Near-Infrared Spectroscopy and Chemometrics for Effective Online Quality Monitoring and Process Control during Pelagic Fishmeal and Oil Processing
Abstract
:1. Introduction
2. Materials and Methods
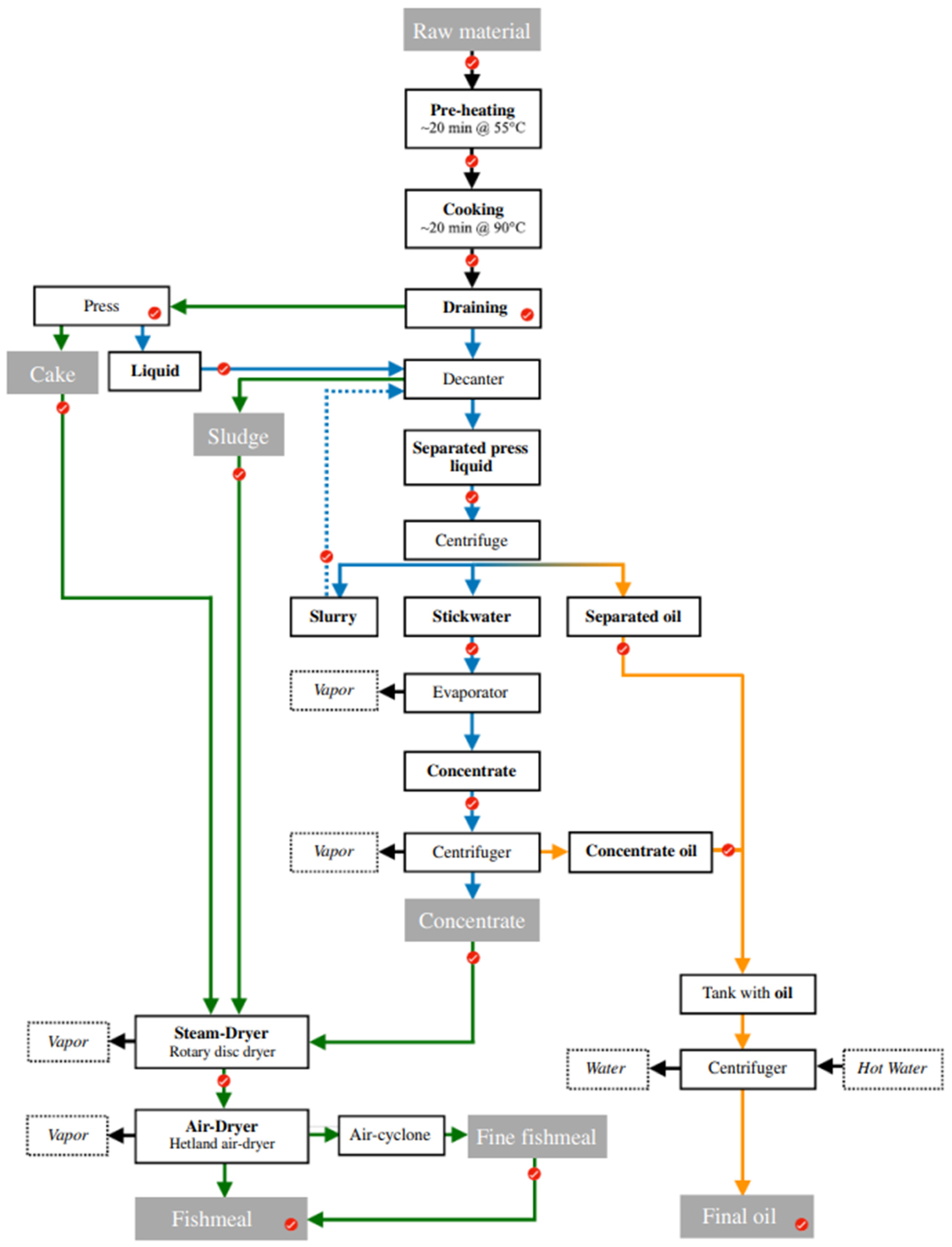
2.1. Raw Materials and Process Description
2.2. Near-Infrared Spectroscopy
2.3. Chemical Reference Measurements
2.4. NIR PCA and PLSR Prediction Models
3. Results and Discussion
3.1. Chemical Changes during Fishmeal and Oil Processing
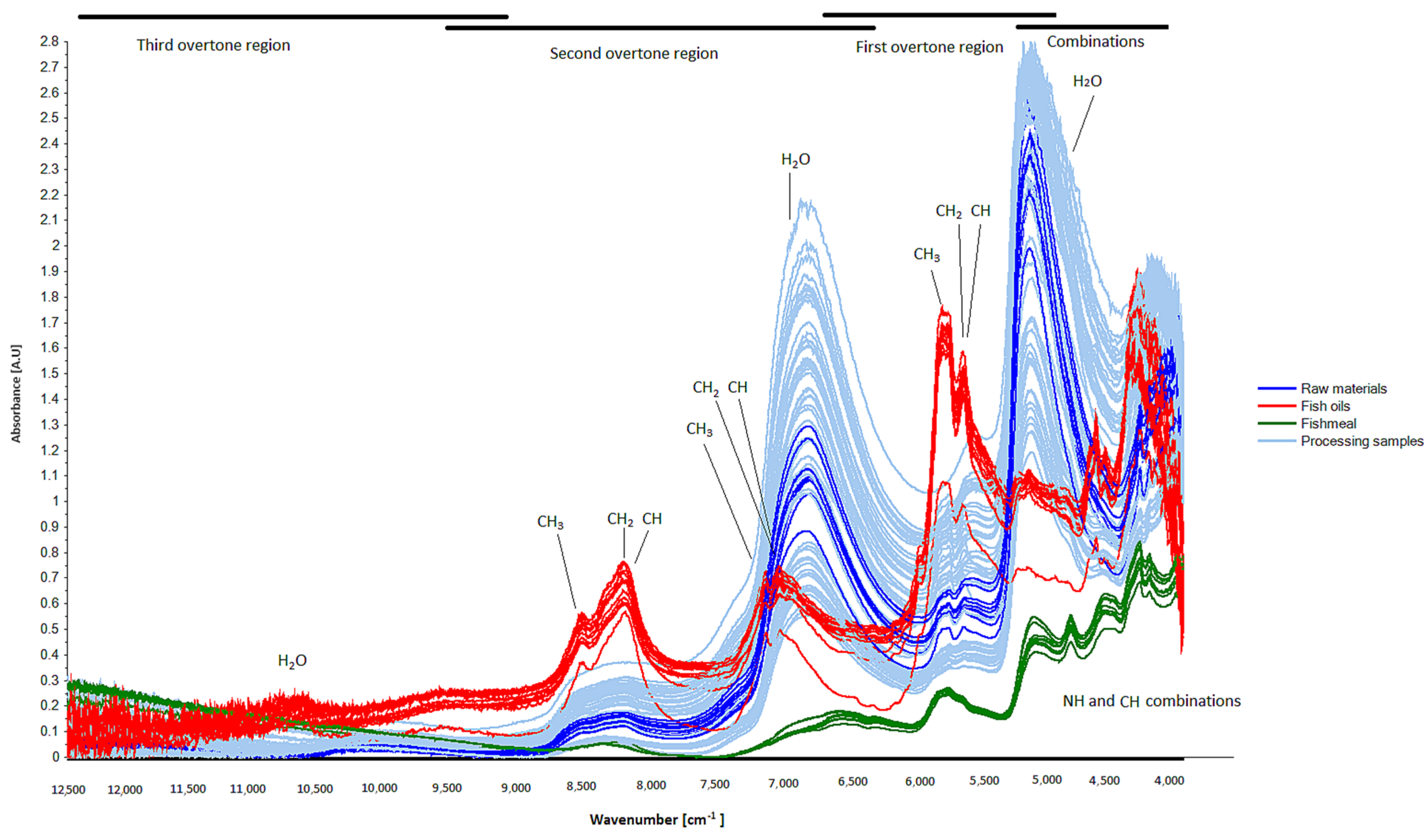
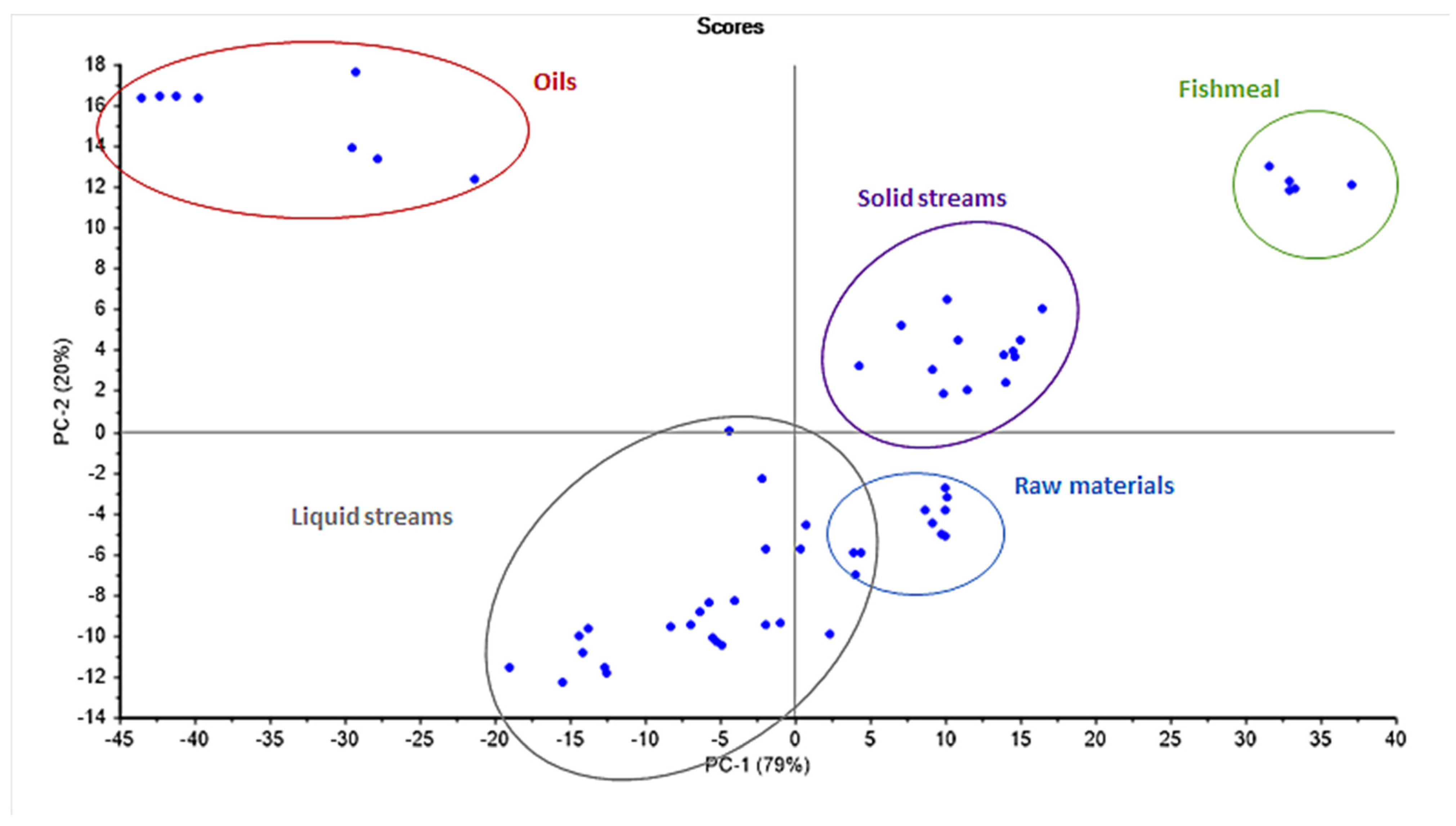
3.2. NIR Spectra and Principal Component Analysis
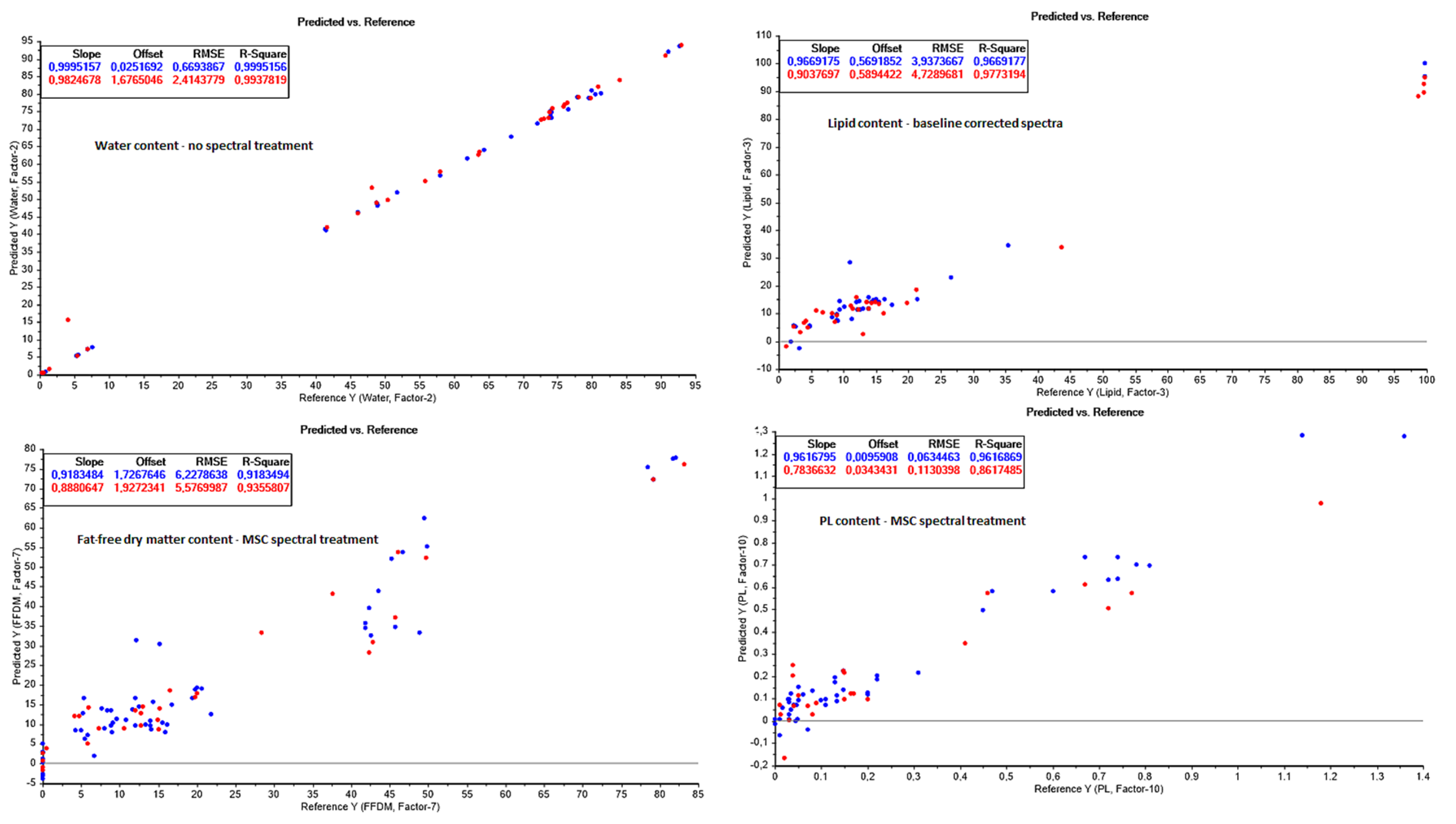
3.3. PLS Quality Prediction Models
3.3.1. Water Content Prediction
3.3.2. Total Lipid Content Prediction
3.3.3. Fat-Free Dry Matter (FFDM) Prediction
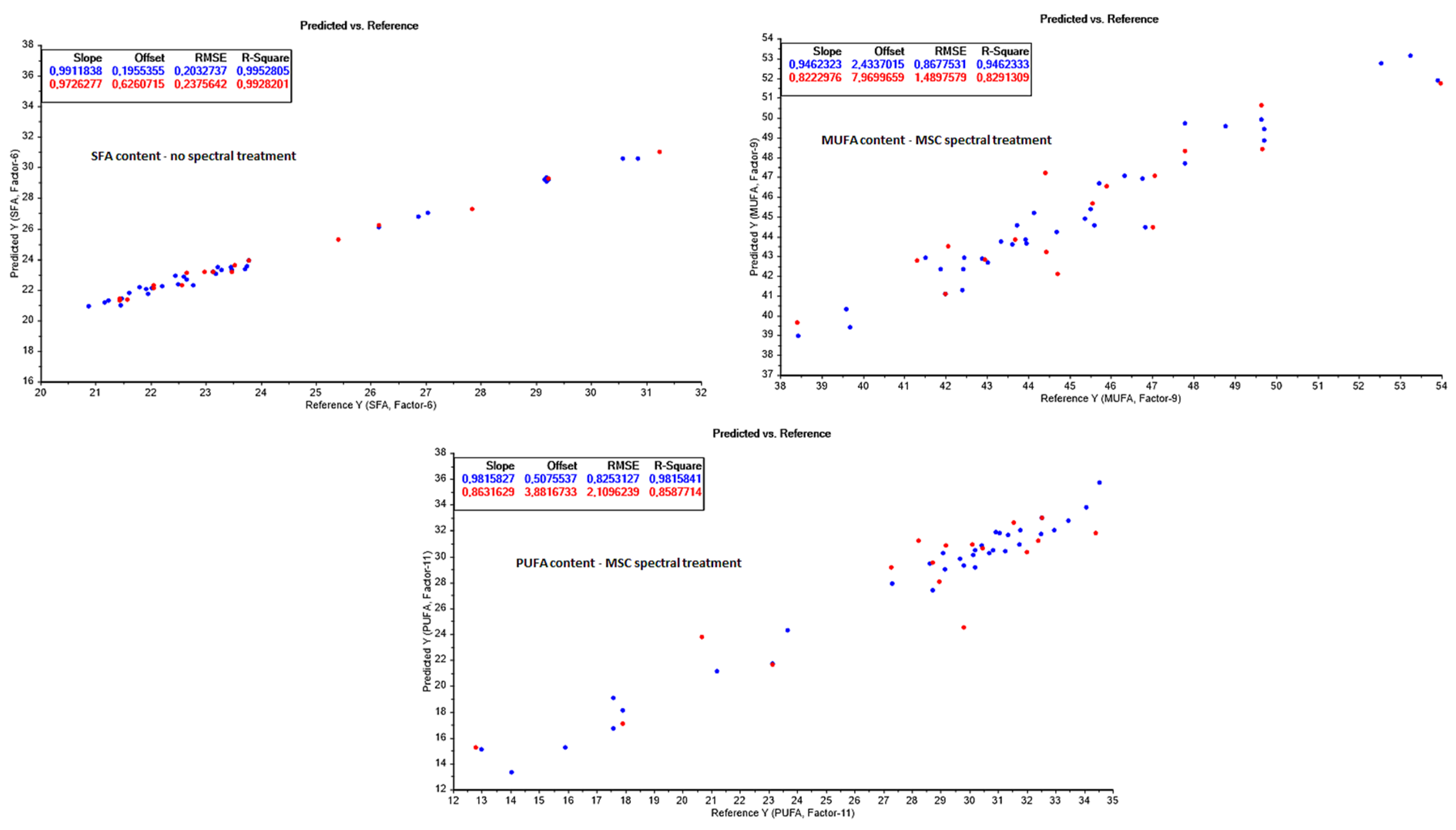
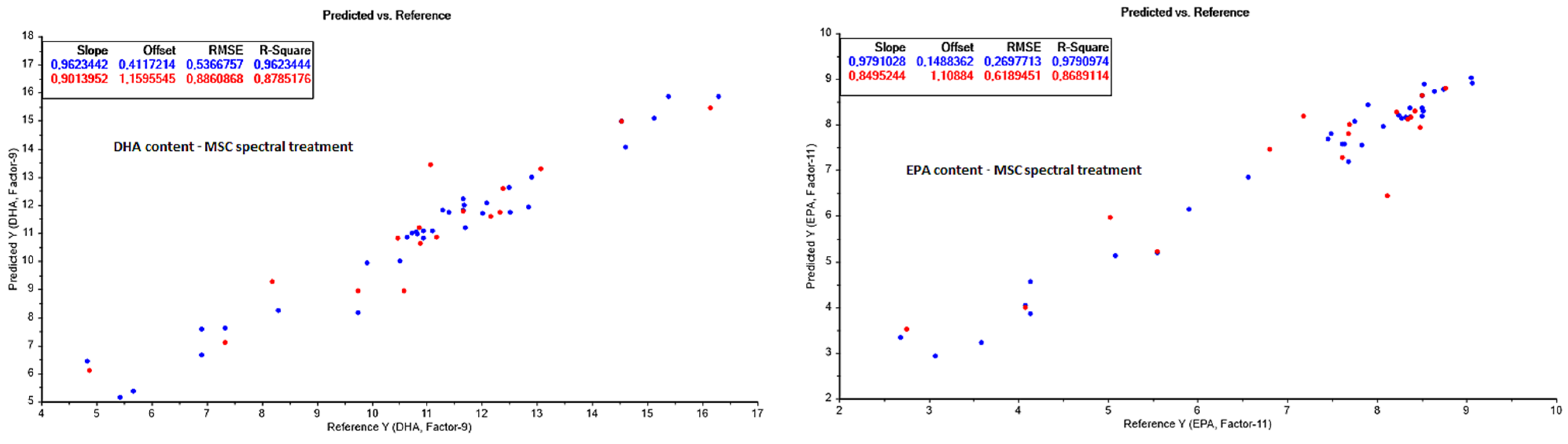
3.3.4. Fatty Acid Composition (FAC) Prediction
3.3.5. Free Fatty Acids (FFA) and Phospholipids (PL) Prediction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Assignment of NIR Overtones and Combination Bands
| Approximate Peak Maximum Wave Numbers (cm−1) | Functional Group Assignment | Dominant Chemical Component |
|---|---|---|
| 10,600 | O-H stretch 2nd overtone | Water |
| 8600–8150 | C-H 2nd overtone | Lipids |
