The Effects of Four Different Thawing Methods on Quality Indicators of Amphioctopus neglectus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Methods
2.2.1. Sample Pretreatment
2.2.2. Thawing Loss Rate
2.2.3. Water-Holding Capacity
2.2.4. Cooking Loss Rate
2.2.5. pH
2.2.6. Color
2.2.7. Texture
2.2.8. Volatile Basic Nitrogen (TVB–N) Value
2.2.9. Extraction and Determination of Mass Concentration of Myofibrillar Protein
2.2.10. Determination the Activity of Ca2+–ATPase
2.2.11. Determination of Total Sulfhydryl Content
2.2.12. Water Distribution
2.2.13. Data Processing and Analysis
3. Results
3.1. Effects of Thawing Methods on the Thawing Time and Water Retention of A. neglectus
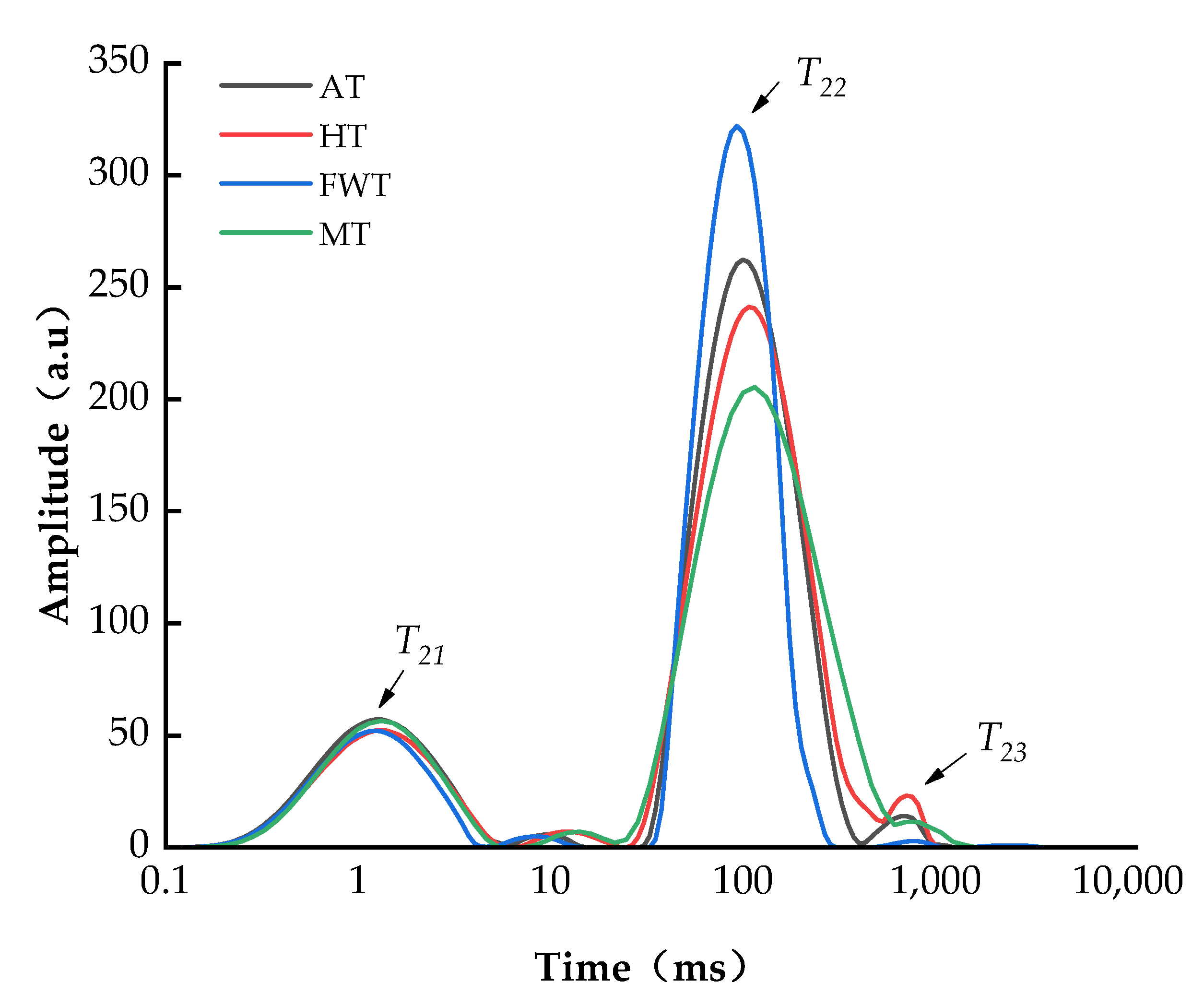
3.2. Effects of Thawing Mode on Water Distribution of A. neglectus
3.3. Impact of Thawing Methods on the Color and pH of A. neglectus
3.4. Effects of Thawing Methods on the Texture of A. neglectus
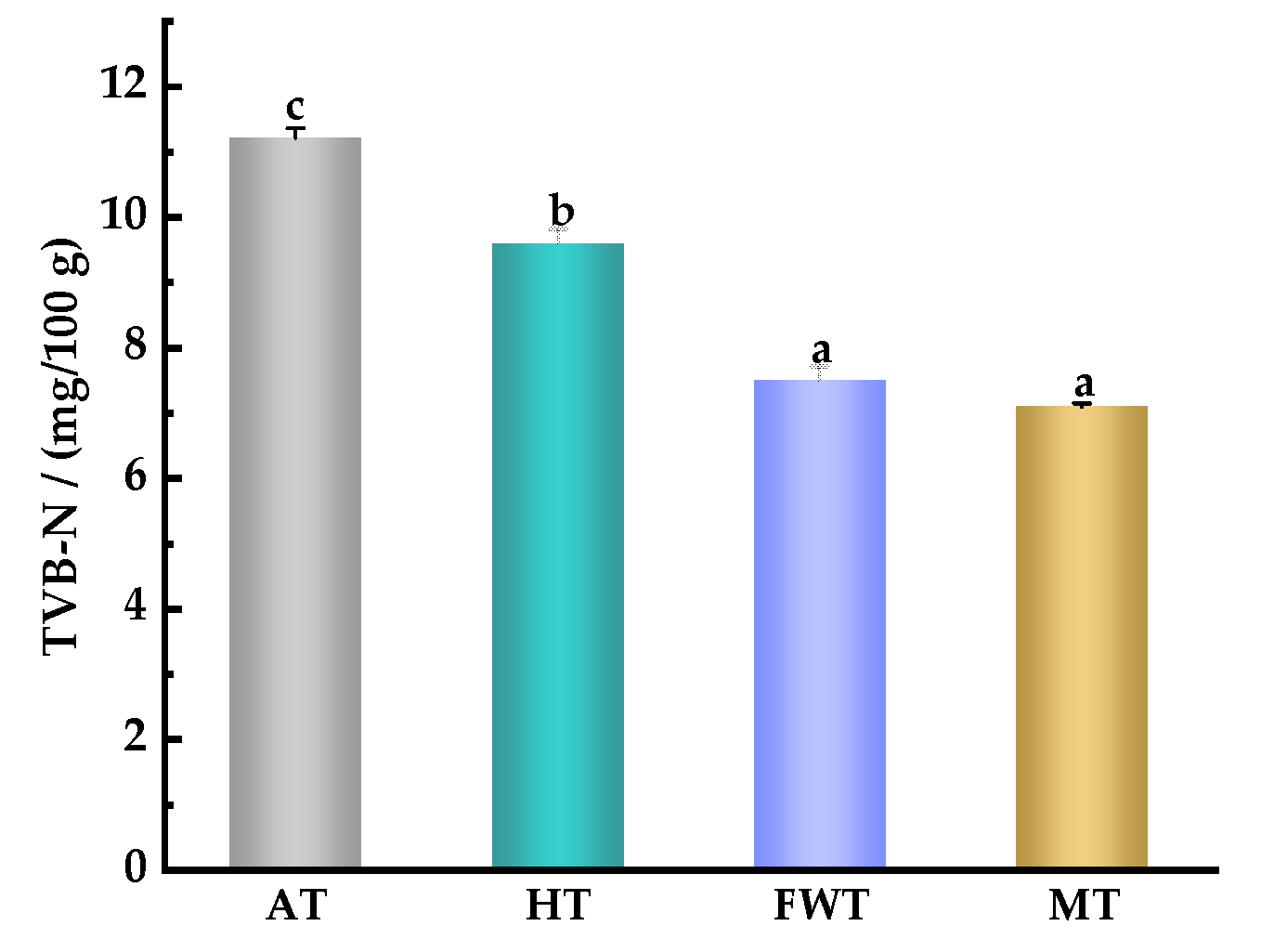
3.5. Effects of Thawing Mode on the Content of Total Volatile Basic Nitrogen (TVB–N) in A. neglectus Muscle
