Lyophilized Avocado Paste Improves Corn Chips’ Nutritional Properties and Sensory Acceptability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Corn Chips Supplemented with AP
2.3. Physicochemical and Proximate Analyses
2.4. Methanolic Extraction
2.5. Quantification of Phenolic Compounds and Flavonoids
2.6. Antioxidant Capacity
2.7. Fatty Acid Profile
2.8. Mineral Content
2.9. Sensory Analysis
2.10. Experimental Design and Statistical Analysis
3. Results
3.1. Physicochemical and Proximate Analyses
3.2. Minerals
3.3. Fatty Acid Profile
3.4. Phenolic Compounds, Total Flavonoids, and Antioxidant Capacity
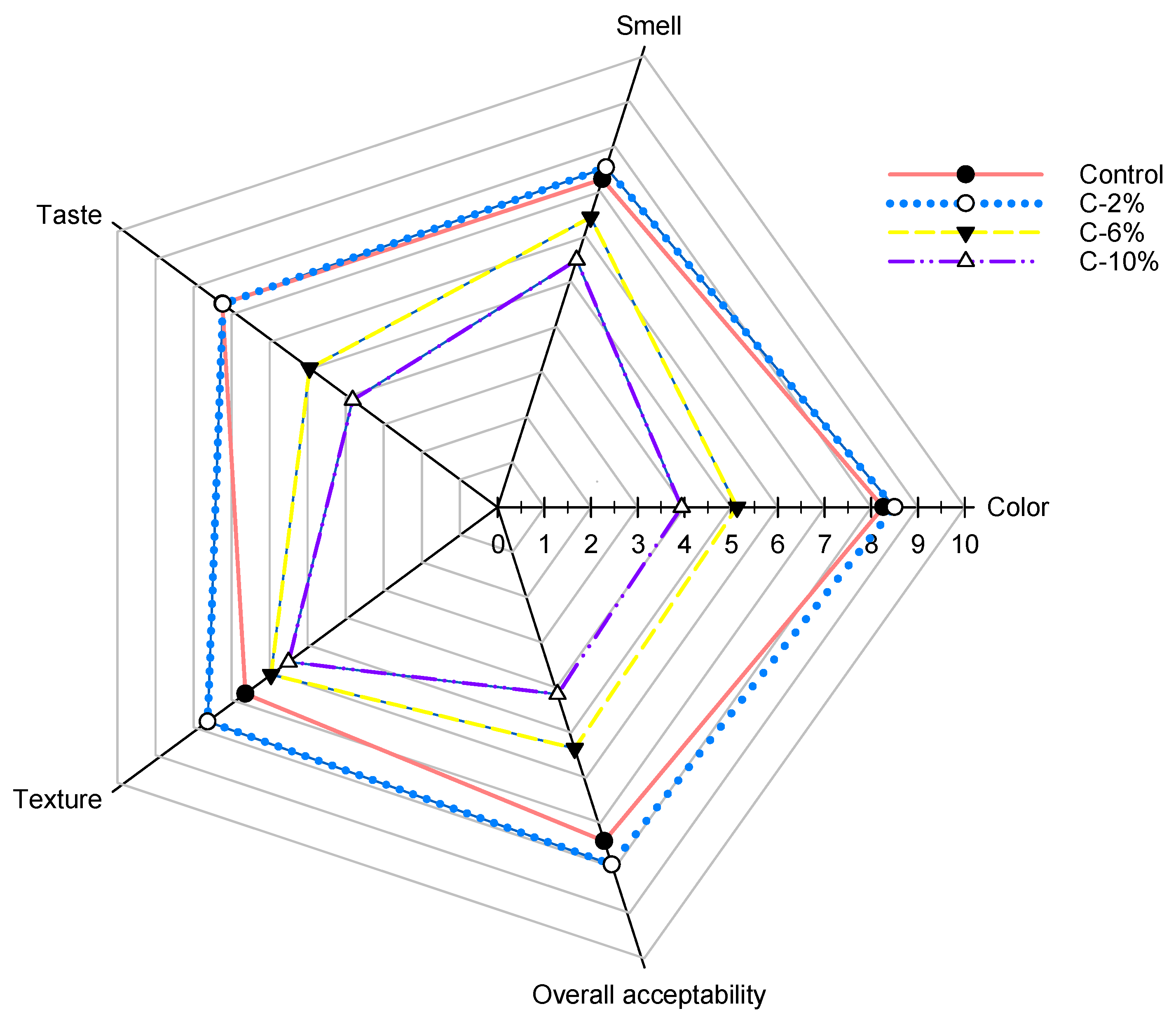
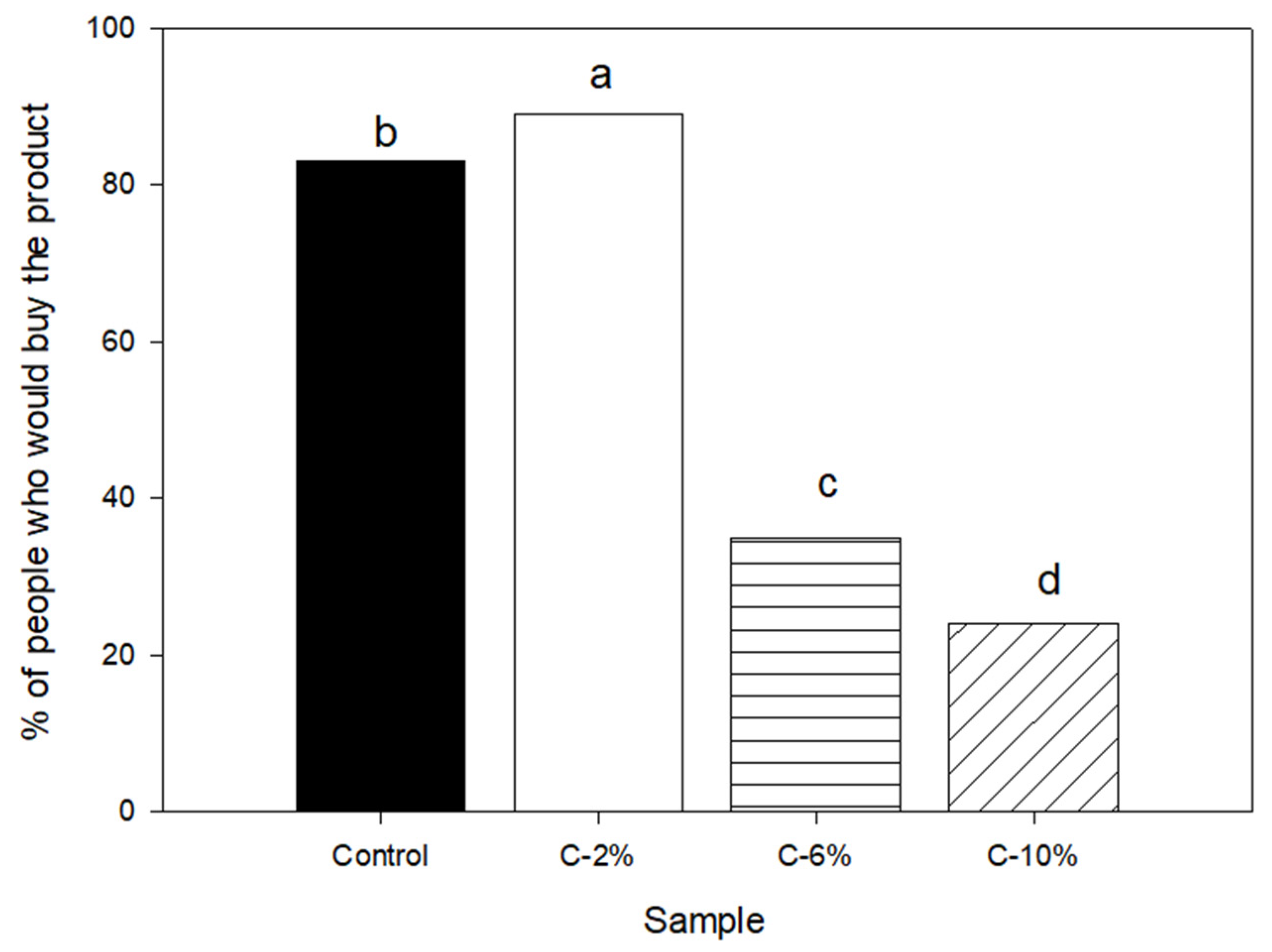
3.5. Sensory Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Castillo-Llamosas, A.; del Rio, P.G.; Perez-Perez, A.; Yanez, R.; Garrote, G.; Gullon, B. Recent advances to recover value-added compounds from avocado byproducts following a biorefinery approach. Curr. Opin. Green Sustain. Chem. 2021, 28, 100433. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive identification of bioactive compounds of avocado peel by liquid chromatography coupled to ultra-high-definition accurate-mass Q-TOF. Food Chem. 2018, 245, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Moreno, N.; Esparza, I.; Bimbela, F.; Gandía, L.M.; Ancín-Azpilicueta, C. Valorization of selected fruit and vegetable wastes as bioactive compounds: Opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2061–2108. [Google Scholar] [CrossRef]
- González-Estudillo, J.C.; González-Campos, J.B.; Nápoles-Rivera, F.; Ponce-Ortega, J.M.; El-Halwagi, M.M. Optimal planning for sustainable production of Avocado in Mexico. Process Integr. Optim. Sustain. 2017, 1, 109–120. [Google Scholar] [CrossRef]
- Permal, R.; Chang, W.L.; Chen, T.; Seale, B.; Hamid, N.; Kam, R. Optimising the spray drying of avocado wastewater and use of the powder as a food preservative for preventing lipid peroxidation. Foods 2020, 9, 1187. [Google Scholar] [CrossRef] [PubMed]
- Zuñiga-Martínez, B.S.; Dominguez-Avila, J.A.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Hernandez-Paredes, J.; Salazar-Lopez, N.J.; Villegas-Ochoa, M.A.; Gonzalez-Aguilar, G.A. Avocado paste from industrial byproducts as an unconventional source of bioactive compounds: Characterization, in vitro digestion and in silico interactions of its main phenolics with cholesterol. J. Food Meas. Charact. 2021, 15, 5460–5476. [Google Scholar] [CrossRef]
- Khajuria, A.; Atienza, V.A.; Chavanich, S.; Henning, W.; Islam, I.; Kral, U.; Liu, M.; Liu, X.; Murthy, I.K.; Oyedotun, T.D.T. Accelerating circular economy solutions to achieve the 2030 agenda for sustainable development goals. Circ. Econ. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Domínguez-Avila, J.A.; Yahia, E.M.; Belmonte-Herrera, B.H.; Wall-Medrano, A.; Montalvo-González, E.; González-Aguilar, G. Avocado fruit and byproducts as potential sources of bioactive compounds. Food Res. 2020, 138, 109774. [Google Scholar] [CrossRef]
