Evaluating How Different Drying Techniques Change the Structure and Physicochemical and Flavor Properties of Gastrodia elata
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Dried G. elata
2.3. Drying Characteristics
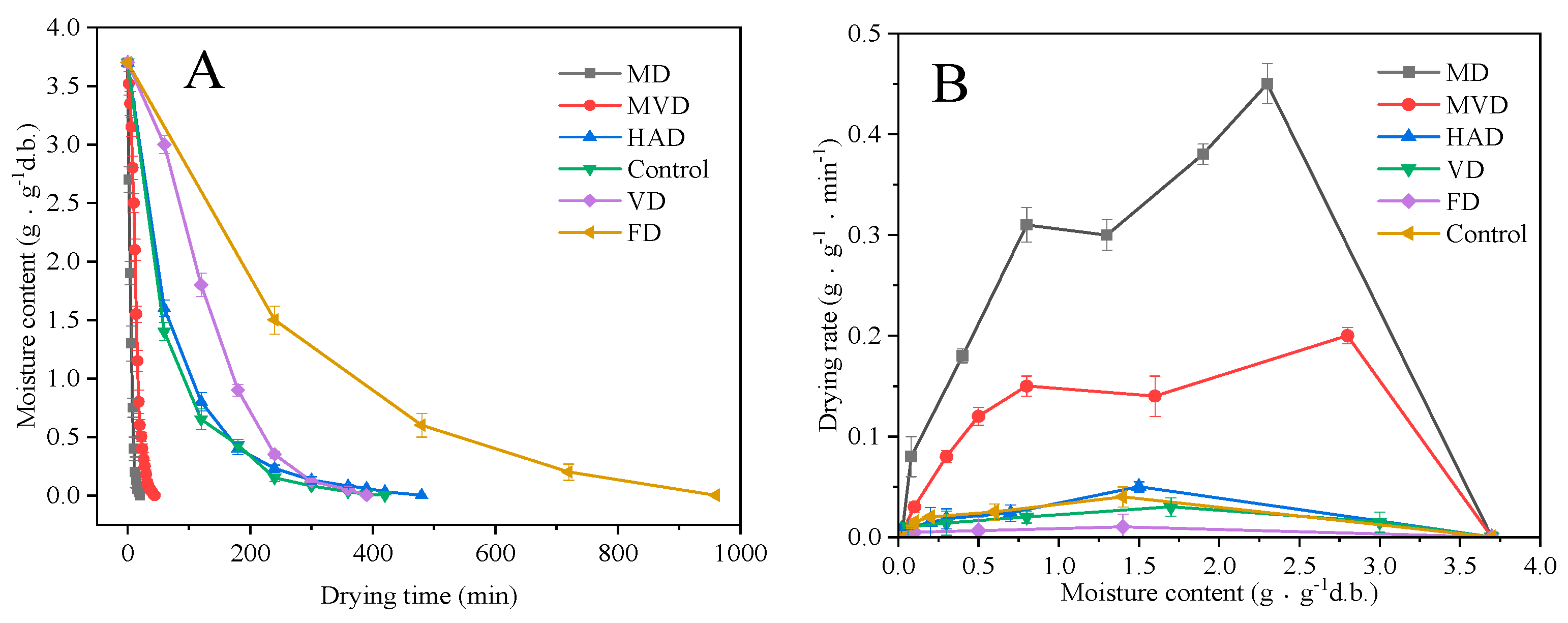
2.3.1. Drying Rate
2.3.2. Low-Field Nuclear Magnetic Resonance Measurement
2.4. Structural Characteristics
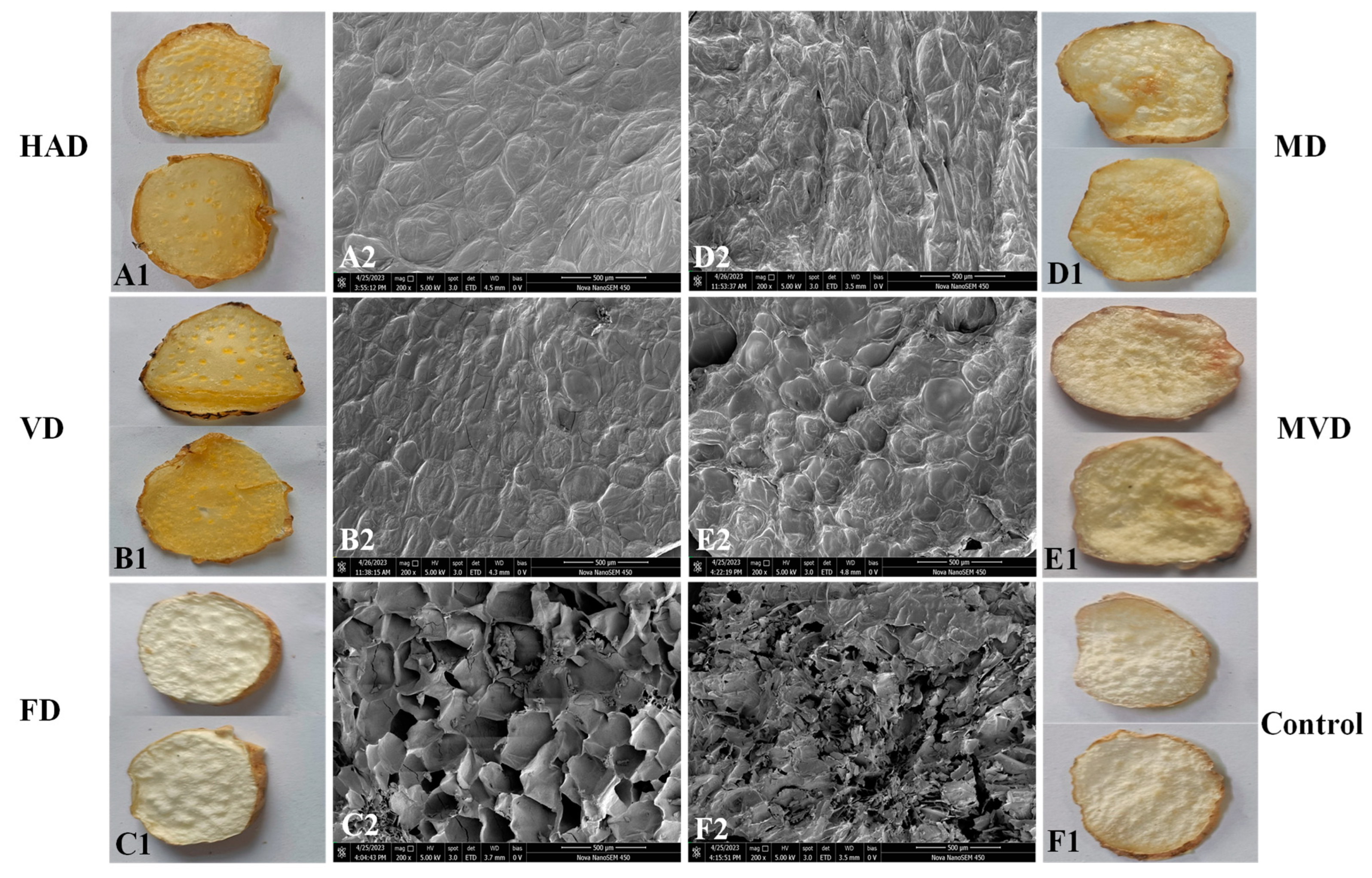
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Physical Characteristics
2.5.1. Color Analysis
2.5.2. Particle-Size Analysis
2.5.3. Water-Holding Capacity and Oil-Holding Capacity
2.6. Determination of Active Ingredients
2.7. Flavor Characteristics
2.7.1. Electronic Noise (E-nose) Analysis
2.7.2. GC-MS Analysis
2.8. Statistics Analysis
3. Results
3.1. Drying Characteristics
3.2. Effect of Drying on Water Status
3.3. Apparent Morphology and Microstructure
3.4. FT-IR Spectra
3.5. Color Analysis
3.6. Particle-Size Analysis
3.7. Water-Holding Capacity (WHC) and Oil-Holding Capacity (OHC)
3.8. Active Ingredients
3.9. E-Nose Analysis
3.10. GC-MS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.; Fan, S.; Shangguan, C.; Zhang, J. Evaluation of the Effects of Simulated in Vitro Digestion by Gastrodia Elata on Biological Activity and Gut Microflora Regulation. Food Biosci. 2022, 50, 102147. [Google Scholar] [CrossRef]
- Zhan, H.D.; Zhou, H.Y.; Sui, Y.P.; Du, X.L.; Wang, W.H.; Dai, L.; Sui, F.; Huo, H.R.; Jiang, T.L. The Rhizome of Gastrodia Elata Blume—An Ethnopharmacological Review. J. Ethnopharmacol. 2016, 189, 361–385. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.F.; Huang, C.L.; Lin, Y.L.; Lee, Y.C.; Yang, Y.C.; Huang, N.K. The Neuroprotective Effects of an Extract of Gastrodia Elata. J. Ethnopharmacol. 2011, 138, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sun, Y.; Weng, Y.; Matsuura, A.; Xiang, L.; Qi, J. Parishin from Gastrodia Elata Extends the Lifespan of Yeast via Regulation of Sir2/Uth1/TOR Signaling Pathway. Oxid. Med. Cell. Longev. 2016, 2016, 4074690. