Distribution and Probabilistic Risk Assessment of Antibiotics, Illegal Drugs, and Toxic Elements in Gastropods from Southeast China
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents and Chemicals
2.2. Sample Collection
2.3. Analysis of Antibiotic Residues and Illegal Drugs
2.4. Analysis of Elements
2.5. Method Validation
2.6. Probabilistic Risk Assessment
3. Results and Discussion
3.1. Method Validation
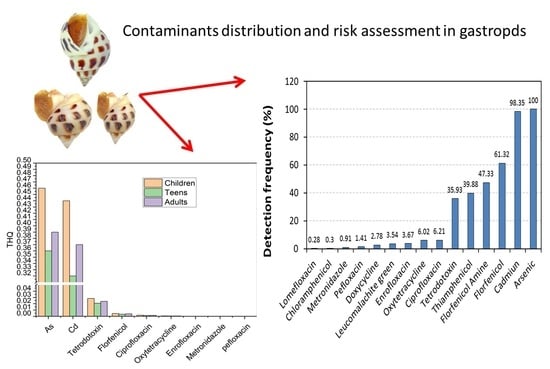
3.2. The Distribution of Antibiotics, Illegal Drugs, and Toxic Elements
3.3. The Effect of Sampling Data
3.4. Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bureau of Fisheries of the Ministry of Agriculture of the People’s Republic of China. China Fishery Statistical Yearbook; 1979–2021; China Agriculture Press: Beijing, China, 2023.
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.X. Antibiotics and food safety in aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Song, S.; Gao, J.; Song, Y.; Xiao, X.; Yang, X.; Jiang, D.; Yang, D. Antibiotic residues in chicken meat in China: Occurrence and cumulative health risk assessment. J. Food Compos. Anal. 2023, 116, 105082. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Gao, L.; Liu, J.; Cai, Y. Investigation of antibiotics in mollusks from coastal waters in the Bohai Sea of China. Environ. Pollut. 2012, 162, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, L.M.; Nobile, M.; Malandra, R.; Panseri, S.; Arioli, F. Occurrence of antibiotics in mussels and clams from various fao areas. Food Chem. 2018, 240, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-D.; Han, J.-L. Determination of steroid hormone residues in farmed fish using high-resolution orbital ion trap mass spectrometry. Anal. Methods 2022, 40, 4146–4152. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, J.C.; Paschoal, J.A.R.; de Queiroz, J.F.; Reyes, F.G.R. Considerations on the use of malachite green in aquaculture and analytical aspects of determining the residues in fish: A review. J. Aquat. Food Prod. Technol. 2011, 20, 273–294. [Google Scholar] [CrossRef]
- Noël, L.; Testu, C.; Chafey, C.; Velge, P.; Guérin, T. Contamination levels for lead, cadmium and mercury in marine gastropods, echinoderms and tunicates. Food Control 2011, 22, 433–437. [Google Scholar] [CrossRef]
- Pan, X.-D.; Han, J.-L. Heavy metals accumulation in bivalve mollusks collected from coastal areas of Southeast China. Mar. Pollut. Bull. 2023, 189, 114808. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-L.; Pan, X.-D.; Chen, Q.; Huang, B.-F. Health risk assessment of heavy metals in marine fish to the population in Zhejiang, China. Sci. Rep. 2021, 11, 11079. [Google Scholar] [CrossRef]
- Han, J.L.; Pan, X.D.; Chen, Q. Distribution and safety assessment of heavy metals in fresh meat from Zhejiang, China. Sci. Rep. 2022, 12, 3241. [Google Scholar] [CrossRef]
- Guidi, L.R.; Santos, F.A.; Ribeiro, A.C.S.; Fernandes, C.; Silva, L.H.; Gloria, M.B.A. Quinolones and tetracyclines in aquaculture fish by a simple and rapid lc-ms/ms method. Food Chem. 2018, 245, 1232–1238. [Google Scholar] [CrossRef]
- ZJFDA. A Report on the Dietary Intake in Zhejiang Province, China; ZJFDA: Hangzhou, China, 2008. Available online: http://www.Zjfda.Gov.Cn/news/detail/13556.Html (accessed on 1 October 2021).