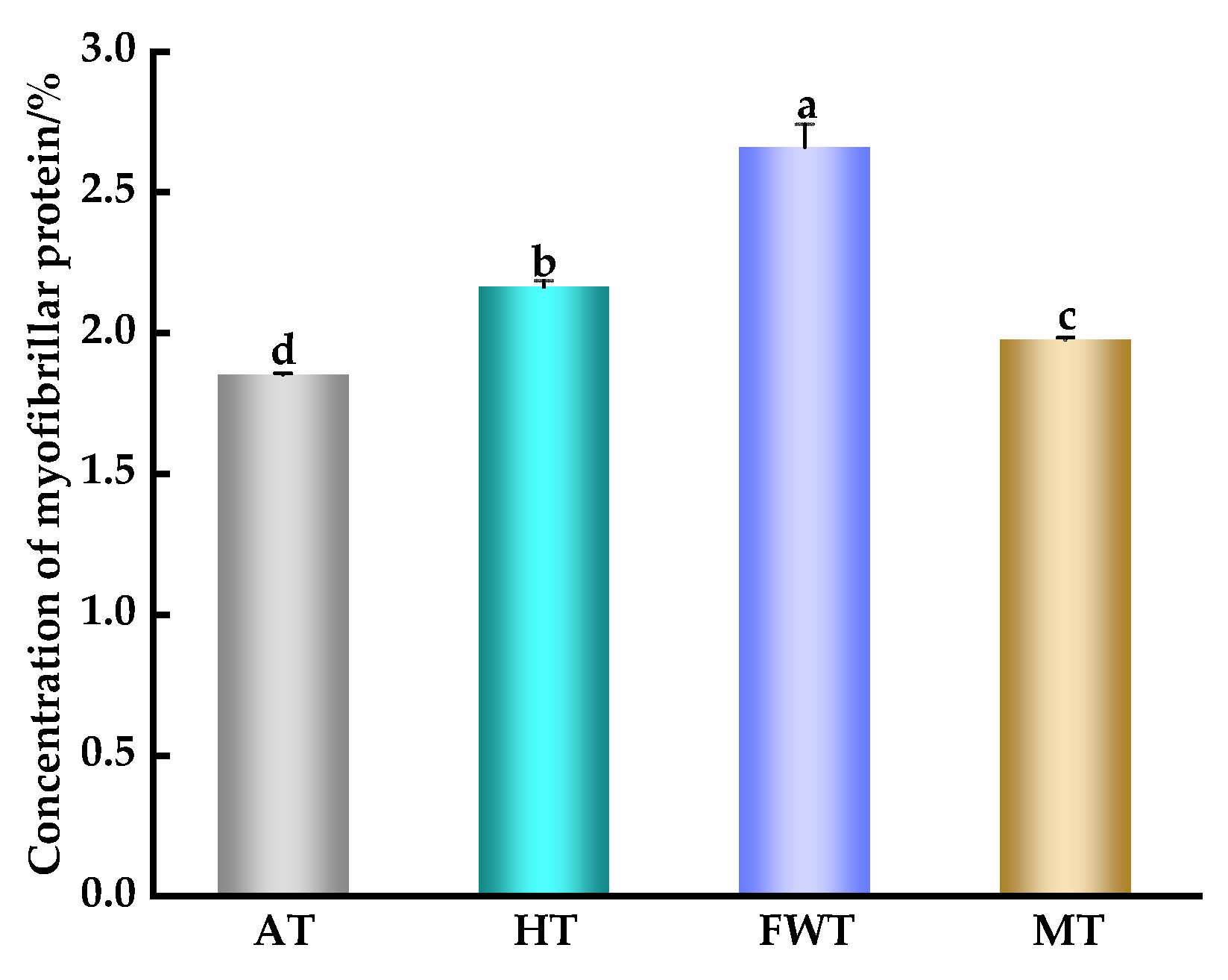
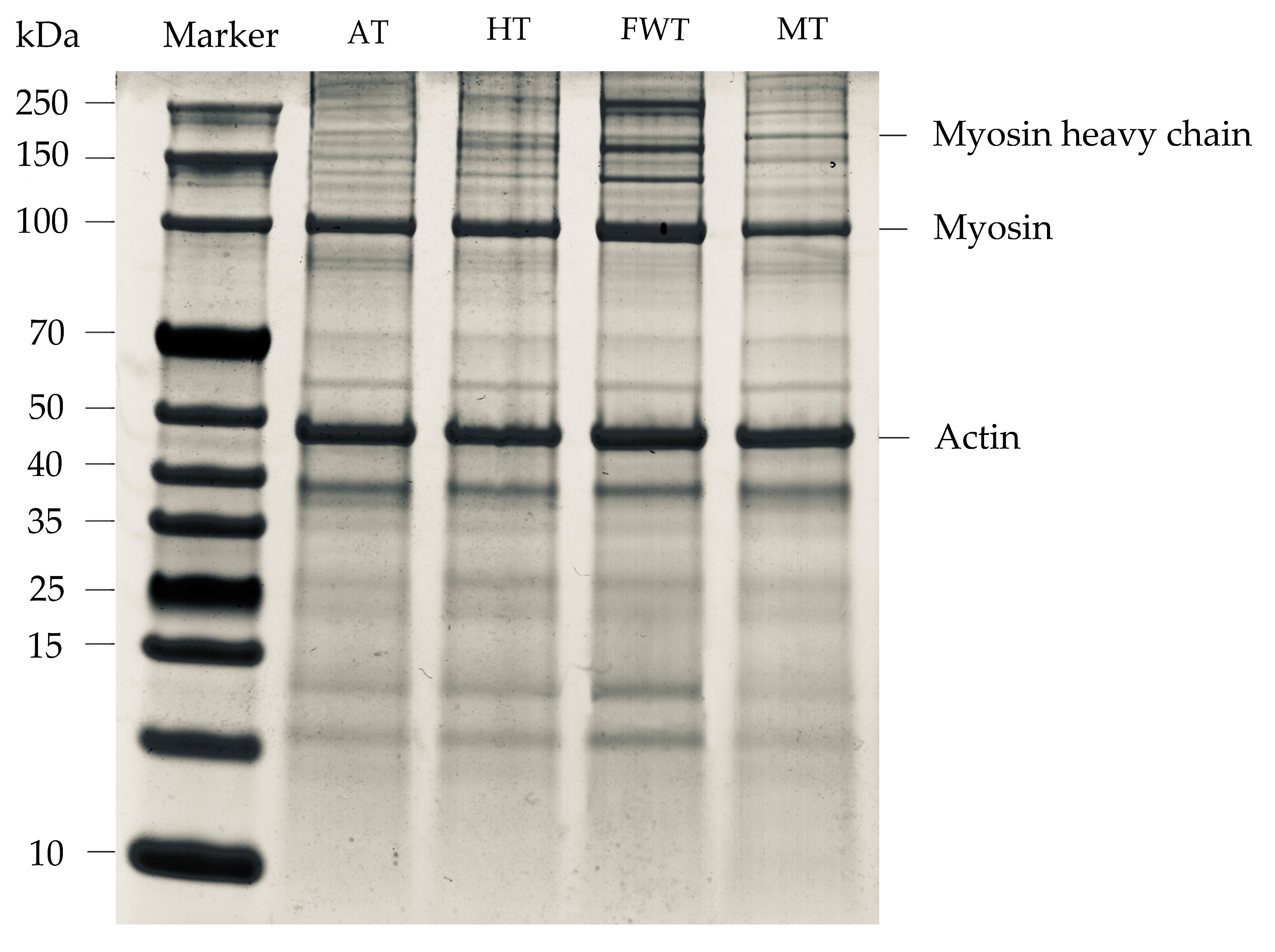
3.6. Impact of Thawing Methods on Protein Oxidation in A. neglectus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, Y.; Zheng, X.; Liu, H.; Sunxie, F. Population genetics and comparative mitogenomic analyses reveal cryptic diversity of Amphioctopus neglectus (Cephalopoda: Octopodidae). Genomics 2020, 112, 3893–3902. [Google Scholar] [CrossRef] [PubMed]
- Méndez Aguilar, F.D.; Olvera Novoa, M.Á.; Rodríguez Morales, S.; Rosas Vázquez, C. Nutritive value of four by-product meals as potential protein sources in diets for Octopus maya. Hidrobiológica 2014, 24, 69–77. [Google Scholar]
- Méndez, L.; Rodríguez, A.; Aubourg, S.P.; Medina, I. Low-Toxicity Solvents for the Extraction of Valuable Lipid Compounds from Octopus (Octopus vulgaris) Waste. Foods 2023, 12, 3631. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Krishnan, S.; Joy, M. Macrocyclic lactones from seafood Amphioctopus neglectus: Newly described natural leads to attenuate angiotensin-II induced cardiac hypertrophy. Biomed. Pharmacother. 