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.L.; Yu, B.; Li, Z.; Jiang, W.X.; Jiang, J.D.; Kong, W.J. Gastrodin Ameliorates Oxidative Stress and Proinflammatory Response in Nonalcoholic Fatty Liver Disease through the AMPK/Nrf2 Pathway. Phyther. Res. 2016, 30, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qian, S. Gastrodin Protects Neural Progenitor Cells Against Amyloid β (1–42)-Induced Neurotoxicity and Improves Hippocampal Neurogenesis in Amyloid β (1–42)-Injected Mice. J. Mol. Neurosci. 2016, 60, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Deng, Y.; Wang, Y.; Liu, Q.; An, J.; Li, M.; Song, N.; Zhang, J.; Cheng, L.; Ma, K. A Comparative Study on Ingredient and Efficiency Difference between Fresh and Steamed Gastrodia Elata Blume: An Herbal Material to a Novel Functional Food. J. Funct. Foods 2021, 82, 104512. [Google Scholar] [CrossRef]
- Subramaniam, S.; Wen, X.Y.; Jing, P. One-Step Microwave Curing-Dehydration of Gastrodia Elata Blume: Relationship between Phytochemicals, Water States and Morphometric Characteristics. Ind. Crops Prod. 2020, 153, 112579. [Google Scholar] [CrossRef]
- Xie, Y.; Li, X.; Zhang, Y.; Zheng, Z. Food and Bioproducts Processing Effects of High-Humidity Hot Air Impingement Steaming on Gastrodia Elata: Steaming Degree, Weight Loss, Texture, Drying Kinetics, Microstructure and Active Components. Food Bioprod. Process. 2021, 127, 255–265. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, R.; Li, H.; Liao, X.; Tang, X.; Wang, X.; Su, Z. How Steaming and Drying Processes Affect the Active Compounds and Antioxidant Types of Gastrodia Elata Bl. f. Glauca S. Chow. Food Res. Int. 2022, 157, 111277. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, Y.; Huang, J.; Zeng, H.; Zheng, B. Effects of Different Drying Methods on the Product Quality and Volatile Compounds of Whole Shiitake Mushrooms. Food Chem. 2016, 197, 714–722. [Google Scholar] [CrossRef]
- Xu, J.; Wang, D.; Lei, Y.; Cheng, L.; Zhuang, W.; Tian, Y. Effects of Combined Ultrasonic and Microwave Vacuum Drying on Drying Characteristics and Physicochemical Properties of Tremella Fuciformis. Ultrason. Sonochem. 2022, 84, 105963. [Google Scholar] [CrossRef]
- Therdthai, N.; Zhou, W. Characterization of Microwave Vacuum Drying and Hot Air Drying of Mint Leaves (Mentha Cordifolia Opiz Ex Fresen). J. Food Eng. 2009, 91, 482–489. [Google Scholar] [CrossRef]
