Comparison of Body Characteristics, Carotenoid Composition, and Nutritional Quality of Chinese Mitten Crab (Eriocheir sinensis) with Different Hepatopancreas Redness
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Color Parameters Measurement
2.3. Hepatopancreas and Gonad Paraffin Section Analysis
2.4. Total Sugar, Lipid, and Protein Analysis
2.5. Carotenoids Compositions Analysis
2.6. Amino Acids and Fatty Acids Analysis
2.7. Statistical Analysis
3. Results
3.1. Differences in Body Characteristics, Hepatopancreas, and Gonad Paraffin Section in Crabs with Different Hepatopancreas Redness
3.2. Differences in Carotenoids and Biochemical Compositions in Hepatopancreas and Gonad of Crabs with Different Hepatopancreas Redness
3.3. Differences in the Compositions of Amino and Fatty Acids in Crabs with Different Hepatopancreas Redness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Czerniejewski, P.; Bienkiewicz, G.; Tokarczyk, G. Nutritional quality and fatty acids composition of invasive Chinese mitten crab from Odra Estuary (Baltic Basin). Foods 2023, 12, 3088. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022; Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Bureau of Fisheries and Fishery Management, Ministry of Agriculture and Rural Affairs of China. 2022 China Fisheries Statistical Yearbook; Chinese Agricultural Press: Beijing, China, 2023; p. 34.
- Chen, D.W.; Zhang, M. Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis). Food Chem. 2007, 104, 1200–1205. [Google Scholar] [CrossRef]
- Chen, D.W.; Zhang, M.; Shrestha, S. Compositional characteristics and nutritional quality of Chinese mitten crab (Eriocheir sinensis). Food Chem. 2007, 103, 1343–1349. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, R.Y.; Jiang, X.D.; Wu, X.G.; Wang, X.C. Dietary supplementation with synthetic astaxanthin and DHA interactively regulates physiological metabolism to improve the color and odor quality of ovaries in adult female Eriocheir sinensis. Food Chem. 2023, 430, 137020. [Google Scholar] [CrossRef] [PubMed]
- Tume, R.K.; Sikes, A.L.; Tabrett, S.; Smith, D.M. Effect of background colour on the distribution of astaxanthin in black tiger prawn (Penaeus monodon): Effective method for improvement of cooked colour. Aquaculture 2009, 296, 129–135. [Google Scholar] [CrossRef]
- Li, Q.Q.; Zu, L.; Cheng, Y.X.; Wade, N.M.; Liu, J.G.; Wu, X.G. Carapace color affects carotenoid composition and nutritional quality of the Chinese mitten crab, Eriocheir sinensis. LWT-Food Sci. Technol. 2020, 126, 109286. [Google Scholar] [CrossRef]
- Jie, H.; Xuan, F.; Shi, H.; Xie, J.; Wei, W.; Gengshen, W.; Wenjun, X. Comparison of nutritional quality of three edible tissues of the wild-caught and pond-reared swimming crab (Portunus trituberculatus) females. LWT-Food Sci. Technol. 2017, 75, 624–630. [Google Scholar] [CrossRef]
- Wade, N.M.; Gabaudan, J.; Glencross, B.D. A review of carotenoid utilisation and function in crustacean aquaculture. Rev. Aquacult. 2017, 9, 141–156. [Google Scholar] [CrossRef]
- Zhang, D.D.; Fan, C.W.; Jiang, X.D.; Cheng, Y.X.; Wu, X.G. Comparison of edible yield, carotenoid composition and nutritional quality between the white carapace strain and green carapace strain of adult Chinese mitten crab, Eriocheir sinensis. J. Fish. China 2022, 47, 099610. [Google Scholar] [CrossRef]
- Wang, S.; Guo, K.; Luo, L.; Zhang, R.; Xu, W.; Song, Y.; Zhao, Z. Fattening in saline and alkaline water improves the color, nutritional and taste quality of adult Chinese mitten crab Eriocheir sinensis. Foods 2022, 11, 2573. [Google Scholar] [CrossRef]
- Ding, Z.; Meng, Q.; Liu, H.; Yuan, S.; Zhang, F.; Sun, M.; Zhao, G.; Pan, J.; Xue, H.; Wang, W. First case of hepatopancreatic necrosis disease in pondreared Chinese mitten crab, Eriocheir sinensis, associated with microsporidian. J. Fish. Dis. 2016, 39, 1043–1051. [Google Scholar] [CrossRef]
- Wang, Q.J.; Jiang, X.D.; Yao, Q.; Long, X.W.; Xu, Y.P.; Wu, M.; Zhang, D.M. Comparative study on the nutrition composition of adult male Chinese mitten crab (Eriocheir sinensis) with different coloured hepatopancreases. Aquac. Res. 2021, 52, 196–207. [Google Scholar] [CrossRef]
- Wang, Q.J.; Zhang, B.Y.; Jiang, X.D.; Long, X.W.; Zhu, W.L.; Xu, Y.P.; Wu, M.; Zhang, D.M. Comparison on nutritional quality of adult female Chinese mitten crab (Eriocheir sinensis) with different colored hepatopancreases. J. Food Sci. 2021, 86, 2075–2090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tao, N.P.; Wu, X.G.; Wang, X.C. Metabolomics of the hepatopancreas in Chinese mitten crabs (Eriocheir sinensis). Food Res. Int. 2022, 152, 110914. [Google Scholar] [CrossRef] [PubMed]
- GB/T 9695.31-2008; Determination of Total Sugar Content in Meat Products. General Administration of Quality Supervision, Inspection and Quarantine of the People‘s Republic of China: Beijing, China, 2008.
- Qin, Y.P.; Li, R.; Liao, Q.L.; Shi, G.P.Y.; Zhou, Y.Y.; Wan, W.T.; Li, J.; Ma, H.T.; Zhang, Y.H.; Yu, Z.N. Comparison of biochemical composition, nutritional quality, and metals concentrations between males and females of three different Crassostrea sp. Food Chem. 2022, 398, 133868. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.5-2016; National Food Safety Standard Determination of Protein in Food. China Food and Drug Administration: Beijing, China; National Health and Family Planning Commission: Beijing, China, 2016.