- Nguyen, L.V.; Nguyen, K.D.A.; Ma, C.T.; Nguyen, Q.T.; Nguyen, H.T.H.; Yang, D.J.; Le Tran, T.; Kim, K.W.; Doan, K.V. p-Coumaric Acid Enhances Hypothalamic Leptin Signaling and Glucose Homeostasis in Mice via Differential Effects on AMPK Activation. Int. J. Mol. Sci. 2021, 22, 1431. [Google Scholar] [CrossRef]
- Qi, M.Y.; Wang, X.T.; Xu, H.L.; Yang, Z.L.; Cheng, Y.; Zhou, B. Protective effect of ferulic acid on STZ-induced diabetic nephropathy in rats. Food Funct. 2020, 11, 3706–3718. [Google Scholar] [CrossRef]
- Nour, O.A.; Ghoniem, H.A.; Nader, M.A.; Suddek, G.M. Impact of protocatechuic acid on high fat diet-induced metabolic syndrome sequelae in rats. Eur. J. Pharmacol. 2021, 907, 174257. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Govea-Salas, M.; Pintado, M.E.; Aguilar, C.N. Process optimization of microwave-assisted extraction of bioactive molecules from avocado seeds. Ind. Crop. Prod. 2020, 154, 112623. [Google Scholar] [CrossRef]
- Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Robles-Sánchez, R.M.; Ayala-Zavala, J.F.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Agro-Industrial Fruit Byproducts as Health-Promoting Ingredients Used to Supplement Baked Food Products. Foods 2022, 11, 3181. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hettiararchchy, N.S.; Horax, R. Quality and estimated glycemic profile of baked protein-enriched corn chips. J. Food Sci. Technol. 2019, 56, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Ruiz, G.C.; Domínguez-Avila, J.A.; Ayala-Zavala, J.F.; Robles-Sánchez, M.; Salazar-López, N.J.; López-Díaz, J.A.; González-Aguilar, G.A. Supplementing corn chips with mango cv.“Ataulfo” peel improves their sensory acceptability and phenolic profile, and decreases in vitro dialyzed glucose. J. Food Process Preserv. 2020, 44, e14954. [Google Scholar] [CrossRef]
- Feng, K.; Hu, W.; Jiang, A.; Xu, Y.; Sarengaowa; Li, X.; Bai, X. Growth Potential of Listeria Monocytogenes and Staphylococcus Aureus on Fresh-Cut Tropical Fruits. J. Food Sci. 2015, 80, M2548–M2554. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. AOAC Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Rana, P.; Sheu, S.-C. Discrimination of four Cinnamomum species by proximate, antioxidant, and chemical profiling: Towards quality assessment and authenticity. J. Food Sci. Technol. 2023, 60, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Yahia, E.; Islas-Osuna, M.; Gutierrez-Martinez, P.; Robles-Sánchez, M.; González-Aguilar, G. Effect of ripeness stage of mango fruit (Mangifera indica L., cv. Ataulfo) on physiological parameters and antioxidant activity. Sci. Hortic. 