Steam Explosion-Assisted Extraction of Polysaccharides from Pleurotus eryngii and Its Influence on Structural Characteristics and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Plant Material
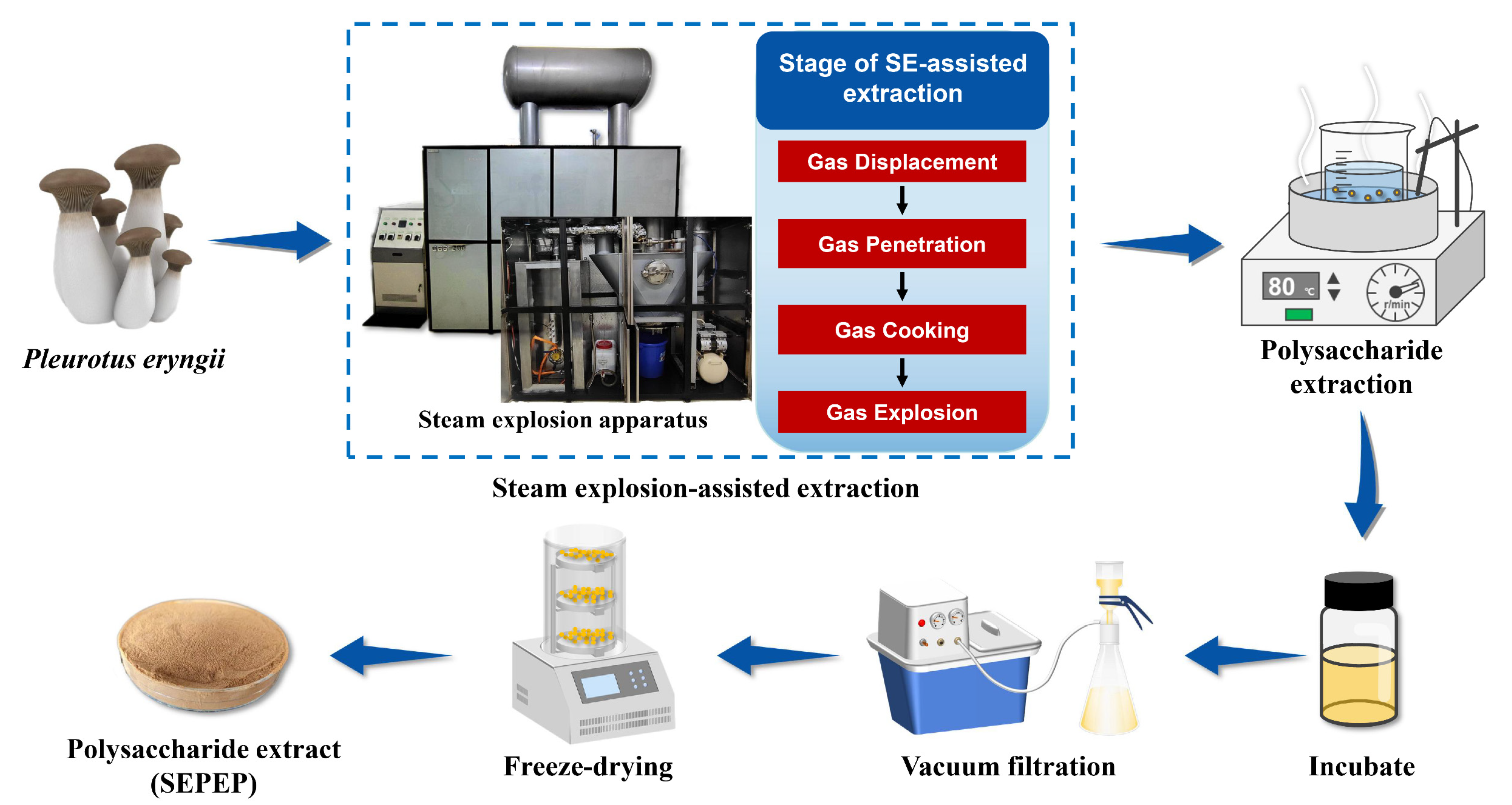
2.2. Steam Explosion (SE)-Assisted Extraction
2.3. Scanning Electron Microscopic (SEM) Observation
2.4. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.5. Determination of Monosaccharide Composition
2.6. DPPH Free Radical Scavenging Assay
2.7. ABTS Free Radical Scavenging Assay
2.8. Hydroxyl Radical Scavenging Capacity Assay
2.9. Statistical Analysis
3. Results and Discussion
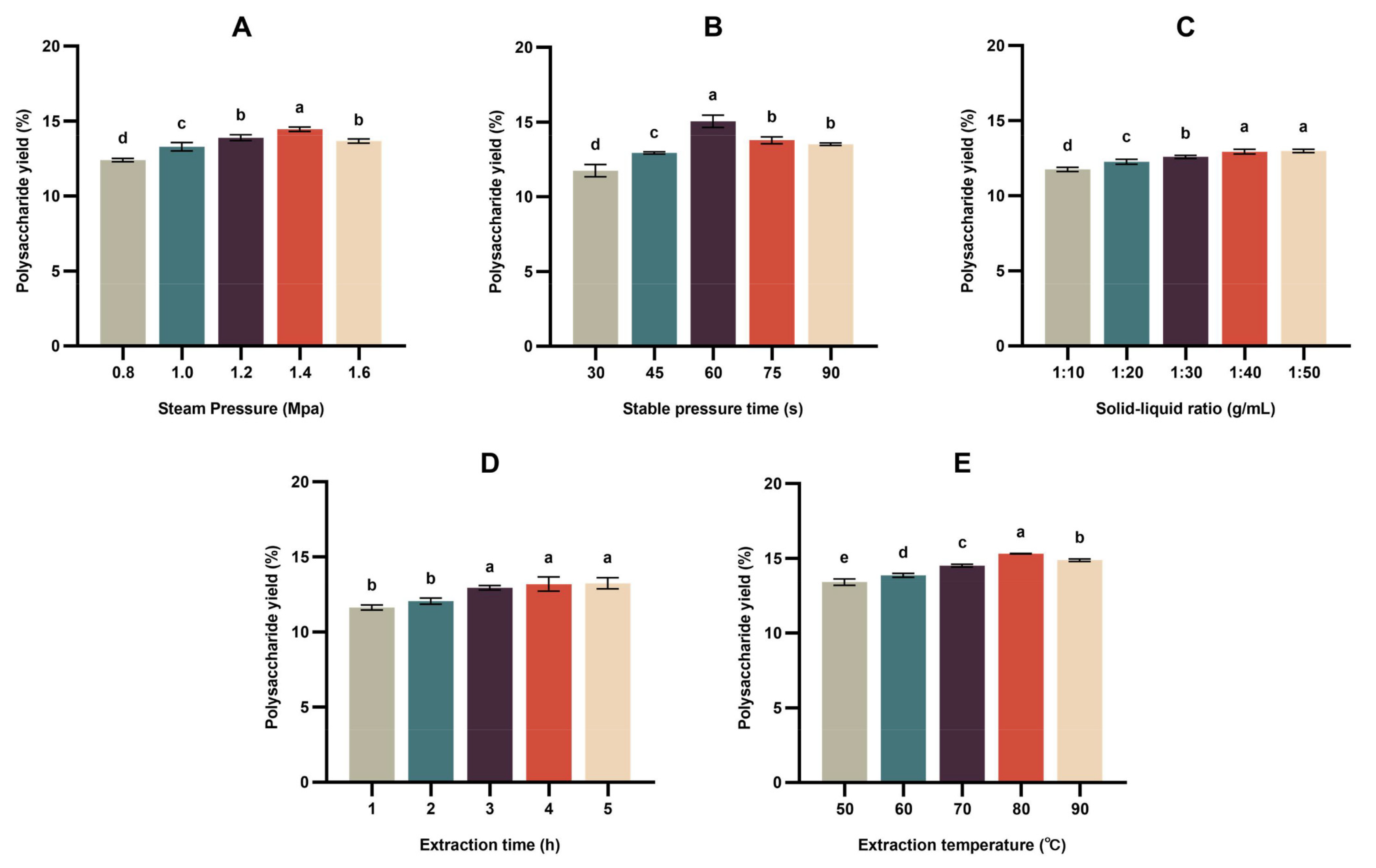
3.1. Determination of Optimal Steam Explosion (SE)-Assisted Extraction Conditions
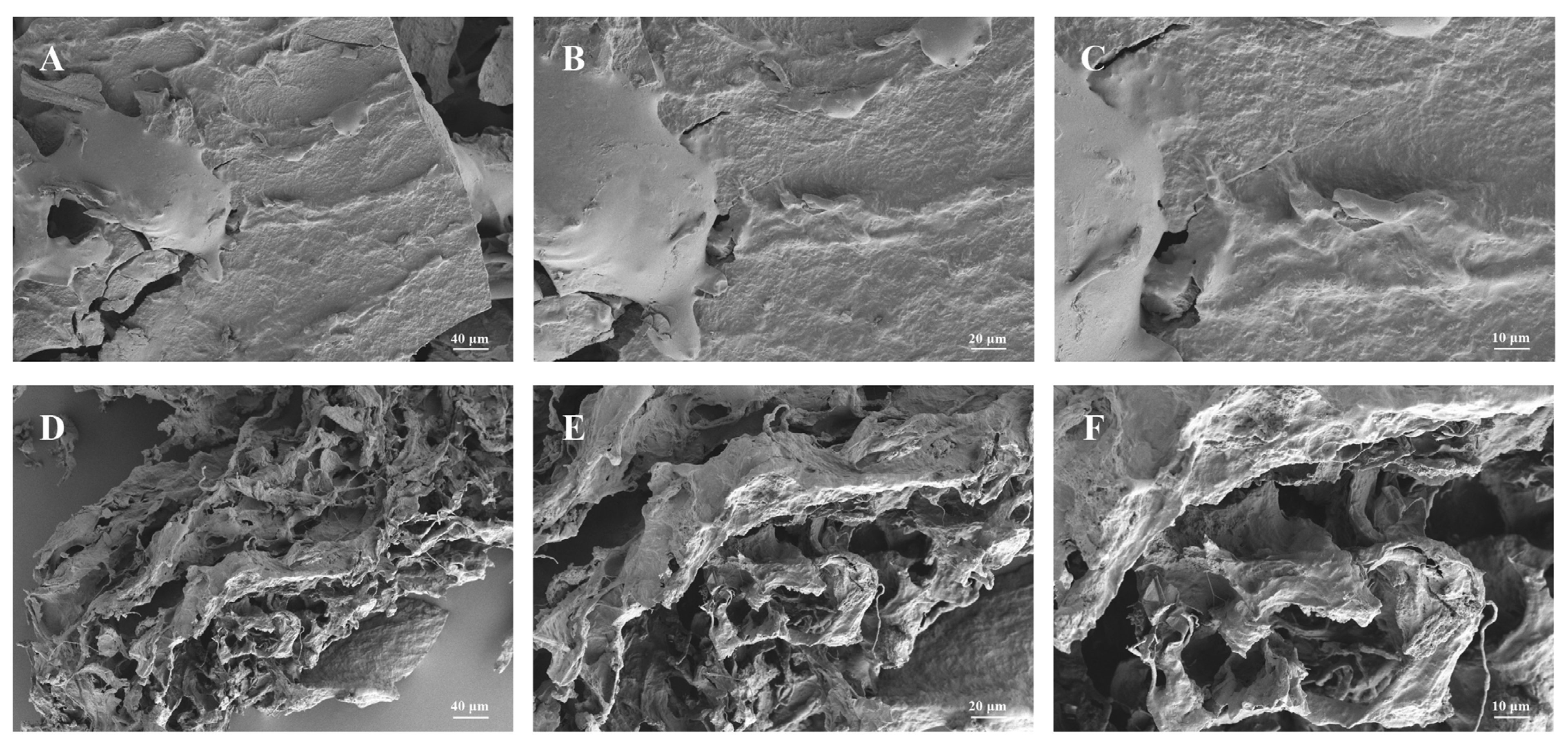
3.2. Effects of Steam Explosion (SE)-Assisted Extraction on the Microstructure of Pleurotus eryngii Polysaccharides
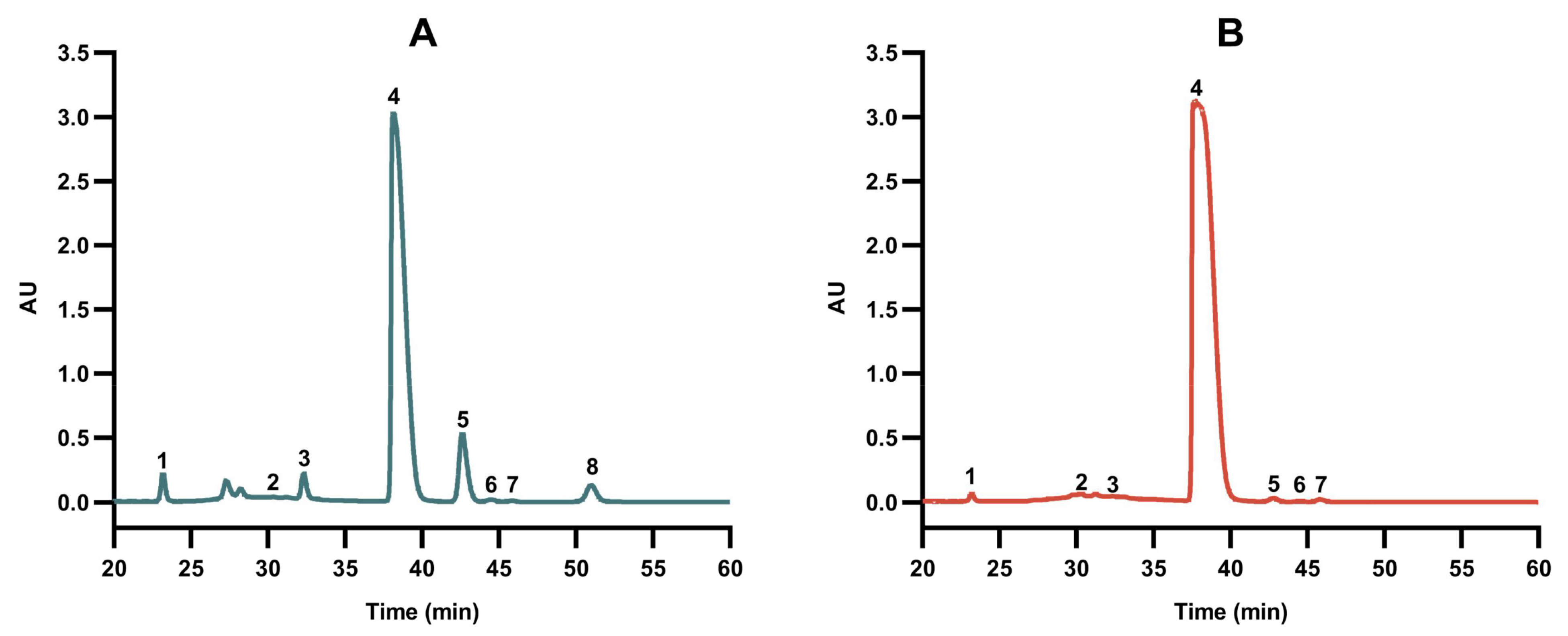
3.3. Monosaccharide Compositional Analysis of Pleurotus eryngii Polysaccharides
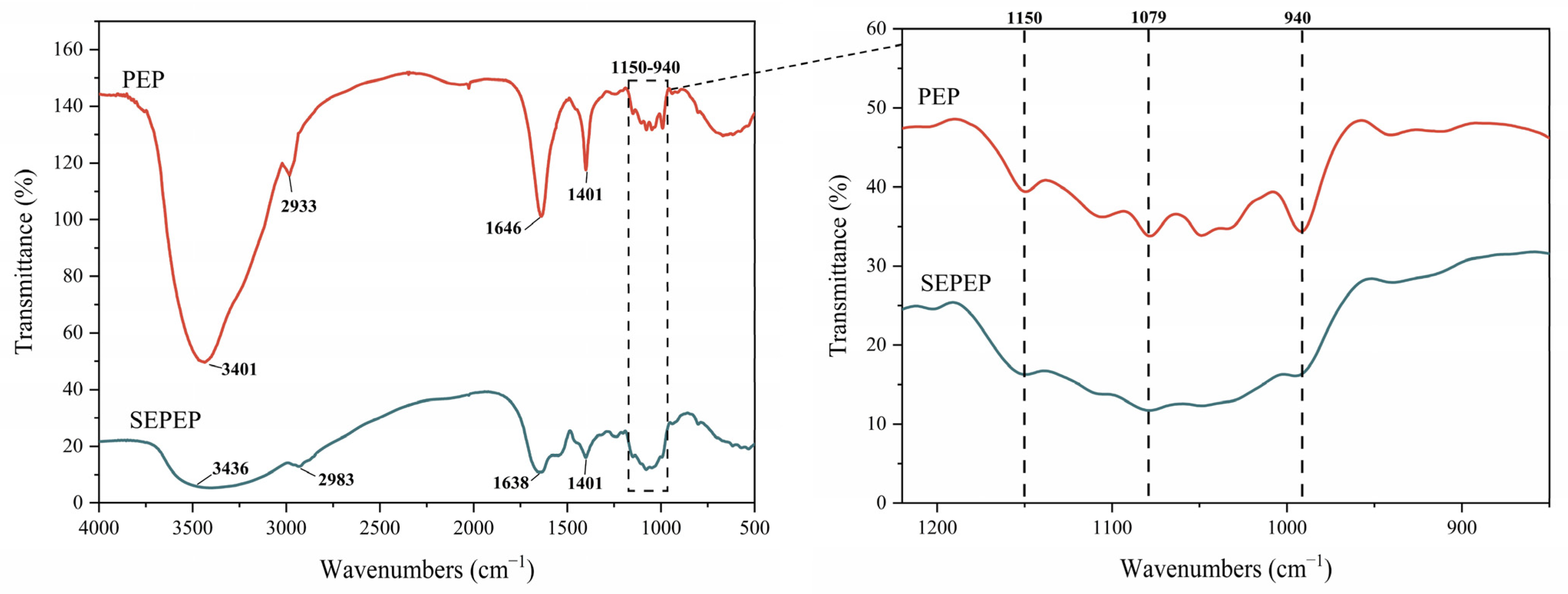
3.4. FTIR Analysis of Pleurotus eryngii Polysaccharides (PEP)
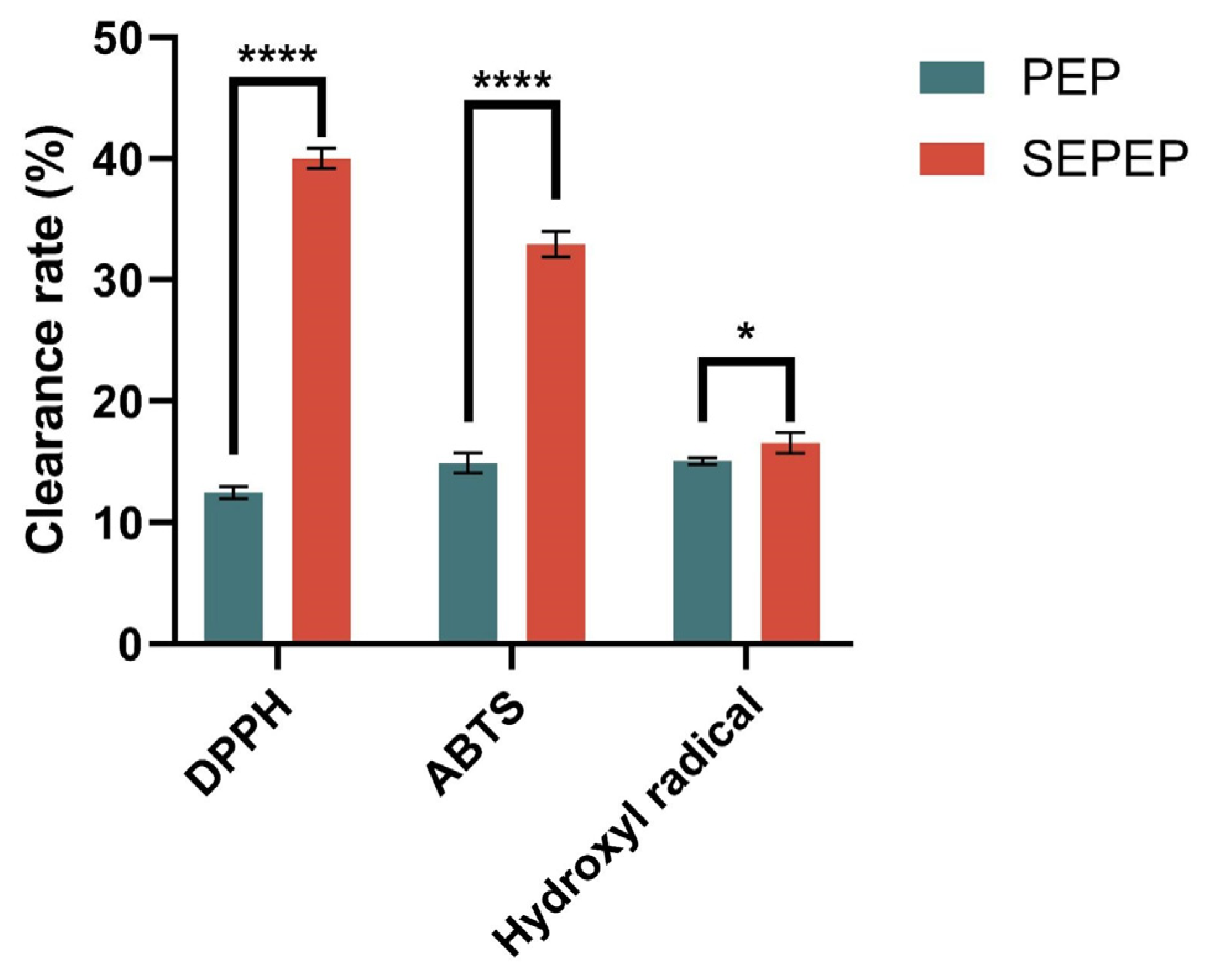
3.5. Effects of Steam Explosion (SE)-Assisted Extraction on the Antioxidant Capacity of Pleurotus eryngii Polysaccharides (PEP)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Huang, W.; Xiong, C.; Zhao, J. Transcriptome analysis reveals the role of nitric oxide in Pleurotus eryngii responses to Cd2+ stress. Chemosphere 2018, 201, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Petraglia, T.; Latronico, T.; Fanigliulo, A.; Crescenzi, A.; Liuzzi, G.M.; Rossano, R. Antioxidant Activity of Polysaccharides from the Edible Mushroom Pleurotus eryngii. Molecules 2023, 28, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-González, J.A.; Serna-Saldívar, S.R.O.; Gutiérrez-Uribe, J.A. Nutritional composition and nutraceutical properties of the Pleurotus fruiting bodies: Potential use as food ingredient. J. Food Compos. Anal. 2017, 58, 69–81. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Zhang, F.; Linhardt, R.J.; Zeng, G.; Zhang, A. Extraction, structure and bioactivities of the polysaccharides from Pleurotus eryngii: A review. Int. J. Biol. Macromol. 2020, 150, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Gong, P.; Li, Z.; Wu, Y.; Zhang, J.; Wang, J.; Yao, W.; Yang, W.; Chen, F. Advances in the mechanisms of polysaccharides in alleviating depression and its complications. Phytomedicine 2023, 109, 154566. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Gong, P.; Wang, R.; Qi, Z.; Deng, Z.; Han, A.; Long, H.; Wang, J.; Yao, W.; et al. Advances in Postharvest Storage and Preservation Strategies for Pleurotus eryngii. Foods 2023, 12, 1046–1064. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Gong, P. Classification, structure and mechanism of antiviral polysaccharides derived from edible and medicinal fungus. Int. J. Biol. Macromol. 2021, 183, 1753–1773. [Google Scholar] [CrossRef]
- Tu, J.; Brennan, M.; Brennan, C. An insight into the mechanism of interactions between mushroom polysaccharides and starch. Curr. Opin. Food Sci. 2021, 37, 17–25. [Google Scholar] [CrossRef]
- Gong, P.; Long, H.; Guo, Y.; Wang, S.; Chen, F.; Chen, X. Isolation, Structural Characterization, and Hypoglycemic Activities In Vitro of Polysaccharides from Pleurotus eryngii. Molecules 2022, 27, 7140–7158. [Google Scholar] [CrossRef]
- Yi, J.; Li, X.; Wang, S.; Wu, T.; Liu, P. Steam explosion pretreatment of Achyranthis bidentatae radix: Modified polysaccharide and its antioxidant activities. Food Chem. 2022, 375, 131746. [Google Scholar] [CrossRef]