2019, 110, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.M.; Silva, F.; Pinto, F.R.; Barroso, S.; Gil, M.M. Quality assessment of chilled and frozen fish—Mini review. Foods 2020, 9, 1739. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Khan, M.I.; Faiz, F. Effect of thawing on frozen meat quality: A comprehensive review. Pak. J. Food Sci. 2013, 23, 198–211. [Google Scholar]
- Sun, Q.; Dong, X.; Zheng, O.; Liu, S.; Kong, B. Protein oxidation/aggregation during ultrasound thawing at different powers impair the gel properties of common carp (Cyprinus carpio) myofibrillar protein. LWT 2024, 191, 115592. [Google Scholar] [CrossRef]
- Choi, E.J.; Park, H.W.; Chung, Y.B.; Park, S.H.; Kim, J.S.; Chun, H.H. Effect of tempering methods on quality changes of pork loin frozen by cryogenic immersion. Meat Sci. 2017, 124, 69–76. [Google Scholar] [CrossRef] [PubMed]
- HY, D.; Lin, S.; Chen, D.; Liang, R.; Sun, N. Effect of Thawing Methods on the Physico-chemical and Taste Charasteristics of Antarctic Krill Meat. J. Chin. Inst. Food Sci. Technol. 2023, 23, 228–238. [Google Scholar]