- Li, Y.H.; Qi, Y.R.; Wu, Z.F.; Wang, Y.Q.; Wang, X.C.; Wang, F.; Yang, M. Comparative Study of Microwave-Vacuum and Vacuum Drying on the Drying Characteristics, Dissolution, Physicochemical Properties, and Antioxidant Capacity of Scutellaria Extract Powder. Powder Technol. 2017, 317, 430–437. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, Y.N.; Li, H.T.; Qi, Y.R.; Wu, Z.F.; Yang, M. Comparative Study of Microwave-Vacuum and Vacuum Drying on the Physicochemical Properties and Antioxidant Capacity of Licorice Extract Powder. Powder Technol. 2017, 320, 540–545. [Google Scholar] [CrossRef]
- González-Cavieres, L.; Pérez-Won, M.; Tabilo-Munizaga, G.; Jara-Quijada, E.; Díaz-Álvarez, R.; Lemus-Mondaca, R. Advances in Vacuum Microwave Drying (VMD) Systems for Food Products. Trends Food Sci. Technol. 2021, 116, 626–638. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Zhou, C.; Sun, Y.; Wu, B.; Yagoub, A.E.G.A.; Aboagarib, E.A.A. Effect of Vacuum and Ethanol Pretreatment on Infrared-Hot Air Drying of Scallion (Allium Fistulosum). Food Chem. 2019, 295, 432–440. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Bhandari, B.; Yang, P. Intelligent Detection of Flavor Changes in Ginger during Microwave Vacuum Drying Based on LF-NMR. Food Res. Int. 2019, 119, 417–425. [Google Scholar] [CrossRef]
- Li, C.; Ren, G.; Zhang, L.; Duan, X.; Wang, Z.; Ren, X.; Chu, Q.; He, T. Effects of Different Drying Methods on the Drying Characteristics and Drying Quality of Cistanche Deserticola. Lwt 2023, 184, 115000. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Qin, L.; Shen, J.; He, Z.; Shao, Q.; Lin, D. Drying Methods Affect Bioactive Compound Contents and Antioxidant Capacity of Bletilla Striata (Thunb.) Reichb.f. Flower. Ind. Crops Prod. 2021, 164, 113388. [Google Scholar] [CrossRef]
- Kutlu, N.; Gercek, Y.C.; Bayram, N.E. The Effect of Different Drying Methods on Bioactive and Nutrition Contents of Bee Bread and Mathematical Modeling of Drying Characteristics. Chem. Biodivers. 2023, 20, e202200962. [Google Scholar] [CrossRef] [PubMed]