- Li, B.; Yang, W.; Nie, Y.; Kang, F.; Goff, H.D.; Cui, S.W. Effect of steam explosion on dietary fiber, polysaccharide, protein and physicochemical properties of okara. Food Hydrocoll. 2019, 94, 48–56. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, M.; Xie, S.; Peng, D.; An, M.; Chen, Y.; Li, P.; Du, B. Effect of steam explosion pretreatment on polysaccharide isolated from Poria cocos: Structure and immunostimulatory activity. J. Food Biochem. 2022, 46, 14355. [Google Scholar] [CrossRef]
- Xue, H.; Li, P.; Bian, J.; Gao, Y.; Sang, Y.; Tan, J. Extraction, purification, structure, modification, and biological activity of traditional Chinese medicine polysaccharides: A review. Front. Nutr. 2022, 9, 1005181. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Yang, W.; Huang, H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci. Technol. 2021, 109, 564–568. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.; Wang, X.; Huang, X.; Fei, Y.; Yu, Y.; Shou, D. Antioxidant property of water-soluble polysaccharides from Poria cocos Wolf using different extraction methods. Int. J. Biol. Macromol. 2016, 83, 103–110. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Wang, J.; Wu, Z.G.; Yang, J.M.; Li, W.; Shen, L.X. Extraction, purification and anti-proliferative activities of polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 2016, 93, 136–144. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waskiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Chen, Y.; Shan, S.; Cao, D.; Tang, D. Steam flash explosion pretreatment enhances soybean seed coat phenolic profiles and antioxidant activity. Food Chem. 2020, 319, 126552. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; He, X.; Li, F.; Zhao, J.; Yin, R.; Ming, J. Effects of steam explosion pretreatment on the composition and biological activities of tartary buckwheat bran phenolics. Food Funct. 2020, 11, 4648–4658. [Google Scholar] [CrossRef]
- Cui, W.; Wang, Y.; Sun, Z.; Cui, C.; Li, H.; Luo, K.; Cheng, A. Effects of steam explosion on phenolic compounds and dietary fiber of grape pomace. LWT 2023, 173, 114350. [Google Scholar] [CrossRef]