2012, 135, 7–13. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Robles-Sánchez, R.M.; Rojas-Graü, M.A.; Odriozola-Serrano, I.; González-Aguilar, G.A.; Martín-Belloso, O. Effect of minimal processing on bioactive compounds and antioxidant activity of fresh-cut ‘Kent’mango (Mangifera indica L.). Postharvest Biol. Technol. 2009, 51, 384–390. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free-Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Botella-Martínez, C.; Viuda-Martos, M.; Pérez-Alvarez, J.A.; Fernández-López, J. Total and Partial Fat Replacement by Gelled Emulsion (Hemp Oil and Buckwheat Flour) and Its Impact on the Chemical, Technological and Sensory Properties of Frankfurters. Foods 2021, 10, 1681. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.; Southgate, D. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bas, C.; Muñoz-Tebar, N.; Candela-Salvador, L.; Sayas-Barberá, E.; Viuda-Martos, M.; Pérez-Alvarez, J.A.; Fernández-López, J. Development of value-added products suitable for food applications from fresh date fruit (Confitera cv.) and its co-products. Food Bioproc. Technol. 2023, 1–13. [Google Scholar] [CrossRef]
- Vollmer, K.; Czerny, M.; Vásquez-Caicedo, A.L.; Iglesias, S.V.; Frank, J.; Carle, R.; Steingass, C.B. Non-thermal processing of pineapple (Ananas comosus [L.] Merr.) juice using continuous pressure change technology (PCT): HS-SPME-GC–MS profiling, descriptive sensory analysis, and consumer acceptance. Food Chem. 2021, 345, 128786. [Google Scholar] [CrossRef]
- Gómez, M.; Martinez, M.M. Fruit and vegetable byproducts as novel ingredients to improve the nutritional quality of baked goods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2119–2135. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Salmerón-Ruiz, M.L.; Domínguez-Avila, J.A.; Villegas-Ochoa, M.A.; Ayala-Zavala, J.F.; González-Aguilar, G.A. Phenolic compounds from ‘Hass’ avocado peel are retained in the indigestible fraction after an in vitro gastrointestinal digestion. J. Food Meas. Charact. 2021, 15, 1982–1990. [Google Scholar] [CrossRef]
- Mehta, D.; Prasad, P.; Sangwan, R.S.; Yadav, S.K. Tomato processing byproduct valorization in bread and muffin: Improvement in physicochemical properties and shelf life stability. J. Food Sci. Technol. 2018, 55, 2560–2568. [Google Scholar] [CrossRef] [PubMed]
- Ajila, C.M.; Aalami, M.; Leelavathi, K.; Rao, U.J.S.P. Mango peel powder: A potential source of antioxidant and dietary fiber in macaroni preparations. Innov. Food Sci. Emerg. 2010, 11, 219–224. [Google Scholar] [CrossRef]
- Mayo-Mayo, G.; Navarrete-García, A.; Maldonado-Astudillo, Y.I.; Jiménez-Hernández, J.; Santiago-Ramos, D.; Arámbula-Villa, G.; Álvarez-Fitz, P.; Ramirez, M.; Salazar, R. Addition of roselle and mango peel powder in tortilla chips: A strategy for increasing their functionality. J. Food Meas. Charact. 2020, 14, 1511–1519. [Google Scholar] [CrossRef]
