Hawthorn Proanthocyanidin Extract Inhibits Colorectal Carcinoma Metastasis by Targeting the Epithelial-Mesenchymal Transition Process and Wnt/β-Catenin Signaling Pathway
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. HPOE Extraction
2.3. Cell Lines and Culture
2.4. Cell Viability Assay
2.5. Cell Treatment Groups
2.6. Scratch-Healing Assay
2.7. Transwell Migration and Invasion Assay
2.8. Target Screening of HPOE
2.9. Construction of Component–Target Networks
2.10. Screening of Disease Targets for CRC
2.11. Construction of Protein–Protein Interaction Networks
2.12. GO Enrichment and KEGG Pathway Analyses
2.13. RNA Extraction and RT-qPCR
2.14. Western Blotting
2.15. Statistical Analysis
3. Results
3.1. HPOE Inhibits HCT116 Cells Migration and Invasion
3.2. Prediction of Potential Targets and Mechanisms Underlying the HPOE-Mediated Inhibition of CRC Metastasis Based on Network Pharmacology
3.2.1. Target Analysis of the Active Components of HPOE
3.2.2. CRC Target Prediction and PPI Network Analysis
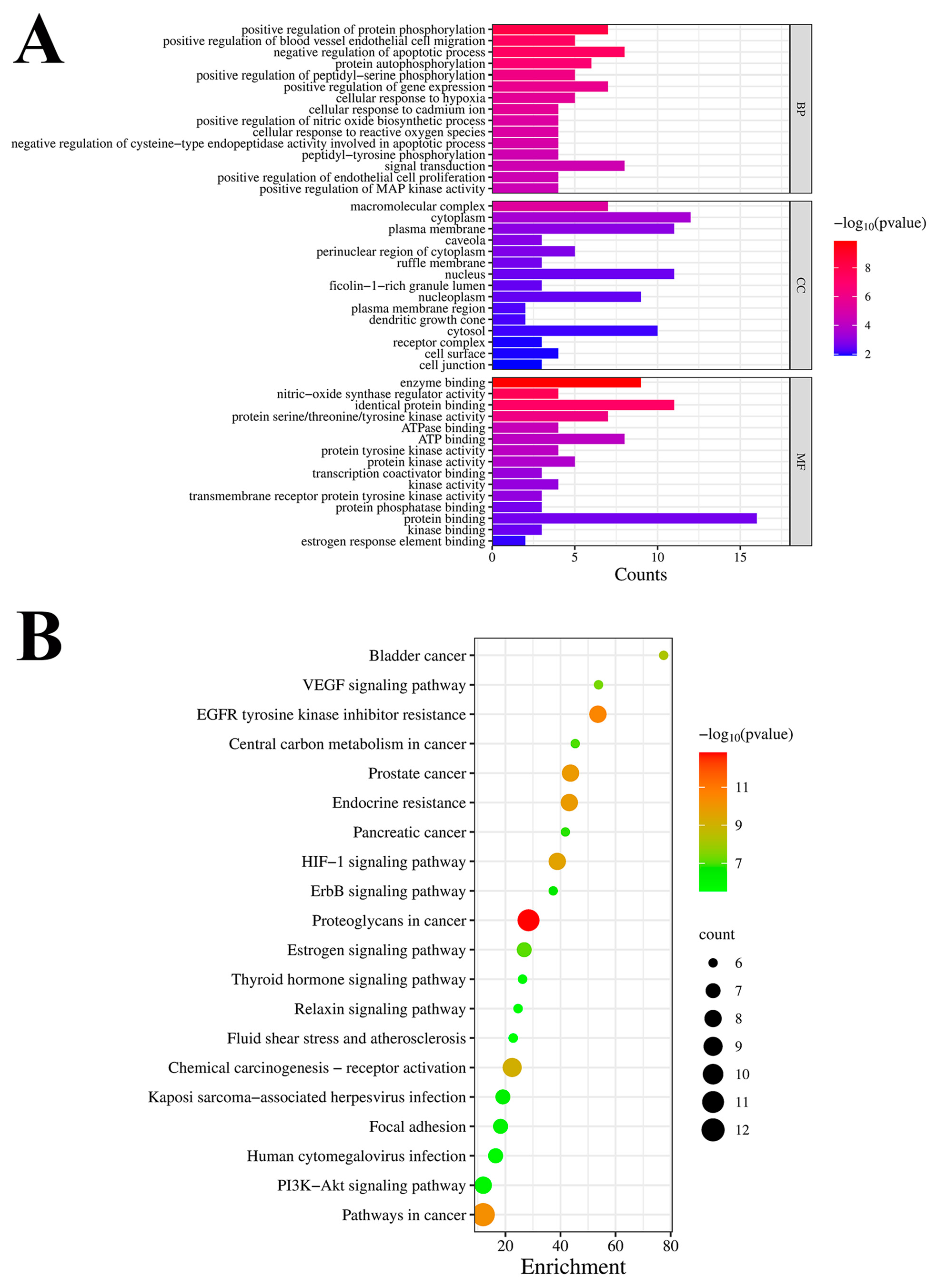
3.2.3. GO Enrichment and KEGG Pathway Analyses
3.3. HPOE Inhibits the Expression of Genes and Proteins Related to EMT in HCT116 Cells
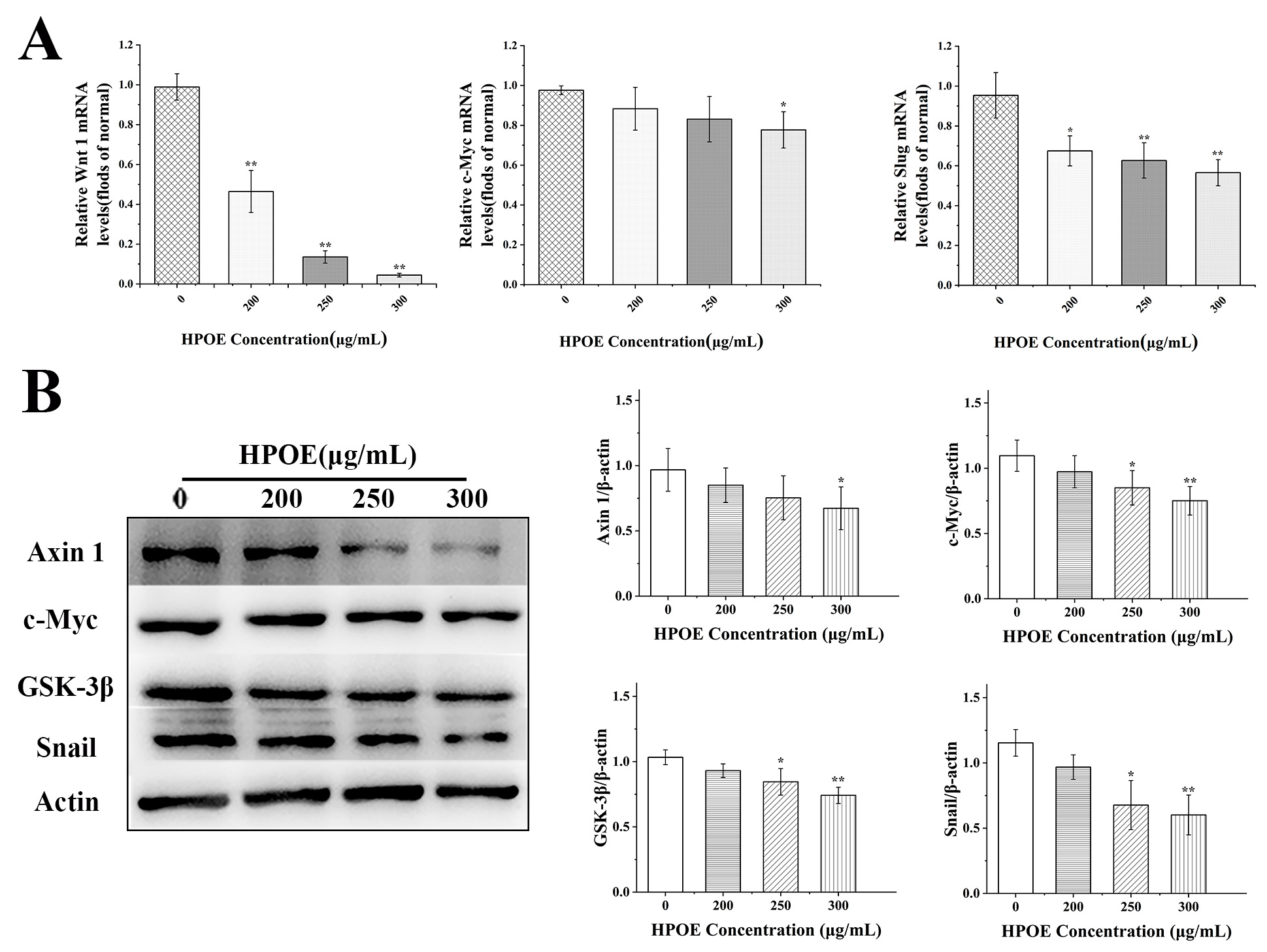
3.4. HPOE Inhibits the Expression of Genes and Proteins Related to the Wnt/β-Catenin Signaling Pathway in HCT116 Cells
3.5. HPOE Inhibits HCT116 Cells’ Migration and Invasion by Inhibiting the Wnt/β-Catenin Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Carcagnì, P.; Leo, M.; Signore, L.; Distante, C. An Investigation about Modern Deep Learning Strategies for Colon Carcinoma Grading. Sensors 2023, 23, 4556. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, A.M. Colorectal Cancer in the Young: Research in Early Age Colorectal Cancer Trends (REACCT) Collaborative. Cancers 2023, 15, 2979. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Liang, Y.; Li, M.; Lin, Y.; Zou, X.; Han, F.; Cao, J.; Li, L. The updates on metastatic mechanism and treatment of colorectal cancer. Pathol. Res. Pract. 2023, 251, 154837. [Google Scholar] [CrossRef]
- Lee, S.-Y.; An, M.; Lee, J. Immune landscape of colorectal cancer lung metastasis. J. Clin. Oncol. 2022, 40, e15542. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, H.; Lv, W.; Zhu, Y. Current status and prospect of immunotherapy for colorectal cancer. Int. J. Color. Dis. 2023, 38, 266. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Fan, X.; Xie, L.; Wu, D.; Liu, R.; Gao, W.; Lou, K.; He, B.; Pu, Y. Nano-enabled colorectal cancer therapy. J. Control. Release 2023, 362, 548–564. [Google Scholar] [CrossRef]