- Horuz, E.; Bozkurt, H.; Karataş, H.; Maskan, M. Effects of Hybrid (Microwave-Convectional) and Convectional Drying on Drying Kinetics, Total Phenolics, Antioxidant Capacity, Vitamin C, Color and Rehydration Capacity of Sour Cherries. Food Chem. 2017, 230, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hao, Q.; Qiao, X.; Li, M.; Qiu, Z.; Zheng, Z.; Zhang, B. An Evaluation of Different Pretreatment Methods of Hot-Air Drying of Garlic: Drying Characteristics, Energy Consumption and Quality Properties. Lwt 2023, 180, 114685. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, M.; Mujumdar, A.S.; Yang, P. Combined LF-NMR and Artificial Intelligence for Continuous Real-Time Monitoring of Carrot in Microwave Vacuum Drying. Food Bioprocess Technol. 2019, 12, 551–562. [Google Scholar] [CrossRef]
- Mothibe, K.J.; Zhang, M.; Mujumdar, A.S.; Wang, Y.C.; Cheng, X. Effects of Ultrasound and Microwave Pretreatments of Apple Before Spouted Bed Drying on Rate of Dehydration and Physical Properties. Dry. Technol. 2014, 32, 1848–1856. [Google Scholar] [CrossRef]
- Oikonomopoulou, V.P.; Krokida, M.K.; Karathanos, V.T. The Influence of Freeze Drying Conditions on Microstructural Changes of Food Products. Procedia Food Sci. 2011, 1, 647–654. [Google Scholar] [CrossRef]
- An, N.N.; Sun, W.H.; Li, B.Z.; Wang, Y.; Shang, N.; Lv, W.Q.; Li, D.; Wang, L.J. Effect of Different Drying Techniques on Drying Kinetics, Nutritional Components, Antioxidant Capacity, Physical Properties and Microstructure of Edamame. Food Chem. 2022, 373, 131412. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, H.H.; Ma, X.M.; Hao, H.H.; Yang, J.H. Effects of Different Drying Methods on the Quality of Daylily Powder. J. Food Process. Preserv. 2022, 46, 1–12. [Google Scholar] [CrossRef]
- Bu, Y.; Zhao, Y.; Zhou, Y.; Zhu, W.; Li, J.; Li, X. Quality and Flavor Characteristics Evaluation of Red Sea Bream Surimi Powder by Different Drying Techniques. Food Chem. 2023, 428, 136714. [Google Scholar] [CrossRef]
- Chu, Q.; Li, L.; Duan, X.; Zhao, M.; Wang, Z.; Wang, Z.; Ren, X.; Li, C.; Ren, G. Effect Mechanism of Different Drying Methods on the Quality and Browning for Daylily. Lwt 2023, 182, 114862. [Google Scholar] [CrossRef]