- Forde, C.G.; Bolhuis, D. Interrelations Between Food Form, Texture, and Matrix Influence Energy Intake and Metabolic Responses. Curr. Nutr. Rep. 2022, 11, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Stribiţcaia, E.; Evans, C.E.; Gibbons, C.; Blundell, J.; Sarkar, A. Food texture influences on satiety: Systematic review and meta-analysis. Sci. Rep. 2020, 10, 12929. [Google Scholar]
- Esmaeili, M.; Ajami, M.; Barati, M.; Javanmardi, F.; Houshiarrad, A.; Mousavi Khaneghah, A. The significance and potential of functional food ingredients for control appetite and food intake. Food Sci. Nutr. 2022, 10, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Chirife, J.; Zamora, M.C.; Motto, A. The correlation between water activity and % moisture in honey: Fundamental aspects and application to Argentine honeys. J. Food Eng. 2006, 72, 287–292. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, S.; Wu, H.; Qin, W.; Zhang, T.; Wang, Y.; Tang, Y.; Qi, S.; Cao, Y.; Gao, X. Cross-sectional study: Relationship between serum trace elements and hypertension. J. Trace Elem. Med. Biol. 2022, 69, 126893. [Google Scholar] [CrossRef]
- Noshin, T.F.; Ali, M.R.; Banik, S. Increased oxidative stress and altered serum macro-minerals and trace elements levels are associated with coronary artery disease. J. Trace Elem. Med. Biol. 2021, 64, 126707. [Google Scholar] [CrossRef]
- McLean, R.M.; Wang, N.X. Potassium. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 96, pp. 89–121. [Google Scholar]
- Castiglione, D.; Platania, A.; Conti, A.; Falla, M.; D’Urso, M.; Marranzano, M. Dietary micronutrient and mineral intake in the mediterranean healthy eating, ageing, and lifestyle (MEAL) study. Antioxidants 2018, 7, 79. [Google Scholar] [CrossRef]
- Spencer, M.; Finlin, B.S.; Unal, R.; Zhu, B.; Morris, A.J.; Shipp, L.R.; Lee, J.; Walton, R.G.; Adu, A.; Erfani, R. Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes 2013, 62, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Becerra, K.; Barron-Cabrera, E.; Muñoz-Valle, J.F.; Torres-Castillo, N.; Rivera-Valdes, J.J.; Rodriguez-Echevarria, R.; Martinez-Lopez, E. A balanced dietary ratio of n-6: n-3 polyunsaturated fatty acids exerts an effect on total fatty acid profile in RBCs and inflammatory markers in subjects with obesity. Healthcare 2023, 11, 2333. [Google Scholar] [CrossRef] [PubMed]
- Zaki, H.; Zaki, M.; Abdulla, M.; Abdel-Latif, E. Evaluation of fatty acid indices and fatty acid content including trans fat of different fried food types using gas-liquid chromatography technique. Adv. Anim. Vet. Sci. 2021, 9, 887–894. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Korish, M.A.; Shiboob, M.M. Fatty acid and cholesterol profiles, hypocholesterolemic, atherogenic, and thrombogenic indices of broiler meat in the retail market. Lipids Health Dis. 2017, 16, 40. [Google Scholar] [CrossRef]