- Ma, Q.; Yu, Y.; Zhou, Z.; Wang, L.; Cao, R. Effects of different treatments on composition, physicochemical and biological properties of soluble dietary fiber in buckwheat bran. Food Biosci. 2023, 53, 102517. [Google Scholar] [CrossRef]
- Tu, J.; Liu, H.; Sun, N.; Liu, S.; Chen, P. Optimization of the Steam Explosion Pretreatment Effect on Total Flavonoids Content and Antioxidative Activity of Seabuckthom Pomace by Response Surface Methodology. Molecules 2019, 24, 60–68. [Google Scholar] [CrossRef]
- Dorado, C.; Cameron, R.G.; Manthey, J.A. Study of Static Steam Explosion of Citrus sinensis Juice Processing Waste for the Isolation of Sugars, Pectic Hydrocolloids, Flavonoids, and Peel Oil. Food Bioprocess Technol. 2019, 12, 1293–1303. [Google Scholar] [CrossRef]
- Yue, P.; Hu, Y.; Tian, R.; Bian, J.; Peng, F. Hydrothermal pretreatment for the production of oligosaccharides: A review. Bioresour. Technol. 2022, 343, 126075. [Google Scholar] [CrossRef]
- Gong, L.; Huang, L.; Zhang, Y. Effect of steam explosion treatment on barley bran phenolic compounds and antioxidant capacity. J. Agric. Food Chem. 2012, 60, 7177–7184. [Google Scholar] [CrossRef]
- Cheng, A.; Hou, C.; Sun, J.; Wan, F. Effect of steam explosion on phenolic compounds and antioxidant capacity in adzuki beans. J. Sci. Food Agric. 2020, 100, 4495–4503. [Google Scholar] [CrossRef]
- Chen, G.Z.; Chen, H.Z. Extraction and deglycosylation of flavonoids from sumac fruits using steam explosion. Food Chem. 2011, 126, 1934–1938. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Y.; Mo, H.; Ma, H.; Zhao, J. Catapult steam explosion significantly increases cellular antioxidant and anti-proliferative activities of Adinandra nitida leaves. J. Funct. Foods 2016, 23, 423–431. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Sun, Y.-Y.; Jia, Y.-Q.; Geng, X.-Q.; Pan, L.-C.; Jiang, W.; Xie, B.-Y.; Zhu, Z.