- Yu, S.; Pan, X.-D.; Han, J.-L. Toxic elements in beans from Zhejiang, Southeast China: Distribution and probabilistic health risk assessment. Foods 2023, 12, 3300. [Google Scholar] [CrossRef] [PubMed]
- US FDA. Cfr-Code of Federal Regulations Title 21 Part 556 Tolerances for Residues of New Animal Drugs in Food; US Food and Drug Administration: Rockville, MD, USA, 2023.
- Zhou, L.-J.; Wang, W.-X.; Lv, Y.-J.; Mao, Z.-G.; Chen, C.; Wu, Q.L. Tissue concentrations, trophic transfer and human risks of antibiotics in freshwater food web in Lake Taihu, China. Ecotoxicol. Environ. Saf. 2020, 197, 110626. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-D.; Han, J.-L. Distribution of cadmium in fresh vegetables marketed in Southeast China and its dietary exposure assessment. Foods 2023, 12, 1204. [Google Scholar] [CrossRef] [PubMed]
- Pipoyan, D.; Stepanyan, S.; Beglaryan, M.; Stepanyan, S.; Mantovani, A. Health risk assessment of toxicologically relevant residues in emerging countries: A pilot study on malachite green residues in farmed freshwater fish of armenia. Food Chem. Toxicol. 2020, 143, 111526. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Malachite green in food. EFSA J. 2016, 14, e04530. [Google Scholar]
- GB2762–2017; Maximum Levels of Contaminants in Foods. Ministry of Health of the People’s Republic of China: Beijing, China, 2017. (In Chinese)
- European Commission. Commission regulation (eu) no 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union 2010, 15, 1–72. [Google Scholar]
- GB 31650–2019; National Food Safety Standard-Maximum Residue Limits for Veterinary Drugs in Foods. Chinese Agriculture Ministry: Beijing, China, 2019.
- Baralla, E.; Demontis, M.P.; Dessì, F.; Varoni, M.V. An overview of antibiotics as emerging contaminants: Occurrence in bivalves as biomonitoring organisms. Animals 2021, 11, 3239. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lou, X.; Yang, G.; Tian, L.; Wang, Y.; Huang, X. Occurrence and human health risk assessment of antibiotics in cultured fish from 19 provinces in China. Front. Cell. Infect. Microbiol. 2022, 12, 964283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Zhou, Y.; Han, Q.; Wang, X.; Song, C.; Wang, S.; Zhao, S. Occurrence, distribution and risk assessment of antibiotics at various aquaculture stages in typical aquaculture areas surrounding the Yellow Sea. J. Environ. Sci. 2023, 126, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.J.; Pan, X.D.; Han, J.L. Antibiotic residues in commercial freshwater fish from southeast China: Distribution and human health risk assessment. Environ. Sci. Pollut. Res. 2024, 31, 23780–23789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Pei, J.; Zhang, R.; Wang, S.; Zeng, W.; Huang, D.; Wang, Y.; Zhang, Y.; Wang, Y.; Yu, K. Occurrence and distribution of antibiotics in mariculture farms, estuaries and the coast of the Beibu Gulf, China: Bioconcentration and diet safety of seafood. Ecotoxicol. Environ. Saf. 2018, 154, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Lu, S.; Huang, C.; Wang, F.; Ren, Y.; Cao, H.; Lin, Q.; Tan, Z.; Wen, X. A survey of chloramphenicol residues in aquatic products of Shenzhen, South China. Food Addit. Contam. A 2021, 38, 914–921. [Google Scholar] [CrossRef]