- Al-Amiri, H.A.; Ahmed, N.; Al-Sharrah, T. Fatty acid profiles, cholesterol composition, and nutritional quality indices of 37 commonly consumed local foods in Kuwait in relation to cardiovascular health. medRxiv 2020, 1–28. [Google Scholar] [CrossRef]
- Dreher, M.L.; Davenport, A.J. Hass Avocado Composition and Potential Health Effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Arreguin-Campos, A.; Cruz-Medrano, M.A.; Bilbao, M.D.D. Spent coffee (Coffea arabica L.) grounds promote satiety and attenuate energy intake: A pilot study. J. Food Biochem. 2020, 44, e13204. [Google Scholar] [CrossRef] [PubMed]
- Karwacka, M.; Rybak, K.; Świeca, M.; Galus, S.; Janowicz, M. The Effect of the Addition of Selected Fruit Pomace Powders and Pectin as Carrier Agents on the Nutritional Value of Freeze-Dried Snacks. Sustainability 2022, 14, 13012. [Google Scholar] [CrossRef]
- Alongi, M.; Verardo, G.; Gorassini, A.; Sillani, S.; Degrassi, C.; Anese, M. Reformulation and food combination as strategies to modulate glycaemia: The case of apple pomace containing biscuits administered with apple juice to healthy rats. Int. J. Food Sci. Nutr. 2021, 72, 174–183. [Google Scholar] [CrossRef]
- Bouazizi, S.; Montevecchi, G.; Antonelli, A.; Hamdi, M. Effects of prickly pear (Opuntia ficus-indica L.) peel flour as an innovative ingredient in biscuits formulation. LWT-Food Sci. Technol. 2020, 124, 109155. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Dominguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, M.W.; Davila-Avina, J.E.; Gonzalez-Aguilar, G.A. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, L.; Ma, C.; Wang, Z.; Hu, X.; Chen, F. Impact of high-hydrostatic pressure and thermal processing on the antioxidant profiles and capacity of tomato juice during storage. Food Innov. Adv. 2023, 2, 124–134. [Google Scholar] [CrossRef]
- Bora, P.; Ragaee, S.; Abdel-Aal, E.-S.M. Effect of incorporation of goji berry byproduct on biochemical, physical and sensory properties of selected bakery products. LWT-Food Sci. Technol. 2019, 112, 108225. [Google Scholar] [CrossRef]
- Ortega-Heras, M.; Gómez, I.; de Pablos-Alcalde, S.; González-Sanjosé, M.L. Application of the just-about-right scales in the development of new healthy whole-wheat muffins by the addition of a product obtained from white and red grape pomace. Foods 2019, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Theagarajan, R.; Malur Narayanaswamy, L.; Dutta, S.; Moses, J.A.; Chinnaswamy, A. Valorisation of grape pomace (cv. Muscat) for development of functional cookies. Int. J. Food Sci. Technol. 2019, 54, 1299–1305. [Google Scholar] [CrossRef]