- Nascimento, R.d.P.d.; Machado, A.P.d.F. The preventive and therapeutic effects of anthocyanins on colorectal cancer: A comprehensive review based on up-to-date experimental studies. Food Res. Int. 2023, 170, 113028. [Google Scholar] [CrossRef]
- Jangde, S.; Purohit, M.R.; Saraf, F.; Merchant, N.; Bhaskar, L.V.K.S. Dietary Phytocompounds for Colon Cancer Therapy; Onco Therapeutics: West Hollywood, CA, USA, 2022; Volume 9, pp. 69–82. [Google Scholar] [CrossRef]
- Lara-Márquez, M.; Báez-Magaña, M.; Raymundo-Ramos, C.; Spagnuolo, P.; Macías-Rodríguez, L.; Salgado-Garciglia, R.; Ochoa-Zarzosa, A.; López-Meza, J.E. Lipid-rich extract from Mexican avocado (Persea americana var. drymifolia) induces apoptosis and modulates the inflammatory response in Caco-2 human colon cancer cells. J. Funct. Foods 2020, 64, 103658. [Google Scholar] [CrossRef]
- Barone, R.; Caruso Bavisotto, C.; Rappa, F.; Gargano, M.L.; Macaluso, F.; Paladino, L.; Vitale, A.M.; Alfano, S.; Campanella, C.; Gorska, M.; et al. JNK pathway and heat shock response mediate the survival of C26 colon carcinoma bearing mice fed with the mushroom Pleurotus eryngii var. eryngii without affecting tumor growth or cachexia. Food Funct. 2021, 12, 3083–3095. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Quiles, J.L.; Battino, M. The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 2: Induction of oxidative stress, alteration of mitochondrial respiration and glycolysis, and suppression of metastatic ability. Food Funct. 2018, 9, 2158–2170. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Hegde, S.K.; Rao, P.; Dinkar, C.; Thilakchand, K.R.; George, T.; Baliga-Rao, M.P.; Palatty, P.L.; Baliga, M.S. Honey Mitigates Radiation-Induced Oral Mucositis in Head and Neck Cancer Patients without Affecting the Tumor Response. Foods 2017, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Kobya Bulut, H.; Güdücü Tüfekci, F. Honey prevents oral mocositis in children undergoing chemotherapy: A quasi-experimental study with a control group. Complement. Ther. Med. 2016, 29, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Li, D.D.; Duan, J.Y.; Sheng, L.M.; Wang, X. Resistance to targeted therapy in metastatic colorectal cancer: Current status and new developments. World J. Gastroenterol. 2023, 29, 926–948. [Google Scholar] [CrossRef] [PubMed]
- Noor, F.; Tahir ul Qamar, M.; Ashfaq, U.A.; Albutti, A.; Alwashmi, A.S.S.; Aljasir, M.A. Network Pharmacology Approach for Medicinal Plants: Review and Assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef] [PubMed]
