley, J.P.; Bertram, H.-J. Synthesis of Lipophilic Clovamide Derivatives and Their Antioxidative Potential against Lipid Peroxidation. J. Agric. Food Chem. 2003, 51, 4596–4602. [Google Scholar] [CrossRef]
- Strobel, N.A.; Fassett, R.G.; Marsh, S.A.; Coombes, J.S. Oxidative Stress Biomarkers as Predictors of Cardiovascular Disease. Int. J. Cardiol. 2011, 147, 191–201. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-Y.; Xu, X.; Li, X.-C. Cardiovascular Diseases: Oxidative Damage and Antioxidant Protection. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3091–3096. [Google Scholar]
- Arlorio, M.; Coisson, J.D.; Travaglia, F.; Locatelli, M.; Bordiga, M.; Zamperone, A.; Pietronave, S.; Brunelleschi, S.; Prat, M. Radical Scavenging Capacity of Cocoa Polyphenols Triggers Anti-Inflammatory Properties in Human Monocytes and Al-lows Protective Effects on H9c2 Cardiomyoblast Exposed to Oxidative Stress. Pol. J. Food Nutr. Sci. 2011, 61, 22–23. [Google Scholar]
- Antonini, S.; Colangelo, D.; Oltolina, F.; Diena, M.; Arlorio, M.; Prat, M. Clovamide Protects Cardiac Progenitor Cells from H2O2-Induced Oxidative Stress. J. Appl. Biotechnol. Bioeng. 2018, 5, 1. [Google Scholar] [CrossRef]
- Kolodziejczyk, J.; Olas, B.; Wachowicz, B.; Szajwaj, B.; Stochmal, A.; Oleszek, W. Clovamide-Rich Extract from Trifolium pallidum Reduces Oxidative Stress-Induced Damage to Blood Platelets and Plasma. J. Physiol. Biochem. 2011, 67, 391–399. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Rocha, J.; Eduardo-Figueira, M.; Barateiro, A.; Fernandes, A.; Brites, D.; Bronze, R.; Duarte, C.M.; Serra, A.T.; Pinto, R.; Freitas, M.; et al. Anti-Inflammatory Effect of Rosmarinic Acid and an Extract of Rosmarinus officinalis in Rat Models of Local and Systemic Inflammation. Basic Clin. Pharmacol. Toxicol. 2015, 116, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, M.I.; Asiri, A.M. Biomedical Features and Therapeutic Potential of Rosmarinic Acid. Arch. Pharmacal Res. 2022, 45, 205–228. [Google Scholar] [CrossRef]
- Zeng, H.; Locatelli, M.; Bardelli, C.; Amoruso, A.; Coisson, J.D.; Travaglia, F.; Arlorio, M.; Brunelleschi, S. Anti-Inflammatory Properties of Clovamide and Theobroma cacao Phenolic Extracts in Human Monocytes: Evaluation of Respiratory Burst, Cytokine Release, NF-κB Activation, and PPARγ Modulation. J. Agric. Food Chem. 2011, 59, 5342–5350. [Google Scholar] [CrossRef]