- Baldán, Y.; Riveros, M.; Fabani, M.P.; Rodriguez, R. Grape pomace powder valorization: A novel ingredient to improve the nutritional quality of gluten-free muffins. Biomass Convers. Biorefinery 2021, 13, 9997–10009. [Google Scholar] [CrossRef]
- Imeneo, V.; Romeo, R.; Gattuso, A.; De Bruno, A.; Piscopo, A. Functionalized biscuits with bioactive ingredients obtained by Citrus lemon pomace. Foods 2021, 10, 2460. [Google Scholar] [CrossRef]
- Chen, W.; Wu, X.; Liu, Z.; Liu, Y.; Liu, Q.; Pointer, M.R.; Liang, J.; Khanh, T.Q. The impact of illuminance level, correlated colour temperature and viewing background on the purchase intention for bread and cakes. Food Qual. Pref. 2022, 98, 104537. [Google Scholar] [CrossRef]
- Michon, C.; O’Sullivan, M.; Sheehan, E.; Delahunty, C.; Kerry, J. Investigation of the influence of age, gender and consumption habits on the liking of jam-filled cakes. Food Qual. Pref. 2010, 21, 553–561. [Google Scholar] [CrossRef]
- Hayes, J.E.; Sullivan, B.S.; Duffy, V.B. Explaining variability in sodium intake through oral sensory phenotype, salt sensation and liking. Physiol. Behav. 2010, 100, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Curutchet, A.; Trias, J.; Tarrega, A.; Arcia, P. Consumer Response to Cake with Apple Pomace as a Sustainable Source of Fibre. Foods 2021, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Sandvik, P.; Nydahl, M.; Kihlberg, I.; Marklinder, I. Consumers’ health-related perceptions of bread–implications for labeling and health communication. Appetite 2018, 121, 285–293. [Google Scholar] [CrossRef] [PubMed]



| Reagent/Solvent | Supplier |
|---|---|
| Folin–Ciocalteu’s phenol reagent | Sigma-Aldrich (St. Louis, MO, USA) |
| 2,2-diphenyl-1-picrylhydrazyl (DPPH) | |
| Phenolic standards (gallic acid and quercetin) | |
| Sodium carbonate > 99% | |
| Sodium chloride ≥ 99.5% | |
| TPTZ (2,4,6-tripyridyl-S-triazine) | |
| Trolox (6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid) | |
| ABTS (2,2′-azino-bis-(3-ethylbenzothiazolne-6-sulfonic acid) diammonium salt) | |
| Ethanol (HPLC grade) | JT Baker (Phillipsburg, NJ, USA) |
| Hexane (HPLC grade) | |
| Acetone (HPLC grade) |
| Variable | Control (C) | C-2% | C-6% | C-10% |
|---|---|---|---|---|
| L* | 68.49 ± 8.74 d | 54.07 ± 5.34 c | 50.14 ± 6.37 b | 43.70 ± 7.58 a |
| C* | 23.55 ± 2.58 a | 29.43 ± 2.62 b | 32.01 ± 2.45 d | 26.27 ± 2.45 c |
| Hue° | 87.10 ± 2.23 d | 79.08 ± 1.91 c | 74.41 ± 2.48 a | 77.62 ± 1.68 b |
| Hardness (N) | 18.77 ± 0.02 c | 13.74 ± 0.25 a | 16.38 ± 0.13 b | 19.30 ± 1.52 d |
| Water activity (Aw) | 0.11 ± 0.01 a | 0.18 ± 0.00 c | 0.10 ± 0.00 a | 0.16 ± 0.00 b |
| Moisture (%) | 8.17 ± 0.10 a | 3.65 ± 0.01 b | 1.72 ± 0.03 c | 4.33 ± 0.08 b |
| Ash (%) | 1.62 ± 0.01 c | 1.52 ± 0.01 b | 1.63 ± 0.01 c | 1.82 ± 0.01 a |
| Protein (%) | 7.76 ± 0.03 a | 7.67 ± 0.01 b | 7.71 ± 0.00 a | 7.75 ± 0.01 a |