- Gharavi-nakhjavani, M.S.; Niazi, A.; Hosseini, H.; Aminzare, M.; Dizaji, R.; Tajdar-oranj, B.; Mirza Alizadeh, A. Malachite green and leucomalachite green in fish: A global systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2023, 30, 48911–48927. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-L.; Chimeddulam, D.; Sheen, L.-Y.; Wu, K.-Y. Probabilistic risk assessment of exposure to leucomalachite green residues from fish products. Food Chem. Toxicol. 2013, 62, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Dadar, M.; Oliveri Conti, G. Antibiotics and malachite green residues in farmed rainbow trout (Oncorhynchus mykiss) from the iranian markets: A risk assessment. Int. J. Food Prop. 2016, 20, 402–408. [Google Scholar] [CrossRef]
- León, V.M.; Moreno-González, R.; Besada, V.; Martínez, F.; Ceruso, C.; García, V.; Schultze, F.; Campillo, J.A. Sea snail (Hexaplex trunculus) and sea cucumber (Holothuria polii) as potential sentinel species for organic pollutants and trace metals in coastal ecosystems. Mar. Pollut. Bull. 2021, 168, 112407. [Google Scholar] [CrossRef]
- Gouveia, N.; Oliveira, A.J.L.A.; Yokota Harayashiki, C.A.; Souza, J.C.; Longo, E.; Cano, N.F.; Maltez, H.F.; Lourenço, R.A.; Turpo-Huahuasoncco, K.V.; Castro, Í.B. Chemical contamination in coastal areas alters shape, resistance and composition of carnivorous gastropod shells. Chemosphere 2022, 307, 135926. [Google Scholar] [CrossRef]
- Barchiesi, F.; Branciari, R.; Latini, M.; Roila, R.; Lediani, G.; Filippini, G.; Scortichini, G.; Piersanti, A.; Rocchegiani, E.; Ranucci, D. Heavy metals contamination in shellfish: Benefit-risk evaluation in central italy. Foods 2020, 9, 1720. [Google Scholar] [CrossRef]
- Ragi, A.S.; Leena, P.P.; Cheriyan, E.; Nair, S.M. Heavy metal concentrations in some gastropods and bivalves collected from the fishing zone of south india. Mar. Pollut. Bull. 2017, 118, 452–458. [Google Scholar] [CrossRef]
- Menon, M.; Mohanraj, R.; Vb, J.; Prasath Rv, A. Bioaccumulation of heavy metals in a gastropod species at the kole wetland agroecosystem, a ramsar site. J. Environ. Manag. 2023, 329, 117027. [Google Scholar] [CrossRef] [PubMed]
- Pepi, M.; Focardi, S. Antibiotic-resistant bacteria in aquaculture and climate change: A challenge for health in the Mediterranean area. Int. J. Environ. Res. Public Health 2021, 18, 5723. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cai, X.; Cao, M.; Liu, H.; Liang, Y.; Hu, L.; Yin, Y.; Li, Y.; Shi, J. Long-term investigation of heavy metal variations in mollusks along the Chinese Bohai Sea. Ecotoxicol. Environ. Saf. 2022, 236, 113443. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lv, L.; An, M.; Wang, T.; Yu, Y. Heavy metals in marine food web from Laizhou Bay, China: Levels, trophic magnification, and health risk assessment. Sci. Total Environ. 2022, 841, 156818. [Google Scholar] [CrossRef] [PubMed]