- Liu, R.; Guo, Y.; Yu, J.; Wei, X.; Zhou, F.; Yuan, X.; Cai, L.; Yu, C. Protective effect of N-(E)-p-Coumaroyltyrosine on LPS-Induced acute Inflammatory Injury and Signaling Pathway Analysis. Fish Shellfish Immunol. 2024, 144, 109242. [Google Scholar] [CrossRef]
- Nichols, E.; Vos, T. Estimating the Global Mortality from Alzheimer’s Disease and other Dementias: A New Method and Results from the Global Burden of Disease Study 2019. Alzheimer’s Dement. 2020, 16, e042236. [Google Scholar] [CrossRef]
- Tyler, S.E.B.; Tyler, L.D.K. Pathways to Healing: Plants with Therapeutic Potential for Neurodegenerative Diseases. IBRO Neurosci. Rep. 2023, 14, 210–234. [Google Scholar] [CrossRef] [PubMed]
- Cimini, A.; Gentile, R.; D’Angelo, B.; Benedetti, E.; Cristiano, L.; Avantaggiati, M.L.; Giordano, A.; Ferri, C.; Desideri, G. Cocoa Powder Triggers Neuroprotective and Preventive Effects in a Human Alzheimer’s Disease Model by Modulating BDNF Signaling Pathway. J. Cell Biochem. 2013, 114, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Reyes, P.K.; de Lara, J.C.-F.; González-Soto, M.; Tejero, M.E. Effects of Cocoa-Derived Polyphenols on Cognitive Function in Humans. Systematic Review and Analysis of Methodological Aspects. Plant Foods Hum. Nutr. 2020, 75, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fallarini, S.; Miglio, G.; Paoletti, T.; Minassi, A.; Amoruso, A.; Bardelli, C.; Brunelleschi, S.; Lombardi, G. Clovamide and Rosmarinic Acid Induce Neuroprotective Effects in In Vitro Models of Neuronal Death. Br. J. Pharmacol. 2009, 157, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-H.; Hu, X.-L.; Lv, X.-Y.; Wang, B.-L.; Lin, J.; Zhang, X.-Q.; Ye, W.-C.; Xiong, F.; Wang, H. Synthesis and Biological Evaluation of Clovamide Analogues with Catechol Functionality as Potent Parkinson’s Disease Agents In Vitro and In Vivo. Bioorganic Med. Chem. Lett. 2019, 29, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, T.; Takase, M.; Shigemori, H. Structure-Activity Relationship of Clovamide and Its Related Compounds for the Inhibition of Amyloid β Aggregation. Bioorganic Med. Chem. 2018, 26, 3202–3209. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, D.; Tsunoda, T.; Shigemori, H. Effects of Clovamide and Its Related Compounds on the Aggregations of Amyloid Polypeptides. J. Nat. Med. 2021, 75, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Betkekar, V.V.; Ohmori, K.; Suzuki, K.; Shigemori, H. Evaluation of Amyloid Polypeptide Aggregation Inhibition and Disaggregation Activity of A-Type Procyanidins. Pharmaceuticals 2021, 14, 1118. [Google Scholar] [CrossRef]