| Lipids (%) | 2.98 ± 0.11 c | 2.08 ± 0.06 d | 2.64 ± 0.00 b | 3.62 ± 0.09 a |
| Fiber (%) | 2.72 ± 0.14 a | 3.38 ± 0.13 b | 4.38 ± 0.00 c | 5.29 ± 0.18 d |
| Carbohydrates (%) | 83.79 ± 0.19 a | 81.92 ± 0.12 b | 81.97 ± 0.19 a | 77.16 ± 0.10 c |
| Variable | Control (C) | C-2% | C-6% | C-10% |
|---|---|---|---|---|
| Ca | 235.41 ± 2.10 d | 243.51 ± 4.46 c | 260.87 ± 3.34 b | 284.90 ± 3.36 a |
| Cu | 0.07 ± 0.01 c | 0.20 ± 0.01 b | 0.20 ± 0.02 b | 0.26 ± 0.02 a |
| Fe | 10.49 ± 0.58 a | 9.13 ± 0.02 b | 9.17 ± 0.13 b | 9.48 ± 0.23 b |
| K | 216.56 ± 2.00 d | 249.58 ± 4.44 c | 261.69 ± 1.59 b | 312.60 ± 2.59 a |
| Mg | 101.48 ± 0.52 b | 104.14 ± 0.22 a | 105.01 ± 0.94 a | 104.59 ± 0.05 a |
| Mn | 0.86 ± 0.50 b | 0.88 ± 0.03 b | 0.95 ± 0.01 a | 0.97 ± 0.02 a |
| Na | 18.57 ± 0.66 c | 31.70 ± 0.87 b | 31.50 ± 0.79 b | 34.73 ± 0.67 a |
| P | 936.08 ± 2.69 a | 827.87 ± 4.94 b | 818.70 ± 2.68 c | 922.99 ± 9.77 a |
| Zn | 9.66 ± 0.26 a | 6.05 ± 0.20 d | 6.96 ± 0.07 c | 8.25 ± 0.27 b |
| Fatty Acid | Control (C) | C-2% | C-6% | C-10% |
|---|---|---|---|---|
| C8:0 | 0.040 ± 0.000 a | 0.022 ± 0.000 b | 0.029 ± 0.000 b | 0.023 ± 0.000 b |
| C10:0 | 0.027 ± 0.000 a | 0.012 ± 0.000 c | 0.018 ± 0.000 b | 0.015 ± 0.000 c |
| C12:0 | 0.051 ± 0.018 a | 0.023 ± 0.000 a | 0.027 ± 0.000 a | 0.029 ± 0.010 a |
| C14:0 | 0.135 ± 0.007 a | 0.097 ± 0.000 b | 0.111 ± 0.00 b | 0.099 ± 0.000 b |
| C14:1 | ND | 0.041 ± 0.010 a | 0.022 ± 0.000 a | 0.046 ± 0.010 a |
| C15:0 | ND | 0.025 ± 0.000 a | 0.037 ± 0.020 a | 0.024 ± 0.010 a |
| C16:0 | 14.228 ± 0.011 c | 14.624 ± 0.040 c | 15.274 ± 0.260 b | 16.143 ± 0.010 a |
| C16:1 | 0.141 ± 0.018 d | 0.724 ± 0.000 c | 1.555 ± 0.100 b | 2.367 ± 0.030 a |
| C17:0 | 0.083 ± 0.010 a | 0.073 ± 0.010 a | 0.087 ± 0.010 a | 0.076 ± 0.010 a |
| C18:0 | 2.795 ± 0.0349 a | 2.452 ± 0.080 b | 2.266 ± 0.070 b,c | 2.126 ± 0.01 c |
| C18:1 | 29.745 ± 0.109 d | 30.873 ± 0.360 c | 33.330 ± 0.260 b | 34.972 ± 0.000 a |
| C18:2 (n-6) | 50.551 ± 0.131 a | 48.891 ± 0.360 b | 45.033 ± 0.08 c | 41.892 ± 0.700 d |
| C18:3 (n-3) | 1.034 ± 0.017 d | 1.130 ± 0.000 c | 1.221 ± 0.010 b | 1.305 ± 0.000 a |
| C18:3 (n-6) | 0.516 ± 0.000 a | 0.487 ± 0.010 b | 0.444 ± 0.000 c | 0.401 ± 0.000 d |
| C20:0 | 0.249 ± 0.037 a | 0.215 ± 0.010 a | 0.207 ± 0.010 a | 0.168 ± 0.030 a |
| C20:5 (n-3) | 0.155 ± 0.000 a | 0.096 ± 0.040 a | 0.146 ± 0.000 a | 0.128 ± 0.010 a |
| C24:00 | 0.241 ± 0.000 a | 0.215 ± 0.000 b | 0.194 ± 0.000 c | 0.187 ± 0.000 c |
| Omega-3 fatty acids | 1.189 ± 0.017 a | 1.226 ± 0.046 a | 1.367 ± 0.005 a | 1.433 ± 0.011 a |
| Omega-6 fatty acids | 51.067 ± 0.131 a | 49.378 ± 0.362 b | 45.476 ± 0.088 c | 42.293 ± 0.070 d |
| Omega-6/Omega-3 ratio | 42.951 ± 0.518 a | 40.291 ± 1.208 a | 33.266 ± 0.184 b | 29.512 ± 0.172 c |