- Mozaffarian, D.; Wu, J.Y. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, M.Y.; Zheng, Y.; Xu, C.H.; Tao, N.P.; Wu, X.G.; Wang, X.C. Brackish water improves the taste quality in meat of adult male Eriocheir sinensis during the postharvest temporary rearing. Food Chem. 2021, 343, 128409. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.F.; Wu, H.R.; Liang, F.; Ge, M.T.; Jiang, S.T.; Lin, L.; Lu, J.F. Comparison of the nutritional quality of three edible tissues from precocious and normal adult female Chinese mitten crabs (Eriocheir sinensis). J. Aquat. Food Prod. Technol. 2020, 30, 49–61. [Google Scholar] [CrossRef]
- Li, Q. Estimates of Genetic Parameters for Colourtraits and Colour Formation Mechanism in Eriocheir sinensis. Ph.D. Thesis, Shanghai Ocean University, Shanghai, China, 2019. [Google Scholar] [CrossRef]
- Parisenti, J.; Beirao, L.H.; Tramonte, V.L.C.G.; Ourique, F.; Brito, C.C.D.; Moreira, C.C. Preference ranking of colour in raw and cooked shrimps. Int. J. Food Sci. Tech. 2011, 46, 2558–2561. [Google Scholar] [CrossRef]
- Long, X.W.; Wu, X.G.; Zhao, L.; Liu, J.G.; Cheng, Y.X. Effects of dietary supplementation with Haematococcus pluvialis cell powder on coloration, ovarian development and antioxidation capacity of adult female Chinese mitten crab, Eriocheir sinensis. Aquaculture 2017, 473, 545–553. [Google Scholar] [CrossRef]
- Wade, N.; Goulter, K.C.; Wilson, K.J.; Hall, M.R.; Degnan, B.M. Esterified astaxanthin levels in lobster epithelia correlate with shell colour intensity: Potential role in crustacean shell colour formation. Comp. Biochem. Phys. B 2005, 141, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, X.G.; Liu, Z.J.; Zheng, H.J.; Cheng, Y.X. Insights into hepatopancreatic functions for nutrition metabolism and ovarian development in the crab Portunus trituberculatus: Gene discovery in the comparative transcriptome of different hepatopancreas stages. PLoS ONE 2014, 9, e84921. [Google Scholar] [CrossRef] [PubMed]
- Harhoğlu, M.M.; Farhadi, A. Factors affecting the reproductive efficiency in crayfish: Implications for aquaculture. Aquac. Res. 2017, 48, 1983–1997. [Google Scholar] [CrossRef]
- Kong, L.; Cai, C.; Ye, Y.; Chen, D.; Wu, P.; Li, E.; Chen, L.; Song, L. Comparison of non-volatile compounds and sensory characteristics of Chinese mitten crabs (Eriocheir sinensis) reared in lakes and ponds: Potential environmental factors. Aquaculture 2012, 364, 96–102. [Google Scholar] [CrossRef]
- Wang, S.; Long, X.; Zu, L.; Wu, X.; Cheng, Y. Gonadal development and nutritional composition of adult Eriocheir sinensis from Xishuangbanna. J. Shanghai Ocean Univ. 2019, 28, 483–490. [Google Scholar] [CrossRef]
- Cheesman, B.; Lee, W.L.; Zagalsky, P.F. Carotenoproteins in invertebrates. Biol. Rev. 1967, 42, 132–160. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Liu, P.; Chen, J.; Zhang, C. Uncovering the nutritive profiles of adult male chinese mitten crab (E. sinensis) harvested from the pond and natural water area of Qin lake based on metabolomics. Foods 2023, 12, 2178. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Li, J.; Liu, P.; Li, J. Transcriptomics revealed the effect of astaxanthin on apoptosis and immunity of the adult prawn of Exopalaemon carinicauda. Fish Shellfish Immun. 2022, 131, 480–486. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, Y.C.; Huai, M.Y.; Li, L.; Man, C.; Pelletier, W.; Wei, H.L.; Yao, R.; Niu, J. Comparison of the retention rates of synthetic and natural astaxanthin in feeds and their effects on pigmentation, growth, and health in rainbow trout (Oncorhynchus mykiss). Antioxidants 2022, 11, 2473. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wang, S.; Sun, B.; Xiong, L.; Cheng, Y. Composition and nutritional qualities of edible tissues of Chinese mitten crab (Eriocheir sinensis) from Ya Lake over different months. J. Food Compos. Anal. 2021, 105, 104199. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, Y.; Wu, M.; Li, X.; Mai, K.; Ai, Q. ω-6 Polyunsaturated fatty acids (linoleic acid) activate both autophagy and antioxidation in a synergistic feedback loop via TOR-dependent and TOR-independent signaling pathways. Cell Death Dis. 2020, 11, 607. [Google Scholar] [CrossRef]