| 7400–7000 | 2nd overtone of C-H combinations | Lipids |
| 6800 | O-H 1st overtone | Water |
| 5750 | C-H 1st overtone of CH3 | Lipids |
| 5500 | C-H 1st overtone of -HC=CH- | Lipids |
| 5200–5000 | O-H stretch 1st overtone of O-H combinations | Water |
| 5100–4500 | N-H combination peptide absorption bands of amide groups | Proteins |
| 4370 | C-H+C-H combination bands -CH2, -CH3 | Lipids |
| 4350–4150 | C-H combination bands C=C stretch, -CH, -CH2 | Lipids |
Appendix B. NIR Prediction Models
| Spectral Data Pre-Treatment | Valid Range of Reference Parameter | Calibration | RMSEC | Cross Validation with Independent Test Set | ||||
|---|---|---|---|---|---|---|---|---|
| n | Optimal LV | n | RMSECV | |||||
| Water (g/100 g sample) | 0.3–93.0 | |||||||
| None | 60 | 2 | 0.9995 | 0.67 | 30 | 0.9938 | 2.41 | |
| Baseline correction | 60 | 3 | 0.9666 | 5.56 | 30 | 0.9631 | 5.88 | |
| 1st derivative | 60 | 3 | 0.9404 | 7.42 | 30 | 0.9348 | 7.82 | |
| 2nd derivative | 60 | 5 | 0.9793 | 4.38 | 30 | 0.8142 | 13.20 | |
| MSC | 60 | 3 | 0.9498 | 6.81 | 30 | 0.9477 | 7.00 | |
| Lipids (g/100 g sample) | 0–100 | |||||||
| None | 60 | 4 | 0.9640 | 4.11 | 30 | 0.9749 | 4.97 | |
| Baseline correction | 60 | 3 | 0.9669 | 3.94 | 30 | 0.9773 | 3.94 | |
| 1st derivative | 60 | 2 | 0.9265 | 5.87 | 30 | 0.9404 | 7.66 | |