- Kato, L.S.; Ferrari, R.G.; Leite, J.V.M. Arsenic in shellfish: A systematic review of its dynamics and potential health risks. Mar. Pollut. Bull. 2020, 161, 111693. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, Q.; Wang, F.; Zhong, S.; Chen, Y.; Guo, Y.; Su, K. Arsenic speciation in shellfish from South China Sea: Levels, estimated daily intake and health risk assessment. Mar. Pollut. Bull. 2022, 178, 113651. [Google Scholar] [CrossRef] [PubMed]
- Anagha, B.; Athira, P.S.; Anisha, P.; Charles, P.E.; Anandkumar, A.; Rajaram, R. Biomonitoring of heavy metals accumulation in molluscs and echinoderms collected from southern coastal india. Mar. Pollut. Bull. 2022, 184, 114169. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sun, L.; Zhu, X.; Bian, G.; Zhou, W.; Cao, Q.; Hong, M. Distribution characteristics and risk assessment of heavy metals in seawater, sediment and shellfish in the inner and outer Daya Bay, Guangdong. Front. Mar. Sci. 2022, 9, 1064287. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, W.; Wang, Y.; Nian, M.; Jiang, F.; Zhang, J.; Chen, Q. Environmental antibiotics exposure in school-age children in Shanghai and health risk assessment: A population-based representative investigation. Sci. Total Environ. 2022, 824, 153859. [Google Scholar] [CrossRef] [PubMed]
- Kitsos, N.; Cassimos, D.; Xinias, I.; Agakidis, C.; Mavroudi, A. Management of antibiotic allergy in children: A practical approach. Allergol. Immunopathol. 2022, 50, 30–38. [Google Scholar] [CrossRef] [PubMed]




| Antibiotic | Correlation Coefficients (r2) | Spiking Recovery (n = 6) | RSDs (%) | LOQ (μg/kg) | |||
|---|---|---|---|---|---|---|---|
| 5 μg/kg | 10 μg/kg | 50 μg/kg | |||||
| QNs | Ciprofloxacin | 0.9990 | 89.5 | 95.5 | 96.2 | 6.7 | 3.0 |
| Enrofloxacin | 0.9991 | 90.2 | 95.6 | 99.4 | 7.5 | 3.0 | |
| Lomefloxacin | 0.9985 | 89.5 | 96.3 | 98.8 | 4.6 | 3.0 | |
| Norfloxacin | 0.9991 | 92.3 | 91.9 | 94.2 | 5.9 | 3.0 | |
| Ofloxacin | 0.9996 | 89.5 | 90.0 | 91.7 | 7.7 | 3.0 | |
| Pefloxacin | 0.9997 | 95.2 | 97.6 | 105.9 | 8.0 | 3.0 | |
| TCs | Chlortetracycline | 0.9985 | 82.8 | 90.4 | 94.3 | 4.9 | 1.5 |
| Doxycycline | 0.9992 | 86.2 | 90.4 | 92.4 | 7.2 | 1.5 | |
| Oxytetracycline | 0.9990 | 86.5 | 95.5 | 94.7 | 8.2 | 1.5 | |
| Tetracycline | 0.9987 | 81.7 | 88.8 | 95.2 | 7.6 | 1.5 | |
| CAPs | Thiamphenicol | 0.9997 | 90.5 | 92.4 | 91.4 | 5.8 | 0.1 |
| Florfenicol | 0.9994 | 94.2 | 99.8 | 104.7 | 7.0 | 0.2 | |
| Florfenicol Amine | 0.9979 | 84.3 | 90.1 | 89.5 | 7.9 | 2.0 | |
| NMZ | Metronidazole | 0.9984 | 90.2 | 92.5 | 101.2 | 8.1 | 2.0 |
| Ilegals | Chloramphenicol | 0.9995 | 95.1 | 99.7 | 107.5 | 9.1 | 0.1 |
| Malachite green | 0.9995 | 95.6 | 97.8 | 103.5 | 5.6 | 1.0 | |
| Leucomalachite green | 0.9990 | 94.2 | 95.0 | 96.3 | 9.2 | 1.0 | |
| Certified (mg/kg) | Tested Values (mg/kg) | Mean (mg/kg) | RSD (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Test 1 | Test 2 | Test 3 | Test 4 | Test 5 | Test 6 | ||||
| As | 12.9 ± 0.6 | 13.1 | 12.5 | 12.8 | 13.8 | 12 | 12.2 | 12.8 | 5.2 |
| Cd | 18.7 ± 0.7 | 16.8 | 17.5 | 18.5 | 19.6 | 18.2 | 19.9 | 18.4 | 6.4 |