- Nogales, C.; Mamdouh, Z.M.; List, M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H.H.H.W. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhu, P.; Wu, D.; Deng, W. A Network Pharmacology Prediction and Molecular Docking-Based Strategy to Explore the Potential Pharmacological Mechanism of Astragalus membranaceus for Glioma. Int. J. Mol. Sci. 2023, 24, 16306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chai, X.; Zhao, F.; Hou, G.; Meng, Q. Food Applications and Potential Health Benefits of Hawthorn. Foods 2022, 11, 2861. [Google Scholar] [CrossRef]
- Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Mousavi Khaneghah, A.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M. Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications. Foods 2020, 9, 436. [Google Scholar] [CrossRef]
- Nam, S.-H. Antimicrobial Activity of Crataegi fructus Extract Used for Potential Application in the Prevention and Treatment of Oral Diseases. Medicina 2024, 60, 13. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Vermeer, M.A.; Trautwein, E.A. Triterpenic Acids Present in Hawthorn Lower Plasma Cholesterol by Inhibiting Intestinal ACAT Activity in Hamsters. Evid. Based Complement. Altern. Med. 2011, 2011, 801272. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yang, Q.; Li, C.; Zheng, J.; Wang, Y.; Duan, B. Preparation, structural characterization, bioactivities, and applications of Crataegus spp. polysaccharides: A review. Int. J. Biol. Macromol. 2023, 253, 126671. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Shang, X.-Y.; Lv, T.-M.; Yao, G.-D.; Lin, B.; Wang, X.-B.; Huang, X.-X.; Song, S.-J. Phenylpropanoid derivatives from the fruit of Crataegus pinnatifida Bunge and their distinctive effects on human hepatoma cells. Phytochemistry 2019, 164, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xu, G.B.; Tang, X.; Zhang, C.; Zhao, W.; Wang, J.; Chen, H. Anti-cancer potential of polysaccharide extracted from hawthorn (Crataegus.) on human colon cancer cell line HCT116 via cell cycle arrest and apoptosis. J. Funct. Foods 2020, 64, 103677. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, C.; Lu, Y.; Yang, S.; Fang, J.; Jiang, Z. Acetylated xylo-oligosaccharide from Hawthorn kernels inhibits colon cancer cells in vitro and in vivo. J. Funct. Foods 2023, 102, 105436. [Google Scholar] [CrossRef]