- Xu, Q.; Pan, H.; Shui, Y.; Xing, Y.; Wu, L.; Zheng, F.; Fan, X.; Bi, X. Effect of Different Drying Technologies on the Characteristics and Quality of Lemon Slices. J. Food Sci. 2022, 87, 2980–2998. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, H.; Peng, Z.; Luo, Q.; Ming, J.; Zhao, G. Characterization of Stipe and Cap Powders of Mushroom (Lentinus Edodes) Prepared by Different Grinding Methods. J. Food Eng. 2012, 109, 406–413. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Simpson, B.K.; Prasher, S.O.; Monpetit, D.; Malcolmson, L. Thermal Processing Effects on the Functional Properties and Microstructure of Lentil, Chickpea, and Pea Flours. Food Res. Int. 2011, 44, 2534–2544. [Google Scholar] [CrossRef]
- Sanchiz, A.; Pedrosa, M.M.; Guillamón, E.; Arribas, C.; Cabellos, B.; Linacero, R.; Cuadrado, C. Influence of Boiling and Autoclave Processing on the Phenolic Content, Antioxidant Activity and Functional Properties of Pistachio, Cashew and Chestnut Flours. Lwt 2019, 105, 250–256. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, G. The Chemical Composition, Pharmacological Effects, Clinical Applications and Market. Pharm. Chem. J. 2017, 51, 211–215. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.Q.; Liu, S.S.; Liu, D.H.; Wang, X.; Wang, Z.M. Transformation Mechanisms of Chemical Ingredients in Steaming Process of Gastrodia Elata Blume. Molecules 2019, 24, 3159. [Google Scholar] [CrossRef]
- Fan, Q.; Chen, C.; Xie, D.; Zhao, S. Impact of Microwave Treatment on Chemical Constituents in Fresh Rhizoma Gastrodiae (Tianma) by UPLC-MS Analysis. J. Chem. 2014, 2014. [Google Scholar] [CrossRef]
- Gómez, A.H.; Wang, J.; Hu, G.; Pereira, A.G. Monitoring Storage Shelf Life of Tomato Using Electronic Nose Technique. J. Food Eng. 2008, 85, 625–631. [Google Scholar] [CrossRef]
- Ge, X.; Liang, Q.; Long, Y.; Shen, H.; Zhang, Q. Assessment of Fresh Alpinia Galanga (A. Galanga) Drying Techniques for the Chemical Composition of Essential Oil and Its Antioxidant and Biological Activity. Food Chem. 2022, 392, 133314. [Google Scholar] [CrossRef]