- Wan, X.H.; Fu, X.; Ababaikeli, G. Docosahexaenoic acid induces growth suppression on epithelial ovarian cancer cells more effectively than Eicosapentaenoic acid. Nutr. Cancer 2016, 68, 320–327. [Google Scholar] [CrossRef]
- Sachindra, N.; Bhaskar, N.; Mahendrakar, N.S. Carotenoids in crabs from marine and fresh waters of India. LWT-Food Sci. Technol. 2005, 38, 221–225. [Google Scholar] [CrossRef]
- Han, T.; Li, X.; Wang, J.; Wang, C.; Yang, M.; Zheng, P. Effects of dietary astaxanthin (AX) supplementation on pigmentation, antioxidant capacity and nutritional value of swimming crab, Portunus trituberculatus. Aquaculture 2018, 490, 169–177. [Google Scholar] [CrossRef]
- Paibulkichakul, C.; Piyatiratitivorakul, S.; Sorgeloos, P.; Menasveta, P. Improved maturation of pond-reared, black tiger shrimp (Penaeus monodon) using fish oil and astaxanthin feed supplements. Aquaculture 2008, 282, 83–89. [Google Scholar] [CrossRef]
- Wang, W.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Hossain, M.S.; Moss, A.S. Effects of dietary astaxanthin supplementation on juvenile kuruma shrimp, Marsupenaeus japonicus. Aquaculture 2018, 491, 197–204. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, S.; Dai, J.; Wang, C.; Chen, S.; Qian, Y.; Gong, Y.; Han, T. Effects of synthetic astaxanthin on the growth performance, pigmentation, antioxidant capacity, and immune response in black tiger prawn (Penaeus monodon). Aquac. Nutr. 2023, 2023, 6632067. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Wang, L.; Li, Y.; Sun, W.; Wu, X. Effects of long-term Haematococcus pluvialis astaxanthin feeding on the growth, coloration, and antioxidant capacity of commercial-sized Oncorhynchus mykiss. Aquac. Rep. 2023, 30, 101603. [Google Scholar] [CrossRef]
- Tejera, N.; Cejas, J.R.; Rodríguez, C.; Bjerkeng, B.; Jérez, S.; Bolaños, A.; Lorenzo, A. Pigmentation, carotenoids, lipid peroxides and lipid composition of skin of red porgy (Pagrus pagrus) fed diets supplemented with different astaxanthin sources. Aquaculture 2007, 270, 218–230. [Google Scholar] [CrossRef]
- Wang, M.; Tang, Y.; Yu, J.; Su, S.; Li, J.; Yu, F.; Li, H.; Song, C.; Du, F.; Xu, P. Molecular insights into the sex-differential regulation of signal transduction in the cerebral ganglion and metabolism in the hepatopancreas of Eriocheir sinensis during reproduction. Genomics 2020, 112, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zu, L.; Li, Q.; Jiang, X.; Xu, W.; Soyano, K.; Cheng, Y.; Wu, X. A comparative evaluation of the nutritional quality of Eriocheir sinensis and Eriocheir japonica (Brachyura, Varunidae). Crustaceana 2020, 93, 567–585. [Google Scholar] [CrossRef]
- Celik, M.; Türeli, C.; Çelik, M.; Yanar, Y.; Erdem, Ü.; Küçükgülmez, A. Fatty acid composition of the blue crab (Callinectes sapidus Rathbun, 1896) in the north eastern Mediterranean. Food Chem. 2004, 88, 271–273. [Google Scholar] [CrossRef]