-Y. Effect of steam explosion pretreatment on the structure and bioactivity of Ampelopsis grossedentata polysaccharides. Int. J. Biol. Macromol. 2021, 185, 194–205. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.M.; Qi, Z.M.; Wang, S.Y.; Liu, S.X.; Li, X.; Wang, H.J.; Xia, X.C. An overview on natural polysaccharides with antioxidant properties. Curr. Med. Chem. 2013, 20, 2899–2913. [Google Scholar] [CrossRef]
- Noda, Y.; Asada, C.; Sasaki, C.; Hashimoto, S.; Nakamura, Y. Extraction method for increasing antioxidant activity of raw garlic using steam explosion. Biochem. Eng. J. 2013, 73, 1–4. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.-R.; Liu, W.; Su, Y.; Han, Q.-H.; Zhao, L.; Zhang, Q.; Lin, D.-R.; et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef]
- Mo, X.H.; Xiang, H.J.; Wei, L.; Xia, L.H.; Chen, X.Y.; Chen, Y.; Zhang, B. Nature-inspired allomelanin nanomedicine alleviates cardiac ischemia/reperfusion injury via scavenging free radicals and ameliorating myocardial microenvironment. Nano Today 2022, 46, 101589. [Google Scholar] [CrossRef]
- Noda, Y.; Asada, C.; Sasaki, C.; Nakamura, Y. Effects of Hydrothermal Methods such as Steam Explosion and Microwave Irradiation on Extraction of Water Soluble Antioxidant Materials from Garlic Husk. Waste Biomass Valorization 2018, 10, 3397–3402. [Google Scholar] [CrossRef]
- Cui, C.; Mei, L.; Wang, D.; Jia, P.; Zhou, Q.; Liu, W. A self-stabilized and water-responsive deliverable coenzyme-based polymer binary elastomer adhesive patch for treating oral ulcer. Nat. Commun. 2023, 14, 7707. [Google Scholar] [CrossRef]
- Wang, J.; Shi, S.; Li, F.; Du, X.; Kong, B.; Wang, H.; Xia, X. Physicochemical properties and antioxidant activity of polysaccharides obtained from sea cucumber gonads via ultrasound-assisted enzymatic techniques. LWT 2022, 160, 113307. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Y.; Feng, X.; Zhang, L.; Ibrahim, S.A.; Huang, W.; Liu, Y. Effects of degradation on the physicochemical and antioxidant properties of carboxymethyl pachymaran. Int. J. Biol. Macromol. 