| 2nd derivative | 60 | 3 | 0.9828 | 2.84 | 30 | 0.5314 | 21.49 | |
| MSC | 60 | 3 | 0.9681 | 3.86 | 30 | 0.9592 | 6.34 | |
| FFDM (g/100 g sample) | 0–84.4 | |||||||
| None | 60 | 4 | 0.8899 | 8.36 | 30 | 0.9063 | 7.01 | |
| Baseline correction | 60 | 4 | 0.8473 | 8.52 | 30 | 0.8969 | 7.06 | |
| 1st derivative | 60 | 5 | 0.6743 | 12.44 | 30 | 0.8590 | 8.25 | |
| 2nd derivative | 60 | 4 | 0.8570 | 8.24 | 30 | 0.8003 | 9.82 | |
| MSC | 60 | 7 | 0.9183 | 6.23 | 30 | 0.9356 | 5.58 | |
| FFA (g/100 g sample) | 0–5 | |||||||
| None | 52 | 1 | 0.0911 | 0.91 | 25 | 0.0242 | 0.99 | |
| MSC | 52 | 2 | 0.0785 | 0.92 | 25 | 0.0583 | 0.98 | |
| PL (g/100 g sample) | 0–1.4 | |||||||
| None | 44 | 6 | 0.4476 | 0.24 | 22 | 0.4841 | 0.22 | |
| Baseline correction | 44 | 8 | 0.6506 | 0.19 | 22 | 0.5453 | 0.20 | |
| 1st derivative | 44 | 9 | 0.8888 | 0.11 | 22 | 0.6033 | 0.19 | |
| 2nd derivative | 44 | 1 | 0.3427 | 0.26 | 22 | 0.3055 | 0.25 | |
| MSC | 44 | 10 | 0.9617 | 0.06 | 22 | 0.8617 | 0.11 | |
| SFA (g/100 g lipids) | 20–32 | |||||||
| None | 34 | 6 | 0.9953 | 0.20 | 17 | 0.9928 | 0.24 | |
| Baseline correction | 34 | 9 | 0.9464 | 0.68 | 17 | 0.6922 | 1.56 | |
| 1st derivative | 34 | 15 | 0.9989 | 0.10 | 17 | 0.6668 | 1.62 | |
| 2nd derivative | 34 | 1 | 0.4147 | 2.26 | 17 | 0.3149 | 2.32 | |