- Jiang, X.; Xie, Z.; Wade, N.M.; Truong, H.H.; Yang, Y.; Wu, X. Using response surfaces to explore the interactive effect of dietary astaxanthin and β-carotene on growth and antioxidant capability of juvenile Chinese mitten crab, Eriocheir sinensis. Aquaculture 2022, 555, 738196. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Cheng, Y.; Zeng, C.; Yang, X.; Lu, J. Effects of dietary phospholipids and highly unsaturated fatty acids on the precocity, survival, growth and hepatic lipid composition of juvenile Chinese mitten crab, Eriocheir sinensis (H. ilne-Edwards). Aquac. Res. 2011, 42, 457–468. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, L.; Long, X.; Liu, J.; Su, F.; Cheng, Y. Effects of dietary supplementation of Haematococcus pluvialis powder on gonadal development, coloration and antioxidant capacity of adult male Chinese mitten crab (Eriocheir sinensis). Aquacult. Res. 2017, 48, 5214–5223. [Google Scholar] [CrossRef]


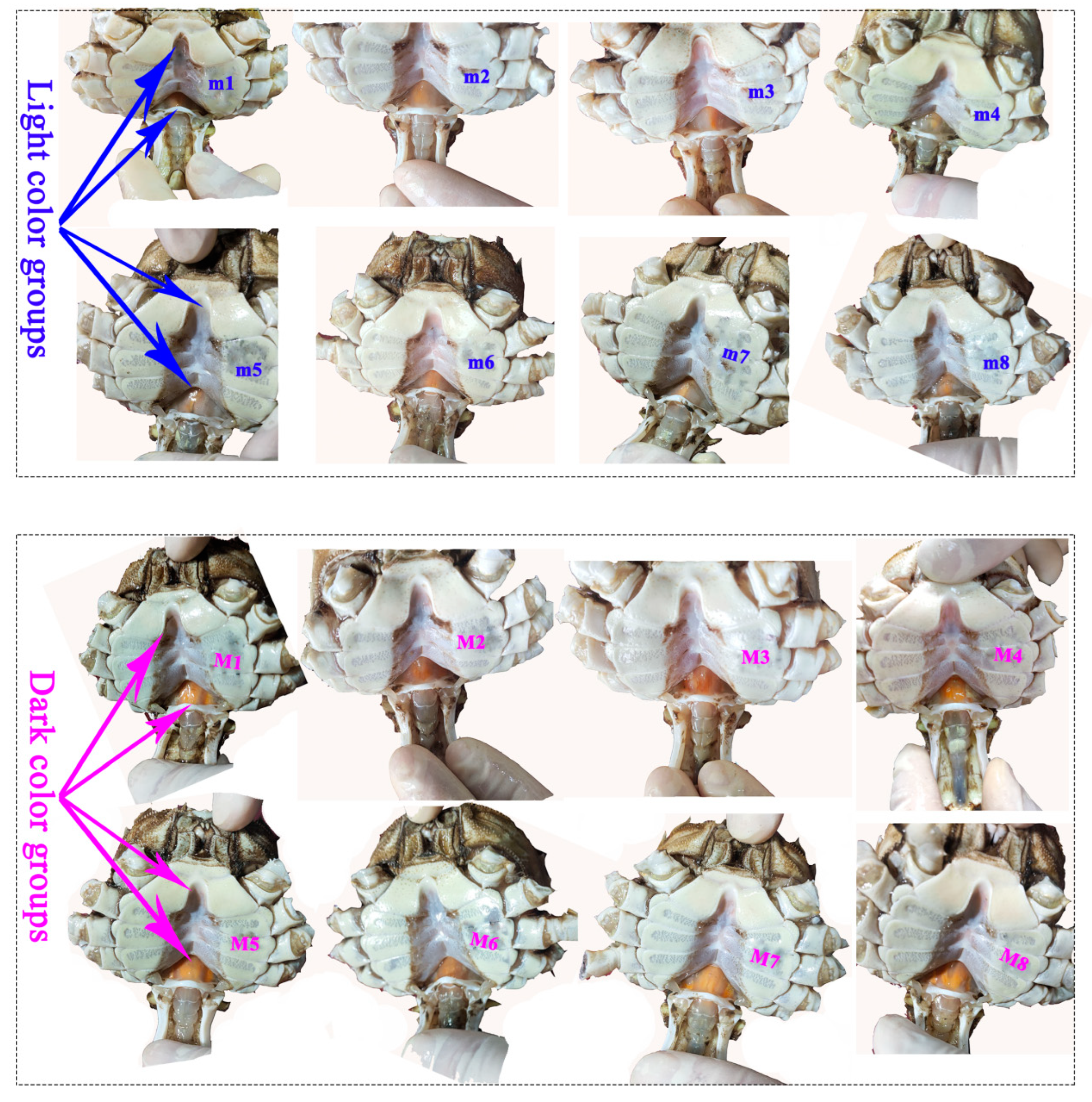

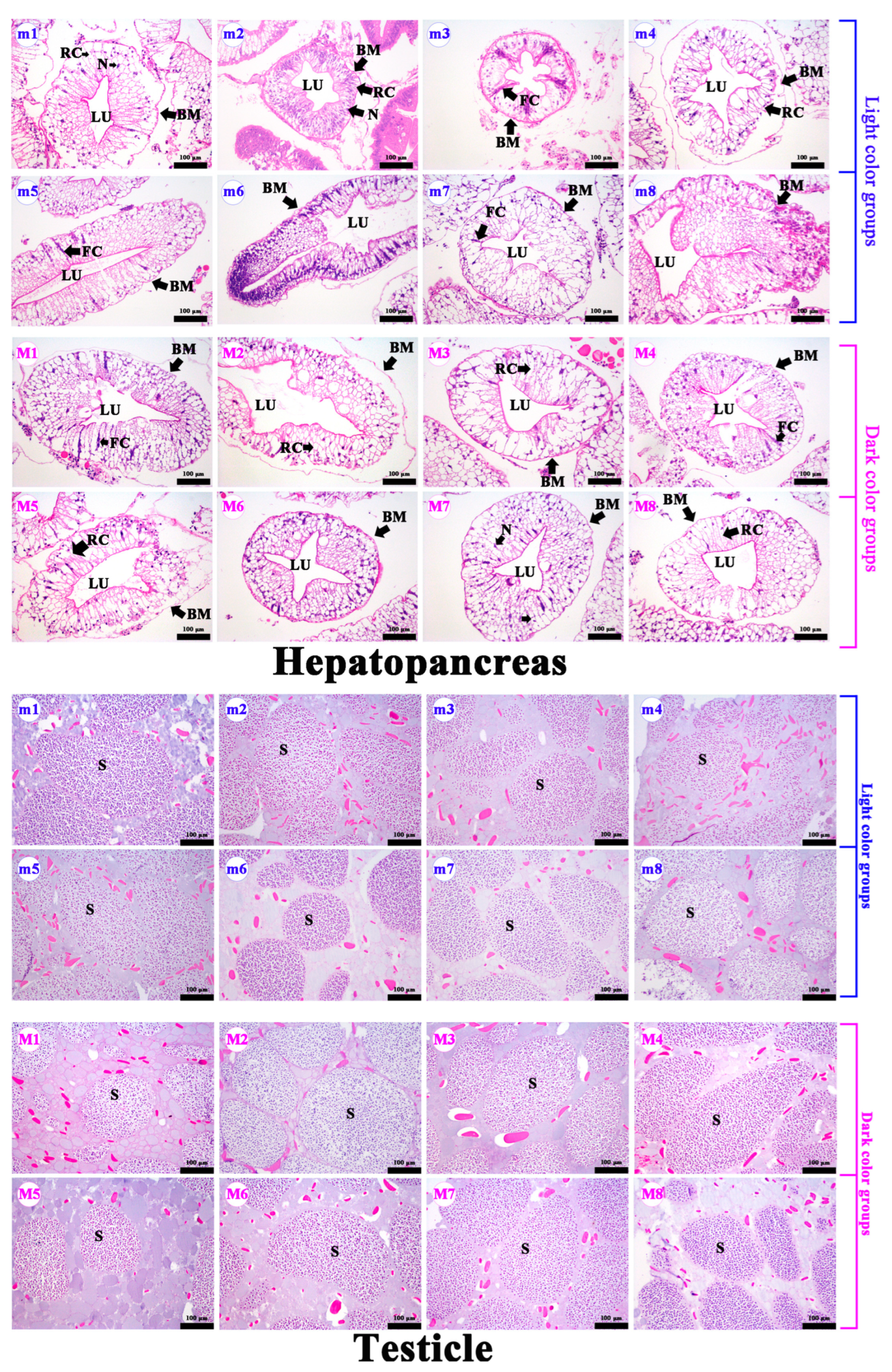

| Index | Females | Males | |||||
|---|---|---|---|---|---|---|---|
| Light Color Group | Dark Color Group | p | Light Color Group | Dark Color Group | p | ||
| Cuirass | L* | 50.06 ± 1.63 | 47.87 ± 1.19 | 0.350 | 49.37 ± 2.30 | 49.86 ± 2.41 | 0.681 |
| a* | 5.30 ± 0.34 | 7.20 ± 0.66 | 0.039 | 3.14 ± 2.04 | 3.87 ± 1.97 | 0.481 | |
| b* | 5.60 ± 0.73 | 4.62 ± 0.98 | 0.485 | 5.70 ± 2.78 | 6.06 ± 2.51 | 0.791 | |
| Abdomen | L* | 29.78 ± 0.88 | 27.88 ± 0.84 | 0.185 | 35.60 ± 2.57 | 32.03 ± 5.64 | 0.359 |
| a* | 5.89 ± 0.78 | 9.81 ± 0.85 | 0.009 | 3.11 ± 2.05 | 6.09 ± 3.25 | 0.037 | |
| b* | 12.41 ± 1.24 | 13.07 ± 1.30 | 0.747 | 6.70 ± 3.08 | 10.95 ± 6.22 | 0.090 | |
| Hepatopancreas | L* | 56.81 ± 1.00 | 55.84 ± 0.74 | 0.496 | 57.52 ± 4.10 | 59.15 ± 3.07 | 0.639 |
| a* | 21.36 ± 0.94 | 29.55 ± 0.40 | 0.000 | 21.83 ± 1.03 | 26.5 ± 1.18 | 0.001 | |
| b* | 55.72 ± 0.83 | 60.35 ± 1.12 | 0.010 | 54.03 ± 4.75 | 58.49 ± 3.37 | 0.222 | |
| Weight (g) | 8.08 ± 0.76 | 8.88 ± 0.87 | 0.547 | 12.36 ± 0.93 | 15.68 ± 2.71 | 0.104 | |
| Hepatopancreas–Somatic Index (HIS/%) | 7.13 ± 0.35 | 6.78 ± 0.41 | 0.574 | 7.38 ± 0.43 | 8.69 ± 0.38 | 0.028 | |
| Gonad | L* | 22.95 ± 0.51 | 23.68 ± 0.61 | 0.421 | 69.89 ± 11.56 | 70.01 ± 7.58 | 0.831 |
| a* | 3.96 ± 0.17 | 4.79 ± 0.29 | 0.041 | −2.00 ± 0.41 | −1.67 ± 0.90 | 0.682 | |
| b* | −0.69 ± 0.43 | 0.60 ± 0.88 | 0.266 | −1.00 ± 0.61 | −0.93 ± 0.24 | 0.203 | |
| Weight (g) | 12.23 ± 0.87 | 14.09 ± 1.37 | 0.323 | 5.56 ± 0.69 | 5.98 ± 0.92 | 0.908 | |
| Gonad–Somatic Index (GIS/%) | 10.84 ± 0.28 | 10.82 ± 0.38 | 0.971 | 3.33 ± 0.17 | 3.29 ± 0.12 | 0.884 | |
| Body characteristics | Weight (g) | 112.73 ± 7.71 | 130.82 ± 12.29 | 0.284 | 173.14 ± 19.63 | 182.23 ± 18.22 | 0.928 |
| Height (mm) | 32.37 ± 0.87 | 33.88 ± 1.20 | 0.379 | 35.39 ± 0.41 | 36.21 ± 0.49 | 0.509 | |
| Carapace width (mm) | 62.89 ± 1.39 | 65.39 ± 2.07 | 0.385 | 68.98 ± 0.81 | 70.41 ± 0.83 | 0.610 | |
| Carapace length (mm) | 57.04 ± 1.36 | 59.32 ± 1.81 | 0.385 | 62.44 ± 0.72 | 62.63 ± 0.98 | 0.686 | |
| Frontal width (mm) | 13.97 ± 0.40 | 14.34 ± 0.24 | 0.488 | 15.03 ± 0.20 | 15.21 ± 0.33 | 0.483 | |
| First orbital margin width (mm) | 36.21 ± 0.74 | 37.14 ± 0.86 | 0.479 | 39.21 ± 0.39 | 39.59 ± 0.65 | 0.708 | |
| Postcarapace length (mm) | 30.02 ± 0.74 | 31.58 ± 1.10 | 0.309 | 33.67 ± 0.47 | 34.12 ± 0.61 | 0.352 | |
| Meropodite length of the third pereopod (mm) | 39.77 ± 1.00 | 41.84 ± 1.47 | 0.315 | 47.75 ± 0.55 | 49.91 ± 0.73 | 0.089 | |
| Propodite length of the third pereopod (mm) | 27.87 ± 0.83 | 30.25 ± 1.15 | 0.156 | 33.55 ± 0.21 | 34.44 ± 0.50 | 0.197 | |
| Dactylopodite length of the fourth pereopod (mm) | 21.20 ± 0.51 | 21.93 ± 0.85 | 0.520 | 24.39 ± 0.50 | 24.42 ± 0.71 | 0.361 | |
| Females | Males | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatopancreas | Ovary | Muscle | Hepatopancreas | Testicle | Muscle | |||||||||||||