- Zhou, P.C.; Xie, J. Effect of different thawing methods on the quality of mackerel (Pneumatophorus japonicus). Food Sci. Biotechnol. 2021, 30, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Xie, J. Quality of cuttlefish as affected by different thawing methods. Int. J. Food Prop. 2022, 25, 33–52. [Google Scholar] [CrossRef]
- Bao, Y.; Ertbjerg, P. Effects of protein oxidation on the texture and water-holding of meat: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3564–3578. [Google Scholar] [CrossRef] [PubMed]
- Gokoglu, N.; Topuz, O.K.; Yerlikaya, P.; Yatmaz, H.A.; Ucak, I. Effects of freezing and frozen storage on protein functionality and texture of some cephalopod muscles. J. Aquat. Food Prod. Technol. 2018, 27, 211–218. [Google Scholar] [CrossRef]
- Jiang, Q.; Jia, R.; Nakazawa, N.; Hu, Y.; Osako, K.; Okazaki, E. Changes in protein properties and tissue histology of tuna meat as affected by salting and subsequent freezing. Food Chem. 2019, 271, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, C.; Lu, J.; Bao, Y.; Han, B.; Zhang, J.; Duan, S.; Song, Z.; Chen, H. Study on the thawing characteristics of beef in ultrasound combined with plasma-activated water. Food Chem. X 2024, 21, 101104. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Hu, R.; Zhao, J.H.; Peng, Y.; Ni, Y.Y. Evaluation of the effects of different thawing methods on texture, colour and ascorbic acid retention of frozen hami melon (Cucumis melo var. saccharinus). Int. J. Food Sci. Technol. 2015, 50, 1116–1122. [Google Scholar] [CrossRef]
- Torres, J.A.; Saraiva, J.A.; Guerra-Rodríguez, E.; Aubourg, S.P.; Vázquez, M. Effect of combining high-pressure processing and frozen storage on the functional and sensory properties of horse mackerel (Trachurus trachurus). Innov. Food Sci. Emerg. Technol. 2014, 21, 2–11. [Google Scholar] [CrossRef]
- GB 5009228-2016. National Food Safety Standard Determination of Volatile Basic Nitrogen in Food. Standards Press of China: Beijing, China, 2016.