2023, 245, 125560. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, B.; Lin, R.; Jia, L.; Deng, P.; Fan, K.; Wang, G.; Wang, L.; Zhang, J. Extraction and antioxidant activities of intracellular polysaccharide from Pleurotus sp. mycelium. Int. J. Biol. Macromol. 2010, 47, 116–119. [Google Scholar] [CrossRef]
- Li, S.; Shah, N.P. Characterization, antioxidative and bifidogenic effects of polysaccharides from Pleurotus eryngii after heat treatments. Food Chem. 2016, 197, 240–249. [Google Scholar] [CrossRef]
- Hao, C.; Yang, J.; Liang, T.; Zhang, J.; Sun, R. Structural elucidation and morphological observation of a polysaccharide from Pleurotus eryngii obtained by alkaline extraction. J. Carbohydr. Chem. 2017, 36, 129–140. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, X.; Tian, X.; Li, R.; Sui, W.; Liu, R.; Wu, T.; Zhang, M. Isolation and Purification, Structural Characterization and Antioxidant Activities of a Novel Hetero-Polysaccharide from Steam Exploded Wheat Germ. Foods 2022, 11, 1245–1258. [Google Scholar] [CrossRef]
- Long, D.; Ye, F.; Zhao, G. Optimization and characterization of wheat bran modified by in situ enhanced CO2 blasting extrusion. LWT Food Sci. Technol. 2014, 59, 605–611. [Google Scholar] [CrossRef]
- de Barros, R.D.; Becarelli, P.; de Oliveira, R.A.; Tognotti, L.; da Silva Bon, E.P. Triticum spelta straw hydrothermal pretreatment for the production of glucose syrups via enzymatic hydrolysis. Biochem. Eng. J. 2019, 151, 107340. [Google Scholar] [CrossRef]
- Chen, L.-C.; Jiang, B.-K.; Zheng, W.-H.; Zhang, S.-Y.; Li, J.-J.; Fan, Z.-Y. Preparation, characterization and anti-diabetic activity of polysaccharides from adlay seed. Int. J. Biol. Macromol. 2019, 139, 605–613. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, Y.-F.; Zhang, B.-W.; Liu, Y.; Wang, J.-H. Purification, structural characterization and immunostimulatory activity of polysaccharides from Umbilicaria esculenta. Int. J. Biol. Macromol. 2021, 181, 743–751. [Google Scholar] [CrossRef]