| Light Color Groups | Dark Color Groups | p | Light Color Groups | Dark Color Groups | p | Light Color Groups | Dark Color Groups | p | Light Color Groups | Dark Color Groups | p | Light Color Groups | Dark Color Groups | p | Light Color Groups | Dark Color Groups | p | |
| Aspartic acid (Asp) + | 0.56 ± 0.39 | 0.51 ± 0.33 | 0.780 | 0.91 ± 0.09 | 0.62 ± 0.23 | 0.008 | 0.09 ± 0.07 | 0.08 ± 0.03 | 0.832 | 0.91 ± 0.35 | 0.77 ± 0.47 | 0.514 | 1.69 ± 1.06 | 2.30 ± 1.55 | 0.372 | 0.08 ± 0.02 | 0.10 ± 0.02 | 0.087 |
| Threonine (Thr) #,* | 1.49 ± 0.25 | 1.45 ± 0.21 | 0.750 | 1.06 ± 0.13 | 0.81 ± 0.21 | 0.012 | 0.40 ± 0.25 | 0.32 ± 0.09 | 0.482 | 1.71 ± 0.32 | 1.59 ± 0.32 | 0.448 | 0.11 ± 0.04 | 0.17 ± 0.08 | 0.069 | 0.40 ± 0.16 | 0.40 ± 0.11 | 0.967 |
| Serine (Ser) * | 1.01 ± 0.28 | 0.99 ± 0.15 | 0.888 | 1.26 ± 0.14 | 1.05 ± 0.21 | 0.032 | 0.46 ± 0.26 | 0.49 ± 0.16 | 0.866 | 1.23 ± 0.28 | 1.08 ± 0.31 | 0.350 | 0.19 ± 0.08 | 0.30 ± 0.14 | 0.102 | 0.45 ± 09.26 | 0.52 ± 0.07 | 0.537 |
| Glutamic acid (Glu) + | 2.33 ± 0.47 | 2.26 ± 0.33 | 0.762 | 2.19 ± 0.29 | 1.71 ± 0.34 | 0.010 | 1.03 ± 0.73 | 0.56 ± 0.12 | 0.178 | 2.69 ± 0.52 | 2.40 ± 0.56 | 0.302 | 1.44 ± 0.64 | 1.85 ± 1.06 | 0.373 | 0.54 ± 0.30 | 0.82 ± 0.19 | 0.093 |
| Glycine (Gly) * | 1.09 ± 0.28 | 1.08 ± 0.18 | 0.926 | 0.63 ± 0.06 | 0.52 ± 0.08 | 0.006 | 3.28 ± 1.02 | 3.88 ± 1.35 | 0.404 | 1.19 ± 0.27 | 1.17 ± 0.27 | 0.869 | 0.45 ± 0.15 | 0.45 ± 0.13 | 1.000 | 4.28 ± 0.84 | 5.42 ± 1.30 | 0.103 |
| Cystine (Cys) | 0.25 ± 0.08 | 0.27 ± 0.05 | 0.545 | 0.04 ± 0.02 | 0.03 ± 0.02 | 0.297 | 0.04 ± 0.02 | 0.03 ± 0.01 | 0.364 | 0.36 ± 0.07 | 0.34 ± 0.07 | 0.659 | 0.05 ± 0.03 | 0.09 ± 0.08 | 0.239 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.380 |
| Valine (Val) # | 1.61 ± 0.32 | 1.54 ± 0.25 | 0.612 | 1.33 ± 0.15 | 0.98 ± 0.34 | 0.020 | 0.30 ± 0.20 | 0.23 ± 0.08 | 0.442 | 1.91 ± 0.38 | 1.78 ± 0.34 | 0.459 | 0.13 ± 0.06 | 0.18 ± 0.09 | 0.148 | 0.26 ± 0.11 | 0.30 ± 0.08 | 0.458 |
| Methionine (Met) # | 0.67 ± 0.19 | 0.59 ± 0.13 | 0.331 | 1.13 ± 0.29 | 0.86 ± 0.26 | 0.069 | 0.24 ± 0.13 | 0.15 ± 0.05 | 0.140 | 0.77 ± 0.20 | 0.69 ± 0.17 | 0.404 | <0.0075 | <0.0075 | - | 0.13 ± 0.06 | 0.19 ± 0.06 | 0.106 |
| Isoleucine (Ile) # | 0.85 ± 0.38 | 0.82 ± 0.35 | 0.909 | 0.65 ± 0.25 | 0.47 ± 0.23 | 0.169 | 0.09 ± 0.06 | 0.07 ± 0.03 | 0.703 | 1.24 ± 0.38 | 1.00 ± 0.31 | 0.198 | 0.13 ± 0.05 | 0.17 ± 0.10 | 0.392 | 0.10 ± 0.04 | 0.12 ± 0.05 | 0.467 |
| Leucine (Leu) # | 1.64 ± 0.84 | 1.47 ± 0.65 | 0.661 | 2.56 ± 1.08 | 2.04 ± 0.95 | 0.320 | 0.22 ± 0.14 | 0.20 ± 0.09 | 0.762 | 2.33 ± 0.79 | 1.91 ± 0.65 | 0.269 | 0.15 ± 0.06 | 0.21 ± 0.14 | 0.242 | 0.18 ± 0.08 | 0.32 ± 0.13 | 0.040 |
| Tyrosine (Tyr) | 0.67 ± 0.54 | 0.45 ± 0.24 | 0.304 | 1.23 ± 0.80 | 1.12 ± 0.71 | 0.781 | 0.22 ± 0.08 | 0.11 ± 0.00 | 0.304 | 1.07 ± 0.52 | 0.74 ± 0.36 | 0.158 | <0.0095 | <0.0095 | - | <0.0095 | 0.19 ± 0.09 | 0.921 |
| Phenylalanine (Phe) #,* | 0.23 ± 0.31 | 0.17 ± 0.15 | 0.642 | 0.34 ± 0.35 | 0.54 ± 0.61 | 0.439 | 0.13 ± 0.05 | 0.09 ± 0.00 | 0.722 | 0.22 ± 0.16 | 0.23 ± 0.15 | 0.929 | <0.0083 | <0.0083 | - | <0.0083 | 0.19 ± 0.07 | - |
| Lysine (Lys) # | 2.29 ± 0.44 | 2.11 ± 0.37 | 0.400 | 1.24 ± 0.32 | 1.00 ± 0.26 | 0.119 | 0.60 ± 0.34 | 0.33 ± 0.11 | 0.119 | 2.63 ± 0.55 | 2.33 ± 0.47 | 0.261 | 0.23 ± 0.10 | 0.31 ± 0.16 | 0.239 | 0.47 ± 0.22 | 0.37 ± 0.07 | 0.333 |
| Histidine (His) # | 0.70 ± 0.13 | 0.67 ± 0.11 | 0.566 | 0.60 ± 0.09 | 0.51 ± 0.11 | 0.088 | 0.28 ± 0.14 | 0.12 ± 0.05 | 0.111 | 0.84 ± 0.17 | 0.77 ± 0.16 | 0.402 | 0.09 ± 0.03 | 0.15 ± 0.06 | 0.044 | 0.19 ± 0.07 | 0.19 ± 0.04 | 1.000 |
| Arginine (Arg) | 2.41 ± 0.72 | 2.39 ± 0.69 | 0.945 | 3.04 ± 0.57 | 2.86 ± 0.42 | 0.495 | 3.42 ± 1.48 | 4.98 ± 1.49 | 0.099 | 3.10 ± 0.89 | 3.01 ± 0.79 | 0.839 | 0.36 ± 0.20 | 0.53 ± 0.10 | 0.053 | 3.83 ± 1.47 | 5.37 ± 0.58 | 0.039 |