- Benjakul, S.; Seymour, T.A.; Morrissey, M.T.; An, H. Physicochemical changes in Pacific whiting muscle proteins during iced storage. J. Food Sci. 1997, 62, 729–733. [Google Scholar] [CrossRef]
- Ge, X.; Wang, H.; Yin, M.; Wang, X. Effect of different thawing methods on the physicochemical properties and microstructure of frozen instant sea cucumber. Foods 2022, 11, 2616. [Google Scholar] [CrossRef]
- Cai, L.; Cao, M.; Regenstein, J.; Cao, A. Recent advances in food thawing technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 953–970. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.H.; Wang, J.F.; Xie, J. Effects of different thawing methods on tuna quality. Food Ferment. Ind. 2019, 45, 189–197. [Google Scholar]
- Shen, Y.; Huang, H.; Wu, Y.Y. Comparison of 5 thawing methods on product quality of frozen purpleback flying squid Sthenoteuthis oualaniensis. J. Food Saf. Qual. 2014, 5, 4092–4096. [Google Scholar]
- Sun, Y.; Luo, W.; He, M.; Zhao, Y.; Sun, J.; Mao, X. Effects of different thawing methods on the physicochemical properties and myofibrillar protein characteristics of Litopenaeus vannamei. LWT 2024, 192, 115668. [Google Scholar] [CrossRef]
- Li, D.; Zhao, H.; Muhammad, A.I.; Song, L.; Guo, M.; Liu, D. The comparison of ultrasound-assisted thawing, air thawing and water immersion thawing on the quality of slow/fast freezing bighead carp (Aristichthys nobilis) fillets. Food Chem. 2020, 320, 126614. [Google Scholar] [CrossRef]
- Yerlikaya, P.; Gökoǧlu, N. Quality changes of blue crab (Callinectes sapidus) meat during frozen storage. J. Food Qual. 2004, 27, 83–89. [Google Scholar] [CrossRef]
- Tan, M.T.; Xie, J.; Wang, J.F. Effects of different thawing methods on quality of squid. Food Sci. 2019, 40, 94–101. [Google Scholar]
- Zhang, G.; Zhu, C.; Walayat, N.; Nawaz, A.; Ding, Y.; Liu, J. Recent development in evaluation methods, influencing factors and control measures for freeze denaturation of food protein. Crit. Rev. Food Sci. Nutr. 2023, 63, 5874–5889. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Xie, Q.; Wang, Y.; Yu, J.; Ma, X. A comprehensive review of the principles, key factors, application, and assessment of thawing technologies for muscle foods. Compr. Rev. Food Sci. Food Saf. 2023, 22, 107–134. [Google Scholar] [CrossRef]
- Speranza, B.; Bevilacqua, A.; Corbo, M.R. Food spoilage and safety: Some key-concepts. In Application of Alternative Food-Preservation Technologies to Enhance Food Safety and Stability; Bentham Publisher: Abu Dhabi, United Arab Emirates, 2010; pp. 17–34. [Google Scholar]
- Felby, C.; Thygesen, L.G.; Kristensen, J.B.; Jørgensen, H.; Elder, T. Cellulose–water interactions during enzymatic hydrolysis as studied by time domain NMR. Cellulose 2008, 15, 703–710. [Google Scholar] [CrossRef]
- Qian, S.; Li, X.; Wang, H.; Mehmood, W.; Zhong, M.; Zhang, C.; Blecker, C. Effects of low voltage electrostatic field thawing on the changes in physicochemical properties of myofibrillar proteins of bovine Longissimus dorsi muscle. J. Food Eng. 2019, 261, 140–149. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Z.; Sun, D.W. Effects of freezing on cell structure of fresh cellular food materials: A review. Trends Food Sci. Technol. 2018, 75, 46–55. [Google Scholar] [CrossRef]