| Analytes | n | Detection Frequency (%) | Concentration (μg/kg Wet Weight) | MRL (μg/kg) | ||
|---|---|---|---|---|---|---|
| Mean | Maximum | |||||
| QNs | Enrofloxacin | 354 | 3.67 | 0.12 | 12.8 | 100 |
| Ciprofloxacin | 354 | 6.21 | 12.14 | 1110 | 100 | |
| Lomefloxacin | 354 | 0.28 | 0.01 | 2.55 | 2 | |
| Norfloxacin | 354 | 0.00 | 0.00 | 0 | 2 | |
| Ofloxacin | 354 | 0.00 | 0.00 | 0 | 2 | |
| Pefloxacin | 354 | 1.41 | 0.03 | 4.32 | 2 | |
| TCs | Chlortetracycline | 216 | 0.00 | 0.00 | 0 | 100 |
| Doxycycline | 216 | 2.78 | 1.32 | 0 | - | |
| Oxytetracycline | 216 | 6.02 | 30.34 | 3410 | 200 | |
| Tetracycline | 50 | 0.00 | 0.00 | 0 | 100 | |
| CAPs | Thiamphenicol | 326 | 39.88 | 3.05 | 136 | - |
| Florfenicol | 287 | 61.32 | 46.03 | 1110 | - | |
| Florfenicol Amine | 262 | 47.33 | 59.04 | 2222 | - | |
| NMZ | Metronidazole | 110 | 0.91 | 0.01 | 0.618 | - |
| Ilegals | Chloramphenicol | 336 | 0.30 | 0.19 | 64 | prohibit |
| Malachite green | 113 | 0.00 | 0.00 | 0 | prohibit | |
| Leucomalachite green | 113 | 3.54 | 0.07 | 2.48 | prohibit | |
| Elements | Cadmiun | 303 | 98.35 | 1.17 | 20.8 | 2.0 (mg/kg) |
| Arsenic | 304 | 100.00 | 6.12 | 48.2 | 0.5 (mg/kg) | |
| Compounds | ADI | Children (μg/kg bw/Day × 10−3) | Teens (μg/kg bw/Day × 10−3) | Adults (μg/kg bw/Day × 10−3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | P50 | P95 | Mean | P50 | P95 | Mean | P50 | P95 | ||
| Florfenicol | 10 | 45.54 | 45.21 | 77.99 | 33.34 | 33.24 | 59.86 | 38.26 | 38.28 | 63.14 |
| Oxytetracycline | 25 | 13.72 | 13.34 | 23.99 | 9.8 | 9.8 | 18.38 | 11.52 | 11.3 | 19.42 |
| Ciprofloxacin | 3 | 5.26 | 5.21 | 9.05 | 3.85 | 3.82 | 6.94 | 4.42 | 4.4 | 7.31 |
| Enrofloxacin | 3 | 0.05 | 0.05 | 0.09 | 0.04 | 0.04 | 0.07 | 0.04 | 0.04 | 0.07 |
| Pefloxacin | 1.6 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.02 |
| Metronidazole | 0.6 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | 0.01 |
| RfD | μg/kg bw/day | μg/kg bw/day | μg/kg bw/day | |||||||
| Cd | 1 | 0.44 | 0.44 | 0.76 | 0.32 | 0.32 | 0.58 | 0.37 | 0.37 | 0.61 |
| As | (0.3) | 2.65 | 2.64 | 4.54 | 1.94 | 1.94 | 3.48 | 2.23 | 2.23 | 3.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.-T.; Pan, X.-D.; Han, J.-L. Distribution and Probabilistic Risk Assessment of Antibiotics, Illegal Drugs, and Toxic Elements in Gastropods from Southeast China. Foods 2024, 13, 1166. https://doi.org/10.3390/foods13081166
Shen H-T, Pan X-D, Han J-L. Distribution and Probabilistic Risk Assessment of Antibiotics, Illegal Drugs, and Toxic Elements in Gastropods from Southeast China. Foods. 2024; 13(8):1166. https://doi.org/10.3390/foods13081166
Chicago/Turabian StyleShen, Hai-Tao, Xiao-Dong Pan, and Jian-Long Han. 2024. "Distribution and Probabilistic Risk Assessment of Antibiotics, Illegal Drugs, and Toxic Elements in Gastropods from Southeast China" Foods 13, no. 8: 1166. https://doi.org/10.3390/foods13081166
APA StyleShen, H.-T., Pan, X.-D., & Han, J.-L. (2024). Distribution and Probabilistic Risk Assessment of Antibiotics, Illegal Drugs, and Toxic Elements in Gastropods from Southeast China. Foods, 13(8), 1166. https://doi.org/10.3390/foods13081166