| Proline (Pro) | 2.13 ± 0.46 | 2.05 ± 0.37 | 0.722 | 2.18 ± 0.32 | 1.68 ± 0.31 | 0.006 | 3.08 ± 1.44 | 2.90 ± 1.12 | 0.821 | 2.70 ± 0.61 | 2.78 ± 0.68 | 0.819 | 0.54 ± 0.31 | 0.62 ± 0.19 | 0.574 | 1.83 ± 0.65 | 2.33 ± 0.25 | 0.111 |
| TAAs | 22.54 ± 4.91 | 21.60 ± 3.74 | 0.674 | 22.20 ± 3.79 | 18.44 ± 4.41 | 0.089 | 17.48 ± 4.60 | 18.77 ± 5.31 | 0.664 | 27.70 ± 6.03 | 25.51 ± 5.48 | 0.460 | 7.19 ± 2.86 | 9.56 ± 4.59 | 0.234 | 18.25 ± 3.27 | 21.50 ± 3.00 | 0.103 |
| EAAs | 8.74 ± 2.35 | 8.13 ± 1.88 | 0.572 | 8.30 ± 2.29 | 6.69 ± 2.35 | 0.188 | 1.84 ± 1.04 | 1.31 ± 0.47 | 0.287 | 10.81 ± 2.67 | 9.52 ± 2.23 | 0.313 | 0.74 ± 0.28 | 1.06 ± 0.54 | 0.171 | 1.54 ± 0.62 | 1.80 ± 0.56 | 0.457 |
| TUAAs | 2.89 ± 0.80 | 2.78 ± 0.49 | 0.738 | 3.10 ± 0.36 | 2.34 ± 0.56 | 0.005 | 1.11 ± 0.78 | 0.63 ± 0.15 | 0.198 | 3.60 ± 0.70 | 3.17 ± 0.92 | 0.313 | 3.13 ± 1.68 | 4.15 ± 2.59 | 0.369 | 0.62 ± 0.31 | 0.91 ± 0.20 | 0.048 |
| TSAAs | 8.47 ± 1.29 | 8.38 ± 1.06 | 0.881 | 6.99 ± 0.76 | 5.65 ± 0.86 | 0.005 | 11.08 ± 3.42 | 11.89 ± 3.41 | 0.694 | 9.60 ± 1.85 | 9.54 ± 2.02 | 0.947 | 2.94 ± 1.06 | 3.77 ± 1.45 | 0.216 | 11.84 ± 2.55 | 13.45 ± 1.81 | 0.236 |
| Females | Males | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatopancreas | Ovary | Muscle | Hepatopancreas | Testicle | Muscle | |||||||||||||
| Light Color Group | Dark Color Group | p | Light Color Group | Dark Color Group | p | Light Color Group | Dark Color Group | p | Light Color Group | Dark Color Group | p | Light Color Group | Dark Color Group | p | Light Color Group | Dark Color Group | p | |
| Saturated fatty acids (SFAs, g/kg tissue weight) | ||||||||||||||||||
| C6:0 | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - | 0.04 ± 0.06 | 0.12 ± 0.00 | 0.449 | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - |
| C10:0 | 0.13 ± 0.05 | 0.14 ± 0.06 | 1.000 | <0.0066 | <0.0066 | - | <0.0066 | <0.0066 | - | 0.12 ± 0.06 | 0.15 ± 0.08 | 0.451 | <0.0066 | <0.0066 | - | <0.0066 | <0.0066 | - |
| C12:0 | 11.04 ± 4.73 | 10.43 ± 5.18 | 0.807 | 0.43 ± 0.15 | 0.43 ± 0.15 | 0.740 | <0.0066 | <0.0066 | - | 10.56 ± 2.54 | 14.07 ± 5.85 | 0.143 | <0.0066 | 0.10 ± 0.00 | - | <0.0033 | <0.0033 | - |
| C13:0 | 0.14 ± 0.02 | 0.14 ± 0.03 | 0.865 | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - | 0.12 ± 0.02 | 0.14 ± 0.03 | 0.138 | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - |
| C14:0 | 6.42 ± 1.25 | 5.60 ± 1.44 | 0.245 | 0.92 ± 0.18 | 0.84 ± 0.17 | 0.378 | <0.0033 | <0.0033 | - | 5.39 ± 0.56 | 6.73 ± 1.84 | 0.083 | <0.0033 | 0.05 ± 0.00 | - | <0.0033 | <0.0033 | - |
| C15:0 | 0.92 ± 0.23 | 0.89 ± 0.18 | 0.795 | 0.16 ± 0.03 | 0.19 ± 0.04 | 0.103 | <0.0033 | <0.0033 | - | 0.69 ± 0.12 | 0.88 ± 0.13 | 0.010 | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - |
| C16:0 | 62.21 ± 9.77 | 56.19 ± 7.11 | 0.181 | 11.52 ± 1.61 | 12.01 ± 1.58 | 0.547 | 0.48 ± 0.08 | 0.53 ± 0.08 | 0.342 | 46.90 ± 6.86 | 57.27 ± 9.07 | 0.022 | 0.31 ± 0.12 | 0.34 ± 0.10 | 0.541 | 0.43 ± 0.07 | 0.47 ± 0.08 | 0.340 |
| C17:0 | 0.68 ± 0.16 | 0.60 ± 0.11 | 0.281 | 0.22 ± 0.04 | 0.25 ± 0.06 | 0.293 | <0.0066 | <0.0066 | - | 0.58 ± 0.11 | 0.64 ± 0.11 | 0.311 | <0.0066 | <0.0066 | - | <0.0066 | <0.0066 | - |
| C18:0 | 9.08 ± 1.90 | 7.88 ± 1.81 | 0.218 | 3.95 ± 0.64 | 3.96 ± 0.66 | 0.979 | 0.35 ± 0.08 | 0.34 ± 0.04 | 0.782 | 7.28 ± 0.94 | 8.13 ± 1.57 | 0.206 | 0.31 ± 0.12 | 0.26 ± 0.04 | 0.295 | 0.37 ± 0.04 | 0.37 ± 0.05 | 0.974 |
| C20:0 | 0.62 ± 0.13 | 0.55 ± 0.14 | 0.331 | 0.21 ± 0.03 | 0.21 ± 0.04 | 0.948 | <0.0066 | <0.0066 | - | 0.52 ± 0.09 | 0.60 ± 0.10 | 0.123 | <0.0066 | 0.07 ± 0.00 | - | <0.0033 | <0.0033 | - |
| C21:0 | 0.24 ± 0.02 | 0.28 ± 0.03 | 0.204 | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - | 0.09 ± 0.12 | 0.13 ± 0.17 | 0.611 | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - |
| C22:0 | 0.52 ± 0.13 | 0.44 ± 0.15 | 0.288 | 0.10 ± 0.02 | 0.11 ± 0.03 | 0.414 | 0.07 ± 0.00 | <0.0033 | - | 0.48 ± 0.10 | 0.55 ± 0.14 | 0.294 | 0.02 ± 0.04 | 0.02 ± 0.04 | 0.966 | <0.0033 | <0.0033 | - |
| C23:0 | <0.0033 | 0.24 ± 0.05 | - | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - | 0.08 ± 0.11 | 0.11 ± 0.15 | 0.694 | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - |
| C24:0 | <0.0066 | 0.14 ± 0.09 | - | <0.0066 | <0.0066 | - | <0.0066 | <0.0066 | - | 0.04 ± 0.07 | 0.08 ± 0.11 | 0.408 | <0.0066 | <0.0066 | - | <0.0033 | <0.0033 | - |
| SFAs | 91.83 ± 11.90 | 83.00 ± 12.75 | 0.174 | 17.53 ± 2.46 | 18.00 ± 2.43 | 0.708 | 0.85 ± 0.17 | 0.87 ± 0.11 | 0.870 | 72.87 ± 8.50 | 89.49 ± 15.93 | 0.021 | 0.64 ± 0.24 | 0.65 ± 0.16 | 0.876 | 0.82 ± 0.20 | 0.84 ± 0.13 | 0.826 |
| Monounsaturated fatty acids (MUFAs, g/kg tissue weight) | ||||||||||||||||||
| C14:1 | 1.11 ± 0.19 | 0.96 ± 0.20 | 0.141 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.201 | <0.0033 | <0.0033 | - | 0.70 ± 0.15 | 0.94 ± 0.23 | 0.027 | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - |
| C16:1 | 32.25 ± 5.10 | 30.13 ± 6.51 | 0.480 | 9.05 ± 1.67 | 9.23 ± 2.23 | 0.858 | 0.09 ± 0.05 | 0.16 ± 0.07 | 0.050 | 20.98 ± 6.09 | 27.39 ± 7.10 | 0.073 | 0.05 ± 0.04 | 0.09 ± 0.04 | 0.050 | 0.07 ± 0.03 | 0.10 ± 0.06 | 0.250 |
| C18:1n9c | 115.51 ± 17.37 | 97.70 ± 11.93 | 0.031 | 24.81 ± 3.17 | 23.81 ± 3.34 | 0.549 | 0.78 ± 0.14 | 0.83 ± 0.12 | 0.530 | 90.59 ± 12.72 | 108.33 ± 16.56 | 0.031 | 0.46 ± 0.13 | 0.61 ± 0.21 | 0.096 | 0.65 ± 0.12 | 0.70 ± 0.10 | 0.421 |
| C20:1 | 2.64 ± 0.81 | 2.12 ± 0.45 | 0.135 | 0.40 ± 0.07 | 0.39 ± 0.10 | 0.954 | 0.04 ± 0.00 | <0.0033 | - | 1.60 ± 0.31 | 1.79 ± 0.33 | 0.254 | 0.01 ± 0.02 | 0.02 ± 0.02 | 0.297 | <0.0033 | <0.0033 | - |
| C22:1n9 | 0.18 ± 0.06 | 0.14 ± 0.07 | 0.237 | 0.14 ± 0.02 | 0.16 ± 0.05 | 0.361 | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.212 | 0.17 ± 0.04 | 0.17 ± 0.07 | 0.986 | 0.03 ± 0.02 | 0.02 ± 0.03 | 0.596 | <0.0033 | <0.0033 | - |
| C24:1 | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - | 0.35 ± 0.18 | 0.37 ± 0.23 | 0.853 | <0.0033 | <0.0033 | - |
| MUFAs | 151.69 ± 22.23 | 131.04 ± 11.29 | 0.034 | 34.47 ± 4.48 | 33.65 ± 4.54 | 0.724 | 0.89 ± 0.19 | 1.00 ± 0.17 | 0.320 | 114.04 ± 17.43 | 138.63 ± 20.40 | 0.021 | 0.89 ± 0.28 | 1.11 ± 0.37 | 0.201 | 0.71 ± 0.14 | 0.80 ± 0.15 | 0.292 |
| Polyunsaturated fatty acids (PUFAs, g/kg tissue weight) | ||||||||||||||||||
| C18:2n6c | 60.41 ± 8.36 | 58.07 ± 17.28 | 0.735 | 16.74 ± 2.35 | 17.43 ± 2.99 | 0.617 | 0.56 ± 0.12 | 0.70 ± 0.12 | 0.078 | 57.79 ± 9.58 | 73.83 ± 15.85 | 0.028 | 0.23 ± 0.09 | 0.37 ± 0.15 | 0.037 | 0.57 ± 0.15 | 0.66 ± 0.11 | 0.268 |
| C18:3n6 | 0.08 ± 0.02 | 0.10 ± 0.02 | 0.105 | <0.0066 | <0.0066 | - | <0.0066 | <0.0066 | - | 0.03 ± 0.04 | 0.06 ± 0.05 | 0.292 | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - |
| C18:3n3 | 6.70 ± 1.74 | 7.32 ± 1.52 | 0.464 | 2.38 ± 0.40 | 3.40 ± 0.48 | 0.000 | 0.06 ± 0.02 | 0.11 ± 0.03 | 0.011 | 4.91 ± 1.32 | 6.61 ± 1.70 | 0.042 | 0.004 ± 0.01 | 0.03 ± 0.02 | 0.007 | 0.06 ± 0.01 | 0.09 ± 0.03 | 0.041 |
| C20:2 | 6.01 ± 1.41 | 4.63 ± 1.13 | 0.049 | 1.23 ± 0.19 | 1.33 ± 0.28 | 0.388 | 0.11 ± 0.03 | 0.09 ± 0.01 | 0.205 | 4.30 ± 1.03 | 4.44 ± 0.97 | 0.784 | 0.10 ± 0.02 | 0.11 ± 0.02 | 0.561 | 0.10 ± 0.01 | 0.09 ± 0.02 | 0.702 |
| C20:3n6 | 0.25 ± 0.11 | 0.19 ± 0.12 | 0.395 | 0.09 ± 0.03 | 0.10 ± 0.05 | 0.598 | <0.0033 | <0.0033 | - | 0.17 ± 0.05 | 0.23 ± 0.09 | 0.089 | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - |
| C20:3n3 | 1.62 ± 0.44 | 1.53 ± 0.32 | 0.661 | 0.37 ± 0.07 | 0.49 ± 0.12 | 0.032 | <0.0033 | 0.04 ± 0.00 | - | 0.99 ± 0.13 | 1.18 ± 0.28 | 0.106 | 0.05 ± 0.03 | 0.06 ± 0.04 | 0.634 | <0.0033 | <0.0033 | - |
| C20:4n6 | 2.90 ± 0.56 | 2.22 ± 0.64 | 0.041 | 2.48 ± 0.48 | 2.13 ± 0.65 | 0.247 | 0.27 ± 0.08 | 0.23 ± 0.05 | 0.266 | 2.78 ± 0.70 | 2.92 ± 0.85 | 0.714 | 0.35 ± 0.12 | 0.43 ± 0.06 | 0.111 | 0.23 ± 0.02 | 0.24 ± 0.02 | 0.371 |
| C20:5n3 (EPA) | 2.27 ± 0.60 | 1.65 ± 0.47 | 0.038 | 3.15 ± 0.58 | 2.47 ± 0.56 | 0.034 | 0.47 ± 0.09 | 0.41 ± 0.04 | 0.199 | 2.49 ± 0.53 | 2.59 ± 0.84 | 0.789 | 0.18 ± 0.07 | 0.21 ± 0.03 | 0.241 | 0.43 ± 0.21 | 0.46 ± 0.07 | 0.764 |
| C22:2 | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - | <0.0033 | 0.03 ± 0.06 | - | <0.0033 | <0.0033 | - | <0.0033 | <0.0033 | - |
| C22:6n3 (DHA) | 1.28 ± 0.31 | 0.85 ± 0.38 | 0.029 | 2.00 ± 0.54 | 1.56 ± 0.79 | 0.214 | 0.25 ± 0.05 | 0.25 ± 0.06 | 0.869 | 1.29 ± 0.30 | 1.21 ± 0.40 | 0.689 | 0.08 ± 0.04 | 0.10 ± 0.02 | 0.095 | 0.31 ± 0.05 | 0.26 ± 0.06 | 0.197 |
| PUFA | 81.49 ± 10.67 | 76.49 ± 18.90 | 0.098 | 28.44 ± 3.30 | 28.93 ± 4.47 | 0.935 | 1.72 ± 0.25 | 1.80 ± 0.18 | 0.526 | 74.75 ± 10.73 | 93.12 ± 18.08 | 0.027 | 0.99 ± 0.27 | 1.31 ± 0.21 | - | 1.69 ± 0.29 | 1.80 ± 0.22 | 0.484 |
| n-3 PUFAs | 11.87 ± 2.66 | 11.35 ± 2.28 | 0.684 | 7.90 ± 1.06 | 7.92 ± 1.43 | 0.966 | 0.78 ± 0.12 | 0.79 ± 0.08 | 0.856 | 9.68 ± 1.26 | 11.61 ± 2.37 | 0.061 | 0.31 ± 0.11 | 0.40 ± 0.08 | 0.073 | 0.80 ± 0.24 | 0.81 ± 0.12 | 0.914 |
| n-6 PUFAs | 63.61 ± 8.50 | 60.51 ± 17.27 | 0.656 | 19.32 ± 2.63 | 19.67 ± 3.28 | 0.815 | 0.83 ± 0.14 | 0.92 ± 0.13 | 0.264 | 60.77 ± 9.56 | 77.36 ± 16.08 | 0.025 | 0.58 ± 0.14 | 0.80 ± 0.14 | 0.009 | 0.79 ± 0.15 | 0.10 ± 0.10 | 0.208 |
| n-3 PUFAs/ n-6 PUFAs | 0.19 ± 0.03 | 0.20 ± 0.06 | 0.680 | 0.41 ± 0.06 | 0.41 ± 0.07 | 0.895 | 0.94 ± 0.12 | 0.86 ± 0.11 | 0.289 | 0.16 ± 0.01 | 0.15 ± 0.02 | 0.448 | 0.52 ± 0.10 | 0.51 ± 0.09 | 0.785 | 1.04 ± 0.34 | 0.91 ± 0.11 | 0.396 |
| UFAs | 233.18 ± 29.60 | 207.53 ± 28.26 | 0.098 | 62.91 ± 7.45 | 62.58 ± 8.28 | 0.807 | 2.61 ± 0.40 | 2.80 ± 0.33 | 0.382 | 188.79 ± 26.62 | 231.75 ± 37.41 | 0.019 | 1.88 ± 0.49 | 2.42 ± 0.57 | 0.043 | 2.40 ± 0.36 | 2.61 ± 0.34 | 0.342 |
| Total fatty acids | 325.00 ± 41.88 | 290.54 ± 47.46 | 0.146 | 80.45 ± 9.76 | 80.58 ± 10.56 | 0.981 | 3.45 ± 0.55 | 3.67 ± 0.46 | 0.478 | 261.65 ± 34.53 | 321.23 ± 52.12 | 0.017 | 2.55 ± 0.47 | 3.08 ± 0.70 | 0.095 | 3.28 ± 0.43 | 3.45 ± 0.45 | 0.525 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Zhang, J.; Wu, Y.; Luo, X.; Xu, Z.; Pan, J.; Zou, G.; Liang, H. Comparison of Body Characteristics, Carotenoid Composition, and Nutritional Quality of Chinese Mitten Crab (Eriocheir sinensis) with Different Hepatopancreas Redness. Foods 2024, 13, 993. https://doi.org/10.3390/foods13070993
Guo H, Zhang J, Wu Y, Luo X, Xu Z, Pan J, Zou G, Liang H. Comparison of Body Characteristics, Carotenoid Composition, and Nutritional Quality of Chinese Mitten Crab (Eriocheir sinensis) with Different Hepatopancreas Redness. Foods. 2024; 13(7):993. https://doi.org/10.3390/foods13070993
Chicago/Turabian StyleGuo, Honghui, Jingang Zhang, Yidi Wu, Xiangzhong Luo, Zhiqiang Xu, Jianlin Pan, Guiwei Zou, and Hongwei Liang. 2024. "Comparison of Body Characteristics, Carotenoid Composition, and Nutritional Quality of Chinese Mitten Crab (Eriocheir sinensis) with Different Hepatopancreas Redness" Foods 13, no. 7: 993. https://doi.org/10.3390/foods13070993
APA StyleGuo, H., Zhang, J., Wu, Y., Luo, X., Xu, Z., Pan, J., Zou, G., & Liang, H. (2024). Comparison of Body Characteristics, Carotenoid Composition, and Nutritional Quality of Chinese Mitten Crab (Eriocheir sinensis) with Different Hepatopancreas Redness. Foods, 13(7), 993. https://doi.org/10.3390/foods13070993