- Jiang, Q.Q.; Wu, C.H.; Dong, K.C.; Chen, S.G.; Ye, X.Q.; Hu, Y.Q. Effect of thawing methods on the characteristics of protein and the quality of muscle in Hairtail (Trichiurus haumela). J. Chin. Inst. Food Sci. Technol. 2016, 16, 17–27. [Google Scholar]
- Xia, X.; Kong, B.; Liu, J.; Diao, X.; Liu, Q. Influence of different thawing methods on physicochemical changes and protein oxidation of porcine longissimus muscle. LWT-Food Sci. Technol. 2012, 46, 280–286. [Google Scholar] [CrossRef]
- López-Bote, C. Chemical and biochemical constitution of muscle. In Lawrie´ s Meat Science; Elsevier: Amsterdam, The Netherlands, 2017; pp. 99–158. [Google Scholar]
- Liu, Y.X.; Cao, M.J.; Liu, G.M. Texture analyzers for food quality evaluation. In Evaluation Technologies for Food Quality; Elsevier: Amsterdam, The Netherlands, 2019; pp. 441–463. [Google Scholar]
- Bekhit, A.E.D.A.; Holman, B.W.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- GB 2733-2015, Fresh and Frozen Animal Aquatic Products. Standards Press of China: Beijing, China, 2015.
- Li, H.Q.; Ma, L.Z.; Chen, S.J.; Wang, Y.Q.; Hu, X.; Huang, H.; Deng, J.C. Effect of different thawing methods on muscle quality of Sthenoteuthis oualaniensis. J. Food Sci. Biotechnol. 2023, 42, 20–28. [Google Scholar]
- Li, F.; Wang, B.; Kong, B.; Shi, S.; Xia, X. Decreased gelling properties of protein in mirror carp (Cyprinus carpio) are due to protein aggregation and structure deterioration when subjected to freeze-thaw cycles. Food Hydrocoll. 2019, 97, 105223. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.; Zhang, W.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Bohrer, B.; Lorenzo, J.M. Protein oxidation in muscle foods: A comprehensive review. Antioxidants 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xue, Y.; Li, Z.; Wang, Y.; Yang, W.; Xue, C. Effects of ozone-induced oxidation on the physicochemical properties of myofibrillar proteins recovered from bighead carp (Hypophthalmichthys nobilis). Food Bioprocess Technol. 2015, 8, 181–190. [Google Scholar] [CrossRef]
- Cai, L.; Wan, J.; Li, X.; Li, J. Effects of different thawing methods on conformation and oxidation of myofibrillar protein from largemouth bass (Micropterus salmoides). J. Food Sci. 2020, 85, 2470–2480. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Li, Z.; Pan, D.; Benjakul, S.; Li, X.; Zhang, B. Investigating the migration hypothesis: Effects of trypsin-like protease on the quality of muscle proteins of red shrimp (Solenocera crassicornis) during cold storage. Food Chem. X 2023, 20, 100906. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Kong, B.; Zheng, O.; Liu, S.; Dong, X. Effect of protein structure changes during different power ultrasound thawing on emulsification properties of common carp (Cyprinus carpio) myofibrillar protein. Ultrason. Sonochem. 2023, 101, 106719. [Google Scholar] [CrossRef] [PubMed]
- Cecil, R. Intramolecular bonds in proteins. I. The role of sulfur in proteins. Proteins 2012, 1, 379–476. [Google Scholar]