- Li, J.; Zhu, M.; Manning-Bog, A.B.; Di Monte, D.A.; Fink, A.L. Dopamine and L-DOPA Disaggregate Amyloid Fibrils: Implications for Parkinson’s and Alzheimer’s Disease. FASEB J. 2004, 18, 962–964. [Google Scholar] [CrossRef]
- Hase, T.; Shishido, S.; Yamamoto, S.; Yamashita, R.; Nukima, H.; Taira, S.; Toyoda, T.; Abe, K.; Hamaguchi, T.; Ono, K.; et al. Rosmarinic acid Suppresses Alzheimer’s Disease Development by Reducing Amyloid β Aggregation by Increasing Monoamine secretion. Sci. Rep. 2019, 9, 8711. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, B.-W.; Lee, H.U.; Choi, D.-K.; Yoon, S.-H. Synthesis of Clovamide Analogues That Inhibit NO Production in Activated BV-2 Microglial Cells. Biol. Pharm. Bull. 2017, 40, 1475–1482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, X.-L.; Lin, J.; Lv, X.-Y.; Feng, J.-H.; Zhang, X.-Q.; Wang, H.; Ye, W.-C. Synthesis and Biological Evaluation of Clovamide Analogues as Potent Anti-Neuroinflammatory Agents in Vitro and In Vivo. Eur. J. Med. Chem. 2018, 151, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; More, S.V.; Park, J.Y.; Jeon, S.B.; Park, S.Y.; Park, E.J.; Yoon, S.H.; Choi, D.K. EOP, A Newly Synthesized Ethyl Pyruvate Derivative, Attenuates the Production of Inflammatory Mediators via p38, ERK and NF-κB Pathways in Lipo-polysaccharide-Activated BV-2 Microglial Cells. Molecules 2014, 19, 19361–19375. [Google Scholar] [CrossRef] [PubMed]
- Yarza, R.; Vela, S.; Solas, M.; Ramirez, M.J. c-Jun N-terminal Kinase (JNK) Signaling as a Therapeutic Target for Alzheimer’s Disease. Front. Pharmacol. 2016, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Park, J.I.; More, S.V.; Park, J.Y.; Kim, B.W.; Jeon, S.B.; Yun, Y.S.; Park, E.J.; Yoon, S.H.; Choi, D.K. Anti-Neuroinflammatory Effects of DPTP, A Novel Synthetic Clovamide Derivative in In Vitro and In Vivo Model of Neuroinflammation. Brain Res. Bull. 2015, 112, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Nignpense, B.E.; Chinkwo, K.A.; Blanchard, C.L.; Santhakumar, A.B. Polyphenols: Modulators of Platelet Function and Platelet Microparticle Generation? Int. J. Mol. Sci. 2019, 21, 146. [Google Scholar] [CrossRef] [PubMed]
- Ludovici, V.; Barthelmes, J.; Nagele, M.P.; Flammer, A.J.; Sudano, I. Polyphenols: Anti-Platelet Nutraceutical? Curr. Pharm. Des. 2018, 24, 146–157. [Google Scholar] [CrossRef]