- Sun, Y.S.; Wang, Z.-W.; Gao, Z.; Zhao, W.; Thakur, K.; Zhou, Q.; Wei, Z.-J. Proanthocyanidin oligomers extract from hawthorn mediates cell cycle arrest, apoptosis, and lysosome vacuolation on HCT116 cells. Curr. Res. Food Sci. 2022, 5, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhao, W.; Zhou, Q.; Chen, H.; Yuan, J.; Zhang, X.; Zhang, Z. Procyanidins from hawthorn (Crataegus pinnatifida) alleviate lipid metabolism disorder via inhibiting insulin resistance and oxidative stress, normalizing the gut microbiota structure and intestinal barrier, and further suppressing hepatic inflammation and lipid accumulation. Food Funct. 2022, 13, 7901–7917. [Google Scholar] [CrossRef]
- Sun, K.; He, S.-B.; Yao, Y.-Z.; Qu, J.-G.; Xie, R.; Ma, Y.-Q.; Zong, M.-H.; Chen, J.-X. Tre2 (USP6NL) promotes colorectal cancer cell proliferation via Wnt/β-catenin pathway. Cancer Cell Int. 2019, 19, 102. [Google Scholar] [CrossRef]
- Zhu, M.; Gong, Z.; Wu, Q.; Shi, X.; Su, Q.; Zhang, Y. Sanguinarine suppresses migration and metastasis in colorectal carcinoma associated with the inversion of EMT through the Wnt/β-catenin signaling. Clin. Transl. Med. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Han, S.; Wang, D.; Huang, Y.; Zeng, Z.; Xu, P.; Xiong, H.; Ke, Z.; Zhang, Y.; Hu, Y.; Wang, F. A reciprocal feedback between colon cancer cells and Schwann cells promotes the proliferation and metastasis of colon cancer. J. Exp. Clin. Cancer Res. 2022, 41, 348. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, Q.; Gao, Z.; Xu, G.B.; Chen, H.; Chitrakar, B.; Sun, Y.; Zhao, W.; Lin, X.; Zhou, K.; et al. Characterization of procyanidin extracts from hawthorn (Crataegus pinnatifida) in human colorectal adenocarcinoma cell line Caco-2, simulated Digestion, and fermentation identified unique and novel prebiotic properties. Food Res. Int. 2023, 165, 112393. [Google Scholar] [CrossRef]
- Park, M.; Park, S.-Y.; Lee, H.-J.; Kim, C.-E. A Systems-Level Analysis of Mechanisms of Platycodon grandiflorum Based on A Network Pharmacological Approach. Molecules 2018, 23, 2841. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Kim, Y.; Kim, H.H.; Jeong, S.; Ahn, D.; Chung, S.J.; Kim, H. Network pharmacology and molecular docking approaches to elucidate the potential compounds and targets of Saeng-Ji-Hwang-Ko for treatment of type 2 diabetes mellitus. Comput. Biol. Med. 2022, 149, 106041. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Tan, Z.; Zhang, W.; Li, Q.; Jiang, Z.; Shen, S.; Lou, S.; Chen, X. Exogenous melatonin improves the chilling tolerance and preharvest fruit shelf life in eggplant by affecting ROS- and senescence-related processes. Hortic. Plant J. 2023, 9, 523–540. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, J.; Chen, B.; Wang, Y.; Yang, S.; Wu, K.; Meng, X. The Role of MMP-9 and MMP-9 Inhibition in Different Types of Thyroid Carcinoma. Molecules 2023, 28, 3705. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wu, S.; Hu, Q.; Wu, H.; Wei, S.; Xie, H.; Sun, K.; Li, X.; Fang, L. Agkihpin, a novel SVAE may inhibit the migration and invasion of liver cancer cells associated with the inversion of EMT induced by Wnt/β-catenin signaling inhibition. Biochem. Biophys. Res. Commun. 2016, 479, 283–289. [Google Scholar] [CrossRef]
- Ma, Y.-S.; Li, W.; Liu, Y.; Shi, Y.; Lin, Q.-L.; Fu, D. Targeting Colorectal Cancer Stem Cells as an Effective Treatment for Colorectal Cancer. Technol. Cancer Res. Treat. 2020, 19, 1533033819892261. [Google Scholar] [CrossRef]
- Li, X.; Cao, D.; Sun, S.; Wang, Y. Anticancer therapeutic effect of ginsenosides through mediating reactive oxygen species. Front. Pharmacol. 2023, 14, 1215020. [Google Scholar] [CrossRef]
- García-Beltrán, J.M.; Mansour, A.T.; Alsaqufi, A.S.; Ali, H.M.; Esteban, M.A. Effects of aqueous and ethanolic leaf extracts from drumstick tree (Moringa oleifera) on gilthead seabream (Sparus aurata L.) leucocytes, and their cytotoxic, antitumor, bactericidal and antioxidant activities. Fish. Shellfish. Immunol. 2020, 106, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.-L.; Chang, C.-H. Oridonin enhances cytotoxic activity of natural killer cells against lung cancer. Int. Immunopharmacol. 2023, 122, 110669. [Google Scholar] [CrossRef]
- Hamad, G.M.; Taha, T.H.; Alshehri, A.; El-Deeb, N.M. Myrrh as a Functional Food with Therapeutic Properties Against Colon Cancer in Traditional Meals. J. Food Process. Preserv. 2017, 41, e12963. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef]
- Yang, P.; Chai, Y.; Wei, M.; Ge, Y.; Xu, F. Mechanism of salidroside in the treatment of endometrial cancer based on network pharmacology and molecular docking. Sci. Rep. 2023, 13, 14114. [Google Scholar] [CrossRef]
- Luo, Y.; Feng, Y.; Song, L.; He, G.-Q.; Li, S.; Bai, S.-S.; Huang, Y.-J.; Li, S.-Y.; Almutairi, M.M.; Shi, H.-L.; et al. A network pharmacology-based study on the anti-hepatoma effect of Radix Salviae Miltiorrhizae. Chin. Med. 2019, 14, 27. [Google Scholar] [CrossRef]
- Damodharan, U.; Ganesan, R.; Radhakrishnan, U.C. Expression of MMP2 and MMP9 (Gelatinases A and B) in Human Colon Cancer Cells. Appl. Biochem. Biotechnol. 2011, 165, 1245–1252. [Google Scholar] [CrossRef]
- Buttacavoli, M.; Di Cara, G.; Roz, E.; Pucci-Minafra, I.; Feo, S.; Cancemi, P. Integrated Multi-Omics Investigations of Metalloproteinases in Colon Cancer: Focus on MMP2 and MMP9. Int. J. Mol. Sci. 2021, 22, 12389. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.; Robinson, A.E.; Engler, J.R.; Petritsch, C.; James, C.D.; Phillips, J.J. Proteoglycans and their roles in brain cancer. FEBS J. 2013, 280, 2399–2417. [Google Scholar] [CrossRef]