- Dong, W.; Hu, R.; Long, Y.; Li, H.; Zhang, Y.; Zhu, K.; Chu, Z. Comparative Evaluation of the Volatile Profiles and Taste Properties of Roasted Coffee Beans as Affected by Drying Method and Detected by Electronic Nose, Electronic Tongue, and HS-SPME-GC-MS. Food Chem. 2019, 272, 723–731. [Google Scholar] [CrossRef]
- Yi, C.; Zhu, H.; Tong, L.; Zhou, S.; Yang, R.; Niu, M. Volatile Profiles of Fresh Rice Noodles Fermented with Pure and Mixed Cultures. Food Res. Int. 2019, 119, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Chi, C.; Zhang, Y.; Li, L.; Chen, L.; Li, X. New Insights into How Starch Structure Synergistically Affects the Starch Digestibility, Texture, and Flavor Quality of Rice Noodles. Int. J. Biol. Macromol. 2021, 184, 731–738. [Google Scholar] [CrossRef] [PubMed]



| Samples | L* | a* | b* | ΔE |
|---|---|---|---|---|
| Control | 88.25 ± 0.21 c | 1.57 ± 0.01 c | 9.58 ± 0.04 e. | - |
| HAD | 86.74 ± 0.01 d | 1.22 ± 0.02 e | 11.63 ± 0.03 c | 2.55 ± 0.03 d |
| VD | 84.45 ± 0.01 e | 2.34 ± 0.02 a | 12.75 ± 0.01 a | 5.05 ± 0.01 a |
| MD | 86.80 ± 0.01 d | 1.82 ± 0.01 b | 12.66 ± 0.05 b | 3.41 ± 0.04 b |
| MVD | 88.61 ± 0.02 b | 1.38 ± 0.02 d | 11.26 ± 0.01 d | 1.79 ± 0.05 e |
| FD | 91.03 ± 0.02 a | 0.35 ± 0.00 f | 8.59 ± 0.04 f | 3.22 ± 0.02 c |
| Samples | D10 | D50 | D90 | Specific Surface Area (cm2/g) | Span | WHC (g/g) | OHC (g/g) |
|---|---|---|---|---|---|---|---|
| HAD | 18.65 ± 0.35 a | 98.00 ± 8.49 c | 289.50 ± 3.54 a | 2432.5 ± 34.7 e | 2.78 ± 0.21 a | 9.42 ± 0.30 b | 2.07 ± 0.06 c |
| VD | 15.05 ± 0.49 c | 98.80 ± 2.12 bc | 289.00 ± 5.66 a | 2947.0 ± 73.6 d | 2.77 ± 0.01 a | 9.37 ± 0.36 b | 1.99 ± 0.11 c |
| MD | 13.75 ± 0.64 d | 93.70 ± 2.69 c | 267.00 ± 5.66 b | 3226.0 ± 46.7 c | 2.71 ± 0.15 ab | 9.80 ± 0.23 b | 2.11 ± 0.06 c |
| MVD | 17.10 ± 0.71 b | 115.50 ± 3.53 a | 300.50 ± 13.44 a | 2912.0 ± 76.4 d | 2.46 ± 0.04 bc | 11.38 ± 0.38 a | 2.21 ± 0.08 bc |
| FD | 7.15 ± 0.11 f | 71.20 ± 0.56 d | 175.50 ± 3.54 c | 4863.5 ± 65.8 a | 2.37 ± 0.04 c | 3.66 ± 0.18 c | 2.55 ± 0.18 a |
| Control | 10.70 ± 0.28 e | 108.50 ± 2.12 ab | 292.50 ± 6.36 a | 4297.5 ± 47.4 b | 2.60 ± 0.01 abc | 2.22 ± 0.08 d | 2.37 ± 0.04 ab |
| Samples | GA(mg/g) | HA(mg/g) | HBA(mg/g) |
|---|---|---|---|