| Total SFA | 17.850 ± 0.032 b,c | 17.758 ± 0.038 c | 18.249 ± 0.224 b | 18.889 ± 0.040 a |
| Total UFA | 82.142 ± 0.021 a,b | 82.242 ± 0.038 a | 81.751± 0.224 b | 81.111 ± 0.040 c |
| Total MUFA | 29.887 ± 0.127 d | 31.638 ± 0.371 c | 34.908 ± 0.141 b | 37.385 ± 0.041 a |
| Total PUFA | 52.256 ± 0.149 a | 50.605 ± 0.408 b | 46.843 ± 0.083 c | 43.726 ± 0.081 d |
| Atherogenic index (AI) | 0.180 ± 0.000 c | 0.182 ± 0.000 c | 0.192± 0.003 b | 0.204 ± 0.000 a |
| Thrombogenic index (TI) | 0.389 ± 0.000 b | 0.388 ± 0.002 b | 0.398 ± 0.005 b | 0.415 ± 0.000 a |
| Variable | Control (C) | C-2% | C-6% | C-10% |
|---|---|---|---|---|
| Total phenolic compounds | 0.93 ± 0.09 a | 2.49 ± 0.22 b | 3.11 ± 0.23 c | 3.56 ± 0.35 d |
| Total flavonoids | 1.17 ± 0.22 a | 3.22 ± 0.29 b | 3.85 ± 0.24 c | 6.61 ± 0.41 d |
| TEAC | 2.28 ± 0.08 a | 4.22 ± 0.18 b | 4.31 ± 0.02 b | 5.27 ± 0.37 |
| FRAP | 1.19 ± 0.03 a | 1.90 ± 0.11 b | 2.07 ± 0.07 bc | 2.21 ± 0.24 |
| DPPH | 1.09 ± 0.02 a | 1.72 ± 0.17 b | 1.90 ± 0.17 c | 2.44 ± 0.19 d |
| ORAC | 7.81 ± 0.56 a | 20.08 ± 1.06 b | 21.52 ± 0.78 b | 22.52 ± 2.68 b |
| Variable | Phenolic Compounds | Flavonoids | ABTS | DPPH | ORAC |
|---|---|---|---|---|---|
| Flavonoids | 0.945 | ||||
| ABTS | 0.973 | 0.942 | |||
| DPPH | 0.878 | 0.948 | 0.910 | ||
| ORAC | 0.997 | 0.891 | 0.976 | 0.835 | |
| FRAP | 0.990 | 0.947 | 0.973 | 0.881 | 0.980 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Robles-Sánchez, R.M.; Ayala-Zavala, J.F.; Viuda-Martos, M.; López-Díaz, J.A.; Villegas-Ochoa, M.A.; Torres-García, G.; González-Aguilar, G.A. Lyophilized Avocado Paste Improves Corn Chips’ Nutritional Properties and Sensory Acceptability. Foods 2024, 13, 1220. https://doi.org/10.3390/foods13081220
Zuñiga-Martínez BS, Domínguez-Avila JA, Robles-Sánchez RM, Ayala-Zavala JF, Viuda-Martos M, López-Díaz JA, Villegas-Ochoa MA, Torres-García G, González-Aguilar GA. Lyophilized Avocado Paste Improves Corn Chips’ Nutritional Properties and Sensory Acceptability. Foods. 2024; 13(8):1220. https://doi.org/10.3390/foods13081220
Chicago/Turabian StyleZuñiga-Martínez, B. Shain, J. Abraham Domínguez-Avila, R. Maribel Robles-Sánchez, J. Fernando Ayala-Zavala, Manuel Viuda-Martos, José Alberto López-Díaz, Mónica A. Villegas-Ochoa, Gerardo Torres-García, and Gustavo A. González-Aguilar. 2024. "Lyophilized Avocado Paste Improves Corn Chips’ Nutritional Properties and Sensory Acceptability" Foods 13, no. 8: 1220. https://doi.org/10.3390/foods13081220
APA StyleZuñiga-Martínez, B. S., Domínguez-Avila, J. A., Robles-Sánchez, R. M., Ayala-Zavala, J. F., Viuda-Martos, M., López-Díaz, J. A., Villegas-Ochoa, M. A., Torres-García, G., & González-Aguilar, G. A. (2024). Lyophilized Avocado Paste Improves Corn Chips’ Nutritional Properties and Sensory Acceptability. Foods, 13(8), 1220. https://doi.org/10.3390/foods13081220