- Jiang, P.; Meng, W.; Shi, F.; Chen, C.; Sun, Y.; Jiao, L. Structural characteristics, antioxidant properties and antiaging activities of galactan produced by Mentha haplocalyx Briq. Carbohydr. Polym. 2020, 234, 115936. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Liu, J.; Li, R.; Zhou, J.; Li, M.; Lu, J.; Zhao, G.; Li, X.; Sui, W.; et al. Steam explosion improves extractability, antioxidant activity and α-glucosidase inhibitory activity of the constituents of Java tea (Clerodendranthus spicatus). Innov. Food Sci. Emerg. Technol. 2023, 86, 103350. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; Zheng, W.; Gao, Y.; Wang, M.; Zhang, Y.; Gao, Q. Charaterization and immunomodulatory activities of polysaccharide isolated from Pleurotus eryngii. Int. J. Biol. Macromol. 2016, 92, 30–36. [Google Scholar] [CrossRef]
| Factors | Levels | |
|---|---|---|
| 1 | 2 | |
| (A) Steam pressure (MPa) | 1.2 | 1.4 |
| (B) Stable pressure time (s) | 60 | 75 |
| (C) Solid-liquid ratio (g/mL) | 1:40 | 1:50 |
| (D) Extraction time (h) | 3 | 4 |
| (E) Extraction temperature (°C) | 80 | 90 |
| Runs | (A) Steam Pressure (MPa) | (B) Stable Pressure Time (s) | (C) Solid-Liquid Ratio (g/mL) | (D) Extraction Time (h) | (E) Extraction Temperature (°C) |
|---|---|---|---|---|---|
| 1 | 1 (1.2) | 1 (60) | 1 (1:40) | 1 (3) | 1 (80) |
| 2 | 1 | 1 | 1 | 2 (4) | 2 (90) |
| 3 | 1 | 2 (75) | 2 (1:50) | 1 | 1 |
| 4 | 1 | 2 | 2 | 2 | 2 |
| 5 | 2 (1.4) | 1 | 2 | 1 | 2 |
| 6 | 2 | 1 | 2 | 2 | 1 |
| 7 | 2 | 2 | 1 | 1 | 2 |
| 8 | 2 | 2 | 1 | 2 | 1 |
| Runs | (A) Steam Pressure (MPa) | (B) Stable Pressure Time (s) | (C) Solid-Liquid Ratio | (D) Extraction Time (h) | (E) Extraction Temperature (°C) | Polysaccharide Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 1.2 | 60 | 1:40 | 3 | 80 | 13.16 |