- Wang, B.; Kong, B.; Li, F.; Liu, Q.; Zhang, H.; Xia, X. Changes in the thermal stability and structure of protein from porcine longissimus dorsi induced by different thawing methods. Food Chem. 2020, 316, 126375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, G.; Xie, Q.; Wang, Y.; Yu, J.; Ma, X. Physicochemical and structural changes of myofibrillar proteins in muscle foods during thawing: Occurrence, consequences, evidence, and implications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3444–3477. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Song, L.L.; Cai, L.L.; Xiong, Y.F.; Yuan, G.F.; Fang, X.B.; Chen, X.E. Effect of different thawing methods on freshness and quality of Scomber japonicus. Food Sci. 2016, 37, 259–265. [Google Scholar]
- Xu, Z.; Zhu, Z.; Tu, M.; Chang, J.; Han, S.; Han, L.; Chen, H.; Tan, Z.; Du, M.; Li, T. Characterizations and the mechanism underlying cryoprotective activity of peptides from enzymatic hydrolysates of pseudosciaena crocea. Foods 2023, 12, 875. [Google Scholar] [CrossRef] [PubMed]



| Thawing Methods | Operation Methods |
|---|---|
| Air thawing (AT) | A sealed bag containing frozen octopi was placed on a tray and thawed in ambient air at room temperature (24 ± 3) °C. |
| Hydrostatic thawing (HT) | A sealed bag containing frozen octopi was placed in a water bath to thaw at a constant temperature at (20 ± 1) °C with a sample–liquid ratio of 1:10. |
| Flowing-water thawing (FWT) | A sealed bag containing frozen octopi was thawed under a uniform tap water flow at a rate of 50 mL/s. |
| Microwave thawing (MT) | The frozen octopi were thawed in a microwave at a power range of 500–600 W. |
| Water Retention Index | AT | HT | FWT | MT |
|---|---|---|---|---|
| Time/min | 400 ± 16.33 c | 115 ± 4.08 b | 39 ± 6.48 a | 20 ± 1.63 a |
| Thawing loss rate/% | 22.06 ± 0.76 b | 11.49 ± 1.68 a | 11.25 ± 0.78 a | 33.28 ± 2.30 c |
| Cooking loss rate/% | 42.65 ± 0.58 d | 36.34 ± 1.00 c | 26.50 ± 0.06 a | 32.75 ± 0.30 b |
| Water-holding capacity/% | 67.61 ± 2.23 c | 70.56 ± 5.67 bc | 76.94 ± 4.09 a | 74.25 ± 1.87 ab |
| Thawing Methods | AT | HT | FWT | MT |
|---|---|---|---|---|
| L* | 34.97 ± 0.83 b | 38.48 ± 5.33 ab | 40.30 ± 0.88 a | 31.43 ± 2.36 c |
| a* | 4.99 ± 0.33 c | 7.01 ± 0.20 a | 6.11 ± 0.15 b | 6.82 ± 0.69 a |
| b* | 9.02 ± 0.16 c | 10.33 ± 0.33 b | 11.01 ± 0.13 a | 8.07 ± 0.11 d |
| W | 34.16 ± 0.83 c | 37.22 ± 5.33 b | 38.99 ± 0.88 a | 30.62 ± 2.36 b |
| pH | 6.94 ± 0.02 a | 6.76 ± 0.02 b | 6.52 ± 0.01 d | 6.63 ± 0.02 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, S.; Li, S.; Chen, X.; Xu, M.; Su, Y.; Qiao, K.; Chen, X.; Chen, B.; Zhong, H.; et al. The Effects of Four Different Thawing Methods on Quality Indicators of Amphioctopus neglectus. Foods 2024, 13, 1234. https://doi.org/10.3390/foods13081234
Zhang H, Liu S, Li S, Chen X, Xu M, Su Y, Qiao K, Chen X, Chen B, Zhong H, et al. The Effects of Four Different Thawing Methods on Quality Indicators of Amphioctopus neglectus. Foods. 2024; 13(8):1234. https://doi.org/10.3390/foods13081234
Chicago/Turabian StyleZhang, Huixin, Shuji Liu, Shuigen Li, Xiaoe Chen, Min Xu, Yongchang Su, Kun Qiao, Xiaoting Chen, Bei Chen, Hong Zhong, and et al. 2024. "The Effects of Four Different Thawing Methods on Quality Indicators of Amphioctopus neglectus" Foods 13, no. 8: 1234. https://doi.org/10.3390/foods13081234





