Characterization of Grape Pomace Extract Microcapsules: The Influence of Carbohydrate Co-Coating on the Stabilization of Goat Whey Protein as a Primary Coating
Abstract
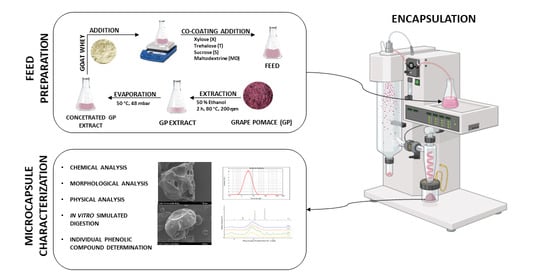
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Grape Pomace
2.3. Grape Pomace Extract Preparation
2.4. Encapsulation via Spray Drying
2.5. Total Phenolic Content Determination
2.6. Surface Phenolic Content Determination
2.7. Encapsulation Efficiency
2.8. Moisture and Dry Matter Content
2.9. Encapsulation Yield
2.10. Bulk Density and Tapped Density
2.11. Hausner Ratio and Carr Index
2.12. Particle Size Distribution
2.13. Determination of the Solubility of the Microcapsules
2.14. Contact Angle and Polarity
2.15. X-ray Powder Diffraction
2.16. Differential Scanning Calorimetry
2.17. Scanning Electron Microscopy
2.18. In Vitro Release of Phenolic Compounds
2.19. In Vitro Simulated Digestion and Bioaccessibility Index
2.20. Determination of Individual Phenolic Compounds
2.21. Accelerated Stability Test
2.22. Statistical Analysis
3. Results and Discussion
3.1. Encapsulation Efficiency and Encapsulation Yield
3.2. Microcapsule Characteristics
3.3. In Vitro Release of Phenolic Compounds from MCs
3.4. In vitro Simulated Digestion
3.5. Accelerated Stability of MCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable Green Processing of Grape Pomace for the Production of Value-Added Products: An Overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Koutelidakis, A. Grape Pomace: A Challenging Renewable Resource of Bioactive Phenolic Compounds with Diversified Health Benefits. MOJFPT 2016, 3, 00065. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; Da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Iora, S.R.F.; Maciel, G.M.; Zielinski, A.A.F.; Da Silva, M.V.; Pontes, P.V.D.A.; Haminiuk, C.W.I.; Granato, D. Evaluation of the Bioactive Compounds and the Antioxidant Capacity of Grape Pomace. Int. J. Food Sci. Tech. 2015, 50, 62–69. [Google Scholar] [CrossRef]
- Mišković Špoljarić, K.; Šelo, G.; Pešut, E.; Martinović, J.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Antioxidant and Antiproliferative Potentials of Phenolic-Rich Extracts from Biotransformed Grape Pomace in Colorectal Cancer. BMC Complement. Med. Ther. 2023, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morgado, B.; Candiracci, M.; Santa-María, C.; Revilla, E.; Gordillo, B.; Parrado, J.; Castaño, A. Obtaining from Grape Pomace an Enzymatic Extract with Anti-Inflammatory Properties. Plant Foods Hum. Nutr. 2015, 70, 42–49. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Zheng, L.; Li, J. Advance on the Bioactivity and Potential Applications of Dietary Fibre from Grape Pomace. Food Chem. 2015, 186, 207–212. [Google Scholar] [CrossRef]
- Muñoz-Bernal, Ó.A.; Coria-Oliveros, A.J.; de la Rosa, L.A.; Rodrigo-García, J.; del Rocío Martínez-Ruiz, N.; Sayago-Ayerdi, S.G.; Alvarez-Parrilla, E. Cardioprotective Effect of Red Wine and Grape Pomace. Food Res. Int. 2021, 140, 110069. [Google Scholar] [CrossRef]