| MSC | 34 | 12 | 0.9889 | 0.31 | 17 | 0.8363 | 1.13 | |
| MUFA (g/100 g lipids) | 38–54 | |||||||
| None | 34 | 9 | 0.9062 | 1.15 | 17 | 0.7968 | 1.62 | |
| Baseline correction | 34 | 5 | 0.6815 | 2.11 | 17 | 0.6200 | 2.22 | |
| 1st derivative | 34 | 4 | 0.3882 | 2.93 | 17 | 0.4230 | 2.74 | |
| 2nd derivative | 34 | 1 | 0.2863 | 3.16 | 17 | 0.2532 | 3.11 | |
| MSC | 34 | 9 | 0.9462 | 0.87 | 17 | 0.8291 | 1.49 | |
| PUFA (g/100 g lipids) | 12–36 | |||||||
| None | 34 | 9 | 0.9371 | 1.53 | 17 | 0.8461 | 2.20 | |
| Baseline correction | 34 | 6 | 0.7060 | 3.30 | 17 | 0.6051 | 3.53 | |
| 1st derivative | 34 | 4 | 0.4791 | 4.39 | 17 | 0.4924 | 4.00 | |
| 2nd derivative | 34 | 1 | 0.3196 | 5.02 | 17 | 0.3087 | 4.67 | |
| MSC | 34 | 11 | 0.9816 | 0.83 | 17 | 0.8588 | 2.11 | |
| DHA (g/100 g lipids) | 4–17 | |||||||
| None | 34 | 8 | 0.9073 | 0.84 | 17 | 0.8146 | 1.09 | |
| Baseline correction | 34 | 6 | 0.7320 | 1.43 | 17 | 0.6302 | 1.55 | |
| 1st derivative | 34 | 4 | 0.5864 | 1.78 | 17 | 0.6197 | 1.57 | |
| 2nd derivative | 34 | 14 | 0.9982 | 0.12 | 17 | 0.3380 | 2.07 | |
| MSC | 34 | 9 | 0.9623 | 0.54 | 17 | 0.8785 | 0.89 | |
| EPA (g/100 g lipids) | 2–10 | |||||||
| None | 34 | 10 | 0.9536 | 0.40 | 17 | 0.8278 | 0.71 | |
| Baseline correction | 34 | 4 | 0.5110 | 1.30 | 17 | 0.6366 | 1.03 | |