| HAD | 2.21 ± 0.26 a | 0.67 ± 0.41 a | 0.177 ± 0.028 a |
| VD | 1.53 ± 0.16 b | 0.47 ± 0.23 a | 0.089 ± 0.025 b |
| MD | 1.53 ± 0.25 b | 0.15 ± 0.10 a | 0.024 ± 0.010 c |
| MVD | 2.32 ± 0.30 a | 0.35 ± 0.16 a | 0.029 ± 0.009 c |
| FD | 2.71 ± 0.37 a | 0.47 ± 0.20 a | 0.019 ± 0.009 c |
| Control | 1.34 ± 0.06 b | 0.37 ± 0.08 a | 0.076 ± 0.016 b |
| Compounds | CAS | Contents (mg/mL) | |||||
|---|---|---|---|---|---|---|---|
| HAD | VD | MD | MVD | FD | Fresh | ||
| aldehydes | |||||||
| 3-Methylbutanal | 590-86-3 | 1.06 | 2.33 | 6.03 | 2.02 | - | - |
| 2-Methylbutanal | 96-17-3 | 2.16 | 5.55 | 11.19 | 2.21 | - | - |
| Hexanal | 66-25-1 | 4.63 | - | 4.39 | 25.02 | 45.64 | |
| Octanal | 124-13-0 | 3.26 | 1.39 | 2.59 | 2.19 | - | 1.84 |
| Benzeneacetaldehyde | 122-78-1 | - | 2.29 | - | 1.45 | - | - |
| Benzaldehyde | 100-52-7 | 12.03 | 11.63 | 11.59 | 12.60 | - | 13.04 |
| Nonanal | 124-19-6 | 15.50 | 7.34 | 6.70 | 13.71 | 8.82 | 10.35 |
| Decanal | 112-31-2 | 2.89 | 3.17 | 2.85 | 3.22 | 7.87 | 3.30 |
| Pentanal | 110-62-3 | - | - | - | - | 2.45 | 3.30 |
| (E)-2-Octenal | 2548-87-0 | - | - | - | - | 6.54 | - |
| Subtotal (number) | 7 | 7 | 7 | 7 | 5 | 6.00 | |
| Subtotal (content) | 41.52 | 33.69 | 45.34 | 37.40 | 50.70 | 77.46 | |
| Alcohols | |||||||
| 4-Hydroxy-4-methyl-2-pentanone | 123-42-2 | 6.07 | 12.34 | 11.83 | 22.60 | 15.69 | 8.32 |
| 2-Ethyl-1-hexanol | 104-76-7 | - | 0.73 | - | 3.18 | - | - |
| 1-Pentanol | 71-41-0 | - | - | - | - | 0.32 | 0.90 |
| 1-Octen-3-ol | 3391-86-4 | - | - | - | - | - | 10.42 |
| 3,5-Octadien-2-ol | 69668-82-2 | - | - | - | - | - | 7.72 |
| Phytol | 150-86-7 | - | - | - | - | - | 1.39 |
| Subtotal (number) | 1 | 2 | 1 | 2 | 2 | 5 | |
| Subtotal (content) | 6.07 | 13.08 | 11.83 | 25.78 | 16.01 | 28.74 | |
| Hydrocarbons | |||||||
| Undecane | 1120-21-4 | 7.00 | 8.04 | 7.05 | 10.66 | 11.50 | 8.52 |
| Dodecane | 112-40-3 | 14.71 | 18.52 | 14.25 | 20.95 | 22.03 | 16.69 |
| Tridecane | 629-50-5 | 7.81 | 8.14 | 7.44 | 15.88 | 13.59 | 7.94 |
| Tetradecane | 629-59-4 | 1.91 | 1.15 | 1.07 | 2.66 | 2.59 | 0.42 |
| Decane | 124-18-5 | 2.92 | - | - | 1.54 | 1.67 | 1.90 |
| 3-Methyl-undecane | 1002-43-3 | - | 2.02 | 1.89 | 1.76 | - | - |