- Luchian, C.E.; Cotea, V.V.; Vlase, L.; Toiu, A.M.; Colibaba, L.C.; Răschip, I.E.; Nadăş, G.; Gheldiu, A.M.; Tuchiluş, C.; Rotaru, L. Antioxidant and Antimicrobial Effects of Grape Pomace Extracts. BIO Web Conf. 2019, 15, 04006. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Antonopoulou, S. The French Paradox Three Decades Later: Role of Inflammation and Thrombosis. Clin. Chim. Acta 2020, 510, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Tsali, A.; Goula, A.M. Valorization of Grape Pomace: Encapsulation and Storage Stability of Its Phenolic Extract. Powder Technol. 2018, 340, 194–207. [Google Scholar] [CrossRef]
- Bassani, A.; Carullo, D.; Rossi, F.; Fiorentini, C.; Garrido, G.D.; Reklaitis, G.V.R.; Bonadies, I.; Spigno, G. Modeling of a Spray-Drying Process for the Encapsulation of High-Added Value Extracts from Food by-Products. Comput. Chem. Eng. 2022, 161, 107772. [Google Scholar] [CrossRef]
- Banožić, M.; Vladić, J.; Banjari, I.; Velić, D.; Aladić, K.; Jokić, S. Spray Drying as a Method of Choice for Obtaining High Quality Products from Food Wastes—A Review. Food Rev. Int. 2021, 39, 1953–1985. [Google Scholar] [CrossRef]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of Grape (Vitis Labrusca Var. Bordo) Skin Phenolic Extract Using Gum Arabic, Polydextrose, and Partially Hydrolyzed Guar Gum as Encapsulating Agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Kalušević, A.M.; Lević, S.M.; Čalija, B.R.; Milić, J.R.; Pavlović, V.B.; Bugarski, B.M.; Nedović, V.A. Effects of Different Carrier Materials on Physicochemical Properties of Microencapsulated Grape Skin Extract. J. Food Sci. Technol. 2017, 54, 3411–3420. [Google Scholar] [CrossRef] [PubMed]
- Ćujić Nikolić, N.; Stanisavljević, N.; Katarina, Š.; Kalušević, A.; Nedović, V.; Samardžić, J.; Janković, T. Application of Gum Arabic in the Production of Spray-Dried Chokeberry Polyphenols, Microparticles Characterisation and In Vitro Digestion Method. Lek. Sirovine 2018, 38, 10–18. [Google Scholar]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Encapsulation of Phenolic-Rich Extract from Banana (Musa Cavendish) Peel. J. Food Sci. Technol. 2020, 57, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Siacor, F.D.C.; Lim, K.J.A.; Cabajar, A.A.; Lobarbio, C.F.Y.; Lacks, D.J.; Taboada, E.B. Physicochemical Properties of Spray-Dried Mango Phenolic Compounds Extracts. J. Agric. Food Res. 2020, 2, 100048. [Google Scholar] [CrossRef]
- Alonso-Moreno, C.; García-Yuste, S. Environmental Potential of the Use of CO2 from Alcoholic Fermentation Processes. The CO-AFP Strategy. Sci. Total Environ. 2016, 568, 319–326. [Google Scholar] [CrossRef]
- Da Rocha, C.B.; Noreña, C.P.Z. Microencapsulation and Controlled Release of Bioactive Compounds from Grape Pomace. Dry. Technol. 2021, 39, 1018–1032. [Google Scholar] [CrossRef]
- Macwan, S.R.; Dabhi, B.K.; Parmar, S.C.; Aparnathi, K.D. Whey and Its Utilization. Int. J. Curr. Microbiol. App. Sci. 2016, 5, 134–155. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Okuro, P.K.; dos Santos, J.G.; Brexó, R.P.; Sato, H.H. Whey Protein as a Key Component in Food Systems: Physicochemical Properties, Production Technologies and Applications. Food Struct. 2017, 14, 17–29. [Google Scholar] [CrossRef]
- El-Hatmi, H.E.-H. Comparison of Composition and Whey Protein Fractions of Human, Camel, Donkey, Goat and Cow Milk. Mljekarstvo 2015, 65, 159–167. [Google Scholar] [CrossRef]
- Araújo, D.F.S.; Guerra, G.C.B.; Pintado, M.M.E.; Sousa, Y.R.F.; Algieri, F.; Rodriguez-Nogales, A.; Araújo, R.F.; Gálvez, J.; Queiroga, R.D.C.R.E.; Rodriguez-Cabezas, M.E. Intestinal Anti-Inflammatory Effects of Goat Whey on DNBS-Induced Colitis in Mice. PLoS ONE 2017, 12, e0185382. [Google Scholar] [CrossRef]