| 1st derivative | 34 | 4 | 0.5146 | 1.30 | 17 | 0.5077 | 1.20 | |
| 2nd derivative | 34 | 1 | 0.3437 | 1.51 | 17 | 0.3335 | 1.40 | |
| MSC | 34 | 11 | 0.9791 | 0.27 | 17 | 0.8689 | 0.62 | |
References
- Hilmarsdóttir, G.S.; Ogmundarson, Ó.; Arason, S.; Gudjónsdóttir, M. Efficiency of fishmeal and fish oil processing of different pelagic fish species: Identification of processing steps for potential optimization toward protein production for human consumption. J. Food Proc. Pres. 2021, 45, e15294. [Google Scholar] [CrossRef]
- Hall, G.M. Fishmeal production and sustainability. In Fish Processing: Sustainability and New Opportunities; Hall, G.M., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 207–235. [Google Scholar]
- Oterhals, Å.; Vogt, G. Impact of extraction, refining and concentration stages on the stability of fish oil. In Food Enrichment with Omega-3 Fatty Acids; Jacobsen, C., Nielsen, N.S., Horn, A.F., Sørensen, A.-D.M., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 111–129. [Google Scholar]
- Cozzolino, D.; Chree, A.; Murray, I.; Scaife, J.R. The assessment of the composition of fishmeal by near infrared reflectance spectroscopy. Aquacult. Nutr. 2002, 8, 147–155. [Google Scholar] [CrossRef]