- Chen, K.; Yong, J.; Zauner, R.; Wally, V.; Whitelock, J.; Sajinovic, M.; Kopecki, Z.; Liang, K.; Scott, K.F.; Mellick, A.S. Chondroitin Sulfate Proteoglycan 4 as a Marker for Aggressive Squamous Cell Carcinoma. Cancers 2022, 14, 5564. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, H.T.; Xu, S.J.; Xia, W.L.; Zhao, Y.; Zhao, X.H.; Zhu, W.B.; Li, F.T.; Li, H.L. Proteoglycan-4 predicts good prognosis in patients with hepatocellular carcinoma receiving transcatheter arterial chemoembolization and inhibits cancer cell migration in vitro. Front. Oncol. 2022, 12, 1023801. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.-L.; Chang, Y.-L.; Liu, H.-Y.; Wu, Y.-T.; Sung, M.-T.; Su, Y.-L.; Huang, C.-C.; Wang, P.-C.; Peng, J.-M. VCAN Hypomethylation and Expression as Predictive Biomarkers of Drug Sensitivity in Upper Urinary Tract Urothelial Carcinoma. Int. J. Mol. Sci. 2023, 24, 7486. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Yao, Y.; Shen, B.; Liu, J.; Pan, Q.; Liu, N.; Li, L.; Huang, J.; Long, Z.; Shao, L. Columbamine suppresses the proliferation and malignization of colon cancer cells via abolishing Wnt/β-catenin signaling pathway. Cancer Manag. Res. 2019, 11, 8635–8645. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.-E.; Kim, H.-J.; Song, I.-S.; Park, C.; Jung, J.W.; Park, D.-S.; Oh, S.-H.; Kim, Y.-S.; Kim, H.-R. Salinomycin suppresses TGF-β1-induced EMT by down-regulating MMP-2 and MMP-9 via the AMPK/SIRT1 pathway in non-small cell lung cancer. Int. J. Med. Sci. 2021, 18, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Lin, J.; Su, J.; Oyang, L.; Wang, H.; Tan, S.; Tang, Y.; Chen, X.; Liu, W.; Luo, X.; et al. Diallyl disulfide inhibits colon cancer metastasis by suppressing Rac1-mediated epithelial-mesenchymal transition. Onco Targets Ther. 2019, 12, 5713–5728. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, M.; Bastias, L.; Montenegro, I.; Werner, E.; Madrid, A.; Godoy, P.; Párraga, M.; Villena, J. Autumn Royal and Ribier Grape Juice Extracts Reduced Viability and Metastatic Potential of Colon Cancer Cells. Evid. Based Complement. Altern. Med. 2018, 2018, 2517080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Liu, H.; Wang, D.; Wang, J.; Liu, M.; Yang, Y.; Zhong, S. A natural selenium polysaccharide from Pleurotus ostreatus: Structural elucidation, anti-gastric cancer and anti-colon cancer activity in vitro. Int. J. Biol. Macromol. 