- De Oliveira, A.H.; Mata, M.E.R.M.C.; Fortes, M.; Duarte, M.E.M.; Pasquali, M.; Lisboa, H.M. Influence of Spray Drying Conditions on the Properties of Whole Goat Milk. Dry. Technol. 2021, 39, 726–737. [Google Scholar] [CrossRef]
- Pradeep Prasanna, P.H.; Charalampopoulos, D. Encapsulation in an Alginate–Goats’ Milk–Inulin Matrix Improves Survival of Probiotic Bifidobacterium in Simulated Gastrointestinal Conditions and Goats’ Milk Yoghurt. Int. J. Dairy. Tech. 2019, 72, 132–141. [Google Scholar] [CrossRef]
- Verruck, S.; De Liz, G.R.; Dias, C.O.; De Mello Castanho Amboni, R.D.; Prudencio, E.S. Effect of Full-Fat Goat’s Milk and Prebiotics Use on Bifidobacterium BB-12 Survival and on the Physical Properties of Spray-Dried Powders under Storage Conditions. Food Res. Int. 2019, 119, 643–652. [Google Scholar] [CrossRef]
- Haque, M.A.; Chen, J.; Aldred, P.; Adhikari, B. Denaturation and Physical Characteristics of Spray-Dried Whey Protein Isolate Powders Produced in the Presence and Absence of Lactose, Trehalose, and Polysorbate-80. Dry. Technol. 2015, 33, 1243–1254. [Google Scholar] [CrossRef]
- Cui, L.; Kimmel, J.; Zhou, L.; Chen, B.; Rao, J. Improving the Functionality of Pea Protein Isolate through Co-Spray Drying with Emulsifying Salt or Disaccharide. Food Hydrocoll. 2021, 113, 106534. [Google Scholar] [CrossRef]
- Imamura, K.; Ogawa, T.; Sakiyama, T.; Nakanishi, K. Effects of Types of Sugar on the Stabilization of Protein in the Dried State. J. Pharm. Sci. 2003, 92, 266–274. [Google Scholar] [CrossRef]
- Wagoner, T.; Vardhanabhuti, B.; Foegeding, E.A. Designing Whey Protein-Polysaccharide Particles for Colloidal Stability. Annu. Rev. Food Sci. Technol. 2016, 7, 93–116. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of Grape Polyphenols Using Maltodextrin and Gum Arabic as Two Alternative Coating Materials: Development and Characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef]
- Kelly, G.M.; O’Mahony, J.A.; Kelly, A.L.; O’Callaghan, D.J. Effect of Hydrolyzed Whey Protein on Surface Morphology, Water Sorption, and Glass Transition Temperature of a Model Infant Formula. J. Dairy Sci. 2016, 99, 6961–6972. [Google Scholar] [CrossRef]
- Boyano-Orozco, L.; Gallardo-Velázquez, T.; Meza-Márquez, O.G.; Osorio-Revilla, G. Microencapsulation of Rambutan Peel Extract by Spray Drying. Foods 2020, 9, 899. [Google Scholar] [CrossRef]
- Lee, C.-W.; Oh, H.-J.; Han, S.-H.; Lim, S. Effects of Hot Air and Freeze Drying Methods on Physicochemical Properties of Citrus “Hallabong” Powders. Food Sci. Biotechnol. 2012, 21, 1633–1639. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static In Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucić-Kojić, A. Physicochemical Characterization and Evaluation of Gastrointestinal In Vitro Behavior of Alginate-Based Microbeads with Encapsulated Grape Pomace Extracts. Pharmaceutics 2023, 15, 980. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static In Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Šelo, G.; Zelić, B.; Planinić, M.; Tišma, M. Recovery of Phenolic Acid and Enzyme Production from Corn Silage Biologically Treated by Trametes Versicolor. Appl. Biochem. Biotechnol. 2017, 181, 948–960. [Google Scholar] [CrossRef]