- Nam, G.S.; Park, H.J.; Nam, K.S. The Antithrombotic Effect of Caffeic Acid Is Associated with A cAMP-Dependent Pathway and Clot Retraction in Human Platelets. Thromb. Res. 2020, 195, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. Caffedymine from Cocoa Has COX Inhibitory Activity Suppressing the Expression of a Platelet Activation Marker, P-Selectin. J. Agric. Food Chem. 2007, 55, 2171–2175. [Google Scholar] [CrossRef]
- Park, J.B.; Schoene, N. Clovamide-Type Phenylpropenoic Acid Amides, N-Coumaroyldopamine and N-Caffeoyldopamine, Inhibit Platelet-Leukocyte Interactions via Suppressing P-Selectin Expression. J. Pharmacol. Exp. Ther. 2006, 317, 813–819. [Google Scholar] [CrossRef]
- Park, J.B.; Schoene, N. Synthesis and Characterization of N-Coumaroyltyramine as a Potent Phytochemical Which Arrests Human Transformed Cells via Inhibiting Protein Tyrosine Kinases. Biochem. Biophys. Res. Commun. 2002, 292, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Wongsakul, A.; Lertnitikul, N.; Suttisri, R.; Jianmongkol, S. N-Trans-p-Coumaroyltyramine Enhances Indomethacin- and Diclofenac-induced Apoptosis Through Endoplasmic Reticulum Stress-dependent Mechanism in MCF-7 Cells. Anticancer Res. 2022, 42, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Peraza-Labrador, A.; Buitrago, D.M.; Coy-Barrera, E.; Perdomo-Lara, S.J. Antiproliferative and Pro-Apoptotic Effects of a Phenolic-Rich Extract from Lycium barbarum Fruits on Human Papillomavirus (HPV) 16-Positive Head Cancer Cell Lines. Molecules 2022, 27, 3568. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Caruso, F.; Rossi, M. Mechanistic Insights into the Inhibition of SARS-CoV-2 Main Protease by Clovamide and Its Derivatives: In Silico Studies. Biophysica 2021, 1, 28. [Google Scholar] [CrossRef]
- Niehues, M.; Stark, T.; Keller, D.; Hofmann, T.; Hensel, A. Antiadhesion as a Functional Concept for Prevention of Pathogens: N-phenylpropenoyl-L-amino Acid Amides as Inhibitors of the Helicobacter pylori BabA outer membrane protein. Mol. Nutr. Food Res. 2011, 55, 1104–1117. [Google Scholar] [CrossRef]
- Canivenc-Lavier, M.-C.; Bennetau-Pelissero, C. Phytoestrogens and Health Effects. Nutrients 2023, 15, 317. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-N.; Lin, C.-C.; Liu, C.-F. Efficacy of Phytoestrogens for Menopausal Symptoms: A Meta-Analysis and Systematic Review. Climacteric 2015, 18, 260–269. [Google Scholar] [CrossRef]
- Patra, S.; Gorai, S.; Pal, S.; Ghosh, K.; Pradhan, S.; Chakrabarti, S. A Review on Phytoestrogens: Current Status and Future Direction. Phytother. Res. 2023, 37, 3097–3120. [Google Scholar] [CrossRef]