| Decyl-cyclopentane | 1795-21-7 | - | - | 0.61 | 0.56 | 0.61 | - |
| 3-Methyl-pentadecane | 19780-34-8 | - | - | 0.31 | 0.57 | - | - |
| Undecyl-cyclopentane | 6785-23-5 | - | 0.66 | 0.54 | 0.57 | 0.82 | - |
| 2-Methyl-tetradecane | 1560-95-8 | 2.42 | 0.63 | - | 0.71 | - | - |
| n-Hexane | 110-54-3 | 3.33 | - | - | 0.43 | - | - |
| D-Limonene | 5989-27-5 | 3.85 | 0.69 | 0.98 | 0.97 | 1.59 | 0.70 |
| 9-Eicosyne | 71899-38-2 | 2.54 | 2.93 | 4.14 | 2.39 | 1.86 | 3.27 |
| Decahydro-2,6-dimethyl-naphthalene | 1618-22-0 | 2.99 | 14.30 | 16.78 | 5.71 | 6.81 | 1.96 |
| 2-Ethyldecahydro-naphthalene | 1618-23-1 | 1.55 | 2.07 | 2.58 | 0.99 | 1.23 | 2.50 |
| Subtotal (number) | 11 | 11 | 12 | 15 | 11 | 9.00 | |
| Subtotal (content) | 51.03 | 59.16 | 57.63 | 66.36 | 64.31 | 43.89 | |
| Others | |||||||
| Acetic acid | 64-19-7 | 3.53 | 3.36 | 4.11 | 3.88 | - | 0.99 |
| Tetramethylpyrazine | 1124-11-4 | 2.17 | 2.36 | 3.76 | 3.14 | 1.65 | 2.67 |
| 2-Ethylfuran | 3208-16-0 | - | - | 1.05 | 0.71 | - | - |
| p-Cresol | 106-44-5 | - | - | - | - | - | 21.52 |
| Hexanoic acid | 142-62-1 | - | - | - | - | - | 51.95 |
| Subtotal (number) | 2 | 2 | 3 | 3 | 1 | 4.00 | |
| Subtotal (content) | 5.70 | 5.72 | 8.92 | 7.73 | 1.65 | 77.13 | |
| Total (number) | 21 | 22 | 23 | 27 | 19 | 24.00 | |
| Total (content) | 104.33 | 111.65 | 123.72 | 137.26 | 132.67 | 227.22 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, R.; Cheng, H.; Li, X.; Zhang, G.; Zheng, J. Evaluating How Different Drying Techniques Change the Structure and Physicochemical and Flavor Properties of Gastrodia elata. Foods 2024, 13, 1210. https://doi.org/10.3390/foods13081210
Ma R, Cheng H, Li X, Zhang G, Zheng J. Evaluating How Different Drying Techniques Change the Structure and Physicochemical and Flavor Properties of Gastrodia elata. Foods. 2024; 13(8):1210. https://doi.org/10.3390/foods13081210
Chicago/Turabian StyleMa, Rong, Hao Cheng, Xinyao Li, Guoquan Zhang, and Jianmei Zheng. 2024. "Evaluating How Different Drying Techniques Change the Structure and Physicochemical and Flavor Properties of Gastrodia elata" Foods 13, no. 8: 1210. https://doi.org/10.3390/foods13081210