- Cassol, L.; Noreña, C.P.Z. Microencapsulation and Accelerated Stability Testing of Bioactive Compounds of Hibiscus Sabdariffa. Food Meas. 2021, 15, 1599–1610. [Google Scholar] [CrossRef]
- Navarro-Flores, M.J.; Ventura-Canseco, L.M.C.; Meza-Gordillo, R.; Ayora-Talavera, T.d.R.; Abud-Archila, M. Spray Drying Encapsulation of a Native Plant Extract Rich in Phenolic Compounds with Combinations of Maltodextrin and Non-Conventional Wall Materials. J. Food Sci. Technol. 2020, 57, 4111–4122. [Google Scholar] [CrossRef]
- Wong, C.W.; Lim, W.T. Storage Stability of Spray-Dried Papaya (Carica Papaya L.) Powder Packaged in Aluminium Laminated Polyethylene (ALP) and Polyethylene Terephthalate (PET). Int. Food Res. J. 2016, 23, 1887–1894. [Google Scholar]
- Baysan, U.; Zungur Bastıoğlu, A.; Coşkun, N.Ö.; Konuk Takma, D.; Ülkeryıldız Balçık, E.; Sahin-Nadeem, H.; Koç, M. The Effect of Coating Material Combination and Encapsulation Method on Propolis Powder Properties. Powder Technol. 2021, 384, 332–341. [Google Scholar] [CrossRef]
- Tontul, I.; Topuz, A. Spray-Drying of Fruit and Vegetable Juices: Effect of Drying Conditions on the Product Yield and Physical Properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
- Kurozawa, L.; Morassi, A.; Vanzo, A.; Park, K.; Hubinger, M. Influence of Spray Drying Conditions on Physicochemical Properties of Chicken Meat Powder. Dry. Technol. 2009, 27, 1248–1257. [Google Scholar] [CrossRef]
- Braga, M.B.; Rocha, S.C.D.S.; Hubinger, M.D. Spray-Drying of Milk–Blackberry Pulp Mixture: Effect of Carrier Agent on the Physical Properties of Powder, Water Sorption, and Glass Transition Temperature. J. Food Sci. 2018, 83, 1650–1659. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 7th ed.; European Treaty Series; European Directorate for the Quality of Medicines and Healthcare: Strasbourg, France, 2010; ISBN 978-92-871-6700-2. [Google Scholar]
- Kim, E.H.-J.; Chen, X.D.; Pearce, D. Effect of Surface Composition on the Flowability of Industrial Spray-Dried Dairy Powders. Colloids Surf. B Biointerfaces 2005, 46, 182–187. [Google Scholar] [CrossRef]
- Aziz, M.G.; Yusof, Y.A.; Blanchard, C.; Saifullah, M.; Farahnaky, A.; Scheiling, G. Material Properties and Tableting of Fruit Powders. Food Eng. Rev. 2018, 10, 66–80. [Google Scholar] [CrossRef]
- Medina-Torres, L.; García-Cruz, E.E.; Calderas, F.; González Laredo, R.F.; Sánchez-Olivares, G.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E.; Rodríguez-Ramírez, J. Microencapsulation by Spray Drying of Gallic Acid with Nopal Mucilage (Opuntia Ficus Indica). LWT Food Sci. Technol. 2013, 50, 642–650. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Pallet, D.; Brat, P.; Hubinger, M.D. Physicochemical and Morphological Characterisation of Açai (Euterpe Oleraceae Mart.) Powder Produced with Different Carrier Agents. Int. J. Food Sci. Technol. 2009, 44, 1950–1958. [Google Scholar] [CrossRef]
- Ong, M.Y.; Yusof, Y.A.; Aziz, M.G.; Chin, N.L.; Amin, N.A.M. Characterisation of Fast Dispersible Fruit Tablets Made from Green and Ripe Mango Fruit Powders. J. Food Eng. 2014, 125, 17–23. [Google Scholar] [CrossRef]
- Adekunle, A.; Shittu, T.; Abioye, A.O.; Adeyanju, J.; Osanaiye, F.G. Physical and Thermal Properties of Baobab Fruit Pulp Powder. Int. J. Eng. Res. Appl. 2013, 3, 925–928. [Google Scholar]