- Powers, C.N.; Setzer, W.N. A Molecular Docking Study of Phytochemical Estrogen Mimics from Dietary Herbal Supplements. Silico Pharmacol. 2015, 3, 4. [Google Scholar] [CrossRef]
- Marinho, S.; Illanes, M.; Ávila-Román, J.; Motilva, V.; Talero, E. Anti-Inflammatory Effects of Rosmarinic Acid-Loaded Nanovesicles in Acute Colitis through Modulation of NLRP3 Inflammasome. Biomolecules 2021, 11, 162. [Google Scholar] [CrossRef]


| Type of Sample | Clovamide Content | References |
|---|---|---|
| mg/g of the product | ||
| Raw beans | 0.052 | [23] |
| Roasted beans | 0.044 | |
| Side products (winnowed) | 0.024 | |
| End products (winnowed) | 0.065 | |
| mg/g of cocoa powder | ||
| Unroasted beans, of Ghana origin | 0.0026 | [24] |
| Roasted beans, of Ghana origin | 0.0012 | |
| Unroasted beans, of Arriba origin | 0.0013 | |
| Roasted beans, of Arriba origin | 0.0005 | |
| Unroasted beans, of Ivory Coast origin | 0.0021 | |
| Roasted beans, of Ivory Coast origin | 0.0011 | |
| mg/g in defatted raw beans | ||
| 18 samples of cocoa beans, originating from 12 countries, 4 continents | 0.12–0.37 | [25] |
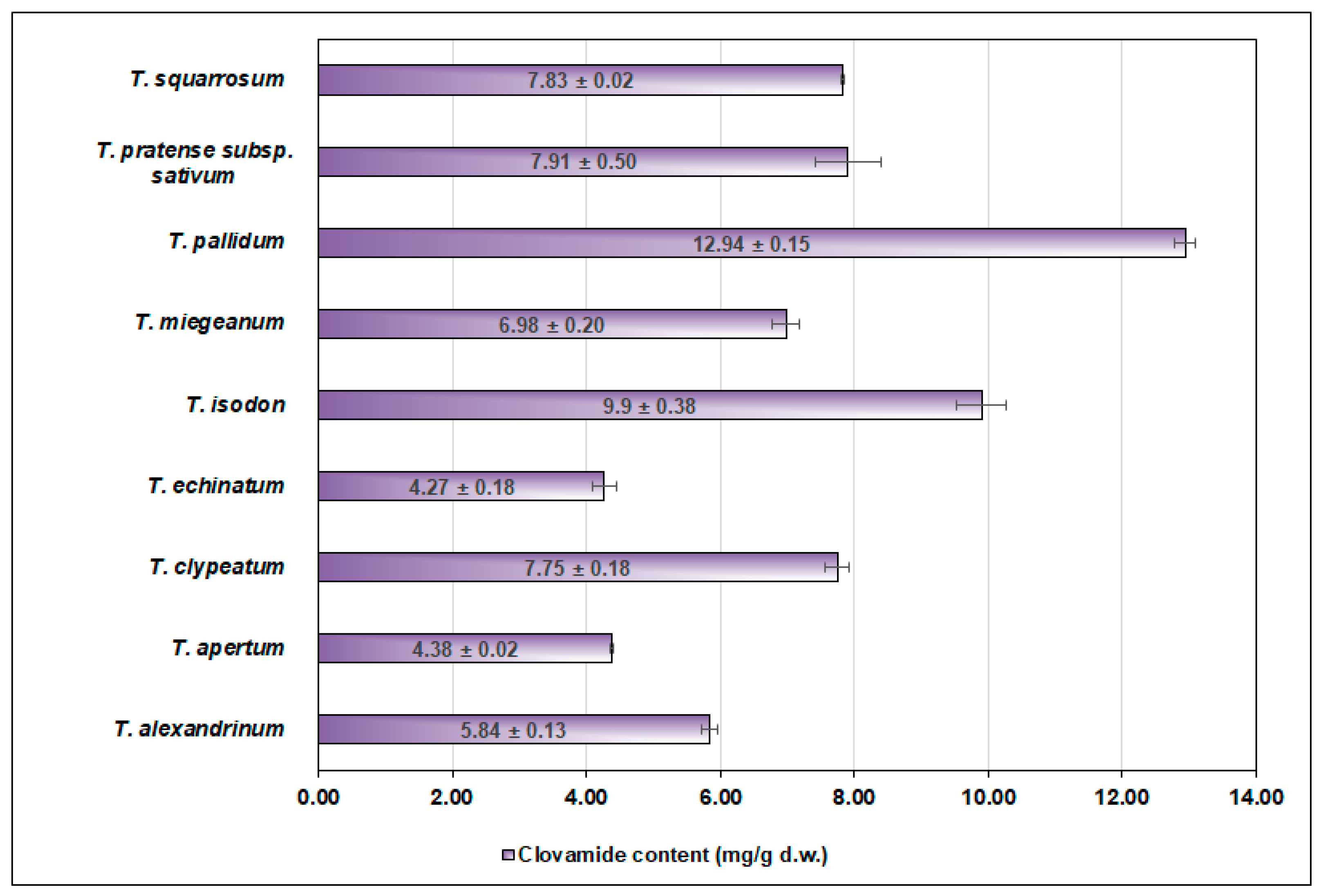
| Tifolium Species | Clovamide % in the Total Phenolics Content |
|---|---|
| T. alexandrinum L. | 21.54 |
| T. apertum Bobrov | 16.06 |
| T. clypeatum L. | 28.32 |
| T. echinatum M.Bieb. | 13.52 |
| T. isodon Murb. | 18.07 |
| T. miegeanum Maire | 14.20 |
| T. pallidum Waldst. & Kit | 36.90 |
| T. pratense subsp. sativum (Schreb.) Ser. | 19.61 |
| T. squarrosum L. | 15.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolodziejczyk-Czepas, J. Clovamide and Its Derivatives—Bioactive Components of Theobroma cacao and Other Plants in the Context of Human Health. Foods 2024, 13, 1118. https://doi.org/10.3390/foods13071118
Kolodziejczyk-Czepas J. Clovamide and Its Derivatives—Bioactive Components of Theobroma cacao and Other Plants in the Context of Human Health. Foods. 2024; 13(7):1118. https://doi.org/10.3390/foods13071118
Chicago/Turabian StyleKolodziejczyk-Czepas, Joanna. 2024. "Clovamide and Its Derivatives—Bioactive Components of Theobroma cacao and Other Plants in the Context of Human Health" Foods 13, no. 7: 1118. https://doi.org/10.3390/foods13071118
APA StyleKolodziejczyk-Czepas, J. (2024). Clovamide and Its Derivatives—Bioactive Components of Theobroma cacao and Other Plants in the Context of Human Health. Foods, 13(7), 1118. https://doi.org/10.3390/foods13071118