| 2 | 1.2 | 60 | 1:40 | 4 | 90 | 13.46 |
| 3 | 1.2 | 75 | 1:50 | 3 | 80 | 9.19 |
| 4 | 1.2 | 75 | 1:50 | 4 | 90 | 10.34 |
| 5 | 1.4 | 60 | 1:50 | 3 | 90 | 13.01 |
| 6 | 1.4 | 60 | 1:50 | 4 | 80 | 12.81 |
| 7 | 1.4 | 75 | 1:40 | 3 | 90 | 13.71 |
| 8 | 1.4 | 75 | 1:40 | 4 | 80 | 15.63 |
| K1j | 46.16 | 52.45 | 55.96 | 49.08 | 50.78 | |
| K2j | 55.16 | 48.87 | 45.35 | 52.24 | 50.53 | |
| k1j | 11.54 | 13.11 | 13.99 | 12.27 | 12.70 | |
| k2j | 13.79 | 12.22 | 11.34 | 13.06 | 12.63 | |
| Rj | 2.25 | 0.89 | 2.65 | 0.79 | 0.06 |
| Samples | PEP | SEPEP |
|---|---|---|
| Polysaccharide yield (%) | 7.52 ± 0.08 | 17.89 ± 0.40 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.; Zheng, P.; Dai, W.; Zheng, Z.; Lin, X.; Hu, J.; Zeng, S.; Lin, S. Steam Explosion-Assisted Extraction of Polysaccharides from Pleurotus eryngii and Its Influence on Structural Characteristics and Antioxidant Activity. Foods 2024, 13, 1229. https://doi.org/10.3390/foods13081229
Qiu J, Zheng P, Dai W, Zheng Z, Lin X, Hu J, Zeng S, Lin S. Steam Explosion-Assisted Extraction of Polysaccharides from Pleurotus eryngii and Its Influence on Structural Characteristics and Antioxidant Activity. Foods. 2024; 13(8):1229. https://doi.org/10.3390/foods13081229
Chicago/Turabian StyleQiu, Jianqing, Peiying Zheng, Wanzhen Dai, Zhijun Zheng, Xiaohui Lin, Jiamiao Hu, Shaoxiao Zeng, and Shaoling Lin. 2024. "Steam Explosion-Assisted Extraction of Polysaccharides from Pleurotus eryngii and Its Influence on Structural Characteristics and Antioxidant Activity" Foods 13, no. 8: 1229. https://doi.org/10.3390/foods13081229
APA StyleQiu, J., Zheng, P., Dai, W., Zheng, Z., Lin, X., Hu, J., Zeng, S., & Lin, S. (2024). Steam Explosion-Assisted Extraction of Polysaccharides from Pleurotus eryngii and Its Influence on Structural Characteristics and Antioxidant Activity. Foods, 13(8), 1229. https://doi.org/10.3390/foods13081229