- Ćujić-Nikolić, N.; Stanisavljević, N.; Šavikin, K.; Kalušević, A.; Nedović, V.; Samardžić, J.; Janković, T. Chokeberry Polyphenols Preservation Using Spray Drying: Effect of Encapsulation Using Maltodextrin and Skimmed Milk on Their Recovery Following In Vitro Digestion. J. Microencapsul. 2019, 36, 693–703. [Google Scholar] [CrossRef]
- Sidlagatta, V.; Chilukuri, S.V.V.; Devana, B.R.; Dasi, S.D.; Rangaswamy, L. Effect of Maltodextrin Concentration and Inlet Air Temperature on Properties of Spray Dried Powder from Reverse Osmosis Concentrated Sweet Orange Juice. Braz. Arch. Biol. Technol. 2020, 63, e20190538. [Google Scholar] [CrossRef]
- Chang, Y.-X.; Yang, J.-J.; Pan, R.-L.; Chang, Q.; Liao, Y.-H. Anti-Hygroscopic Effect of Leucine on Spray-Dried Herbal Extract Powders. Powder Technol. 2014, 266, 388–395. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the Carriers on the Microstructure of Mango Powder Obtained by Spray Drying and Its Functional Characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Chiou, D.; Langrish, T.A.G. Crystallization of Amorphous Components in Spray-Dried Powders. Dry. Technol. 2007, 25, 1427–1435. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, W. Characterisation of Spray Dried Soy Sauce Powders Made by Adding Crystalline Carbohydrates to Drying Carrier. Food Chem. 2015, 168, 417–422. [Google Scholar] [CrossRef]
- Oliveira, B.E.; Junior, P.C.G.; Cilli, L.P.; Contini, L.R.F.; Venturini, A.C.; Yoshida, C.M.P.; Braga, M.B. Spray-Drying of Grape Skin-Whey Protein Concentrate Mixture. J. Food Sci. Technol. 2018, 55, 3693–3702. [Google Scholar] [CrossRef]
- Wijiani, N.; Isadiartuti, D.; Rijal, M.A.S.; Yusuf, H. Characterization and Dissolution Study of Micellar Curcumin-Spray Dried Powder for Oral Delivery. Int. J. Nanomed. 2020, 15, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, C.G.; Borges, C.D.; Zambiazi, R.C.; Rutz, J.K.; da Luz, S.R.; Krumreich, F.D.; Benvenutti, E.V.; Nunes, M.R. Encapsulation of the Phenolic Compounds of the Blackberry (Rubus Fruticosus). LWT Food Sci. Technol. 2014, 58, 527–533. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Zajac, N. Digestion and Absorption of Phenolic Compounds Assessed by In Vitro Simulation Methods. A Review. Rocz. Państwowego Zakładu Hig. 2013, 64, 2. [Google Scholar]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic Compounds: Current Industrial Applications, Limitations and Future Challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Dag, D.; Kilercioglu, M.; Oztop, M.H. Physical and Chemical Characteristics of Encapsulated Goldenberry (Physalis Peruviana L.) Juice Powder. LWT Food Sci. Technol. 2017, 83, 86–94. [Google Scholar] [CrossRef]
- Hosseini, S.M.H.; Emam-Djomeh, Z.; Sabatino, P.; Van Der Meeren, P. Nanocomplexes Arising from Protein-Polysaccharide Electrostatic Interaction as a Promising Carrier for Nutraceutical Compounds. Food Hydrocoll. 2015, 50, 16–26. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Lević, S.; Kalušević, A.; Špoljarić, I.; Đorđević, V.; Komes, D.; Mršić, G.; Nedović, V. Efficiency Assessment of Natural Biopolymers as Encapsulants of Green Tea (Camellia Sinensis L.) Bioactive Compounds by Spray Drying. Food Bioprocess. Technol. 2015, 8, 2444–2460. [Google Scholar] [CrossRef]
- Liao, M.; Ma, L.; Miao, S.