2022, 201, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Network, Cancer Genome Atlas. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, A.; Zhao, X.; Liu, Y.; Liu, S.; Wang, D. Anti-colon cancer activity of water-soluble polysaccharides extracted from Gloeostereum incarnatum via Wnt/β-catenin signaling pathway. Food Sci. Hum. Wellness 2021, 10, 460–470. [Google Scholar] [CrossRef]
- Brown, M.A.; Ried, T. Shifting the Focus of Signaling Abnormalities in Colon Cancer. Cancers 2022, 14, 784. [Google Scholar] [CrossRef]
- Alfiya, D.; Lisa, D.; Jingang, H.; Clara, D.; Neng-Yu, L.; Katrin, P.-Z.; Christian, B.; Alexander, W.; Oliver, D.; Georg, S.; et al. Inactivation of tankyrases reduces experimental fibrosis by inhibiting canonical Wnt signalling. Ann. Rheum. Dis. 2013, 72, 1575. [Google Scholar] [CrossRef]
- Gao, P.; Yang, J.; Gao, X.; Xu, D.; Niu, D.; Li, J.; Wen, Q. Salvianolic acid B improves bone marrow-derived mesenchymal stem cell differentiation into alveolar epithelial cells type I via Wnt signaling. Mol. Med. Rep. 2015, 12, 1971–1976. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward (5′-3′) | Reverse (3′-5′) |
|---|---|---|
| Wnt 1 | CGATGGTGGGGTATTGTGAAC | CCGGATTTTGGCGTATCAGAC |
| Slug | CGAACTGGACACACATACAGTG | CTGAGGATCTCTGGTTGTGGT |
| N-cadherin | TCAGGCGTCTGTAGAGGCTT | ATGCACATCCTTCGATAAGACTG |
| c-Myc | GGAGGCTATTCTGCCCATTTG | CGAGGTCATAGTTCCTGTTGGTG |
| Vimentin | AACCTGGCCGAGGACATCA | TCAAGGTCAAGACGTGCCAGA |
| β-catenin | CAACTAAACAGGAAGGGATGGA | CTATACCACCCACTTGGCAGAC |
| GAPDH | GTCAACGGATTTGGTCGTATTG | CTCCTGGAAGATGGTGATGGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Sun, Y.; Wu, M.; Zhou, L.; Zheng, Y.; Ren, T.; Li, M.; Zhao, W. Hawthorn Proanthocyanidin Extract Inhibits Colorectal Carcinoma Metastasis by Targeting the Epithelial-Mesenchymal Transition Process and Wnt/β-Catenin Signaling Pathway. Foods 2024, 13, 1171. https://doi.org/10.3390/foods13081171
Wang Z, Sun Y, Wu M, Zhou L, Zheng Y, Ren T, Li M, Zhao W. Hawthorn Proanthocyanidin Extract Inhibits Colorectal Carcinoma Metastasis by Targeting the Epithelial-Mesenchymal Transition Process and Wnt/β-Catenin Signaling Pathway. Foods. 2024; 13(8):1171. https://doi.org/10.3390/foods13081171
Chicago/Turabian StyleWang, Ziwei, Yasai Sun, Mengying Wu, Liangfu Zhou, Yu Zheng, Ting Ren, Meijiao Li, and Wen Zhao. 2024. "Hawthorn Proanthocyanidin Extract Inhibits Colorectal Carcinoma Metastasis by Targeting the Epithelial-Mesenchymal Transition Process and Wnt/β-Catenin Signaling Pathway" Foods 13, no. 8: 1171. https://doi.org/10.3390/foods13081171