- Hilmarsdóttir, G.S.; Ogmundarson, Ó.; Arason, S.; Gudjónsdóttir, M. Identification of environmental hotspots in fishmeal and fish oil production for process optimization purposes. J. Clean. Prod. 2022, 343, 130880. [Google Scholar] [CrossRef]
- Romotowska, P.E.; Karlsdóttir, M.G.; Gudjónsdóttir, M.; Kristinsson, H.G.; Arason, S. Seasonal and geographical variation in chemical composition and lipid stability of Atlantic mackerel (Scomber scombrus) caught in Icelandic waters. J. Food Comp. Anal. 2016, 49, 9–18. [Google Scholar] [CrossRef]
- Cozzolino, D.; Chree, A.; Murray, I.; Scaife, J.R. Usefulness of near infrared spectroscopy to monitor the extent of heat treatment in fish meal. Int. J. Food Sci. Technol. 2009, 44, 1579–1584. [Google Scholar] [CrossRef]
- McClure, F.W. 204 years of near infrared technology: 1800–2003. J. Near Infrared Spectro 2003, 11, 487–518. [Google Scholar] [CrossRef]
- Stevik, A.M.; Duun, A.S.; Rustad, T.; O´Farrel, M.; Schulerud, H.; Ottestad, S. Ice fraction assessment by near infrared spectroscopy enhancing automated superchilling process lines. J. Food Eng. 2010, 100, 169–177. [Google Scholar] [CrossRef]
- Bøknæs, N.; Jensen, K.N.; Andersen, C.M.; Martens, H. Fresh assessment of thawed and chilled cod fillets packed in Modified atmosphere using near infrared spectroscopy. LWT–Food Sci. Technol. 2002, 35, 628–634. [Google Scholar] [CrossRef]
- Nilsen, H.; Esaiassen, M. Predicting sensory score of cod (Gadus morhua) from visible spectroscopy. LWT–Food Sci. Technol. 2005, 38, 95–99. [Google Scholar] [CrossRef]
- Ellekjær, M.R.; Isaksson, T.; Solheim, R. Assessment of sensory quality of meat sausages using near infrared spectroscopy. J. Food Sci. 2006, 59, 456–464. [Google Scholar] [CrossRef]
- Blanco, M.; Villaroya, I. NIR spectroscopy: A rapid-response analytical tool. Trends Anal. Chem. 2002, 21, 240–250. [Google Scholar] [CrossRef]
- Gomes, A.A.; Azcarate, S.M.; Dias Diniz, P.H.G.; de Sousa Fernandes, D.D.; Veras, G. Variable selection in the chemometric treatment of food data: A tutorial review. Food Chem. 2022, 370, 131072. [Google Scholar] [CrossRef] [PubMed]
- Rinnan, Å.; van den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Hilmarsdóttir, G.S.; Ogmundarson, Ó.; Arason, S.; Gudjónsdóttir, M. The effects of varying heat treatments on lipid composition during pelagic fishmeal production. Processes 2020, 8, 1142. [Google Scholar] [CrossRef]
- ISO 6496; Animal Feeding Stuffs—Determination of Moisture and Other Volatile Matter Content. International Organization for Standardization: Geneva, Switzerland, 1999. Available online: https://www.iso.org/obp/ui/#iso:std:iso:6496:ed-2:v1:en (accessed on 10 March 2020).
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lowry, R.R.; Tinsley, I.J. Rapid colorimetric determination of free fatty acids. J. Am. Oil Chem. Soc. 1976, 53, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Bernárdez, M.; Pastoriza, L.; Sampedro, G.; Herrera, J.J.R.; Cabo, M.L. Modified method for the analysis of free fatty acids in fish. J. Agric. Food Chem. 2005, 53, 1903–1906. [Google Scholar] [CrossRef]
- Stewart, J.C.M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef]
- AOCS. Official Method and Recommended Practices of the AOC, 7th ed.; AOCS Official Method Ce 1b-89; AOCS: Champaign, IL, USA, 1998. [Google Scholar]
- Fournier, V.; Destaillats, F.; Juanéda, P.; Dionisi, F.; Lambelet, P.; Sébédio, J.-L.; Berdeaux, O. Thermal degradation of long-chain polyunsaturated fatty acids during deodorization of fish oil. Eur. J. Lipid Sci. Technol. 2006, 108, 33–42. [Google Scholar] [CrossRef]
- Larsen, R.; Eilertsen, K.-E.; Elvevoll, E.O. Health benefits of marine foods and ingredients. Biotech. Adv. 2011, 29, 508–518. [Google Scholar] [CrossRef]
- Zheng, J.-S.; Hu, X.-J.; Zhao, Y.-M.; Yang, J.; Li, D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: Meta-analysis of data from 21 independent prospective cohort studies. BMJ 2013, 346, f3706. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.O.; Kawamura, S.; Matsuo, M.; Kato, M.; Koseki, K. Combined analysis of near-infrared spectra, colour, and physicochemical information of brown rice to develop accurate calibration models for determining amylose content. Food Chem. 2019, 286, 297–306. [Google Scholar] [CrossRef] [PubMed]
- FAO. The Production of Fish Meal and Oil; FAO Fisheries Technical Paper—142; Aquaculture Feed and Fertilizer Resources Information System; FAO: Rome, Italy, 1986; ISBN 92-5-102464-2. [Google Scholar]
- Jacobsen, C. Some strategies for the stabilization of long chain n-3 PUFA-enriched foods: A review. Eur. J. Lipid Sci. Technol. 2015, 117, 1853–1866. [Google Scholar] [CrossRef]
- Dang, T.T.H.; Gudjónsdóttir, M.; Ren, D.; Karlsdóttir, M.G.; Nguyen, M.V.; Tómasson, T.; Arason, A. Effects of pre and postrigor freezing and temperature stability during frozen storage on physicochemical stability of Atlantic herring (Clupea harengus) muscle). J. Food Proc. Pres. 2018, 42, e13754. [Google Scholar] [CrossRef]
- Goldstein, E.D.; Helser, T.E.; Vollenweider, J.J.; Sreenivasan, A.; Sewall, F.F. Rapid and reliable assessment of fish physiological condition for fisheries research and management using Fourier Transform Near-infrared spectroscopy. Front. Mar. Sci. 2021, 8, 690934. [Google Scholar] [CrossRef]
- Watts, P.; Long, G.; Meek, M.E. Chloroform. In Concise International Chemical Assessment Document 58; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Windsor, M.L. Fish Protein Concentrate. FAO Fisheries and Aquaculture Department. Available online: http://www.fao.org/3/x5917e00.htm#Contents (accessed on 7 February 2020).
- Wold, J.P.; Isaksson, T. Non-destructive determination of fat and moisture in whole Atlantic salmon by near-infrared diffuse spectroscopy. J. Food Sci. 2006, 62, 734–736. [Google Scholar] [CrossRef]
- ISO 5983-2; Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content—Part 2: Block Digestion and Steam Distillation Method. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/52199.html (accessed on 5 January 2022).
- ISO 16634-1; Food products—Determination of the Total Nitrogen Content by Combustion According to the Dumas Principle and Calculation of the Crude Protein Content—Part 1: Oilseeds and Animal Feeding Stuffs. International Organization for Standardization: Geneva, Switzerland, 2008. Available online: https://www.iso.org/obp/ui/#iso:std:iso:16634:-1:ed-1:v1:en (accessed on 5 January 2022).
- Kristinsson, H.G. Aquatic food protein hydrolysates. In Maximising the Value of Marine By-Products; Shahidi, F., Ed.; Woodhead Publishing Series in Food Science; Technology and Nutrition: Sawston, UK, 2007; pp. 229–248. [Google Scholar]
- Azizian, H.; Kramer, J.K.G. A rapid method for the quantification of fatty acids in fats and oils with emphasis on trans fatty acids using fourier transform near infrared spectroscopy (FT-NIR). Lipids 2005, 40, 855–867. [Google Scholar] [CrossRef]
- Shiroma, C.; Rodriguez-Saona, L. Application of NIR and MIR spectroscopy in quality control of potato chips. J. Food Comp. Anal. 2009, 22, 596–605. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudjónsdóttir, M.; Hilmarsdóttir, G.S.; Ögmundarson, Ó.; Arason, S. Near-Infrared Spectroscopy and Chemometrics for Effective Online Quality Monitoring and Process Control during Pelagic Fishmeal and Oil Processing. Foods 2024, 13, 1186. https://doi.org/10.3390/foods13081186
Gudjónsdóttir M, Hilmarsdóttir GS, Ögmundarson Ó, Arason S. Near-Infrared Spectroscopy and Chemometrics for Effective Online Quality Monitoring and Process Control during Pelagic Fishmeal and Oil Processing. Foods. 2024; 13(8):1186. https://doi.org/10.3390/foods13081186
Chicago/Turabian StyleGudjónsdóttir, María, Gudrún Svana Hilmarsdóttir, Ólafur Ögmundarson, and Sigurjón Arason. 2024. "Near-Infrared Spectroscopy and Chemometrics for Effective Online Quality Monitoring and Process Control during Pelagic Fishmeal and Oil Processing" Foods 13, no. 8: 1186. https://doi.org/10.3390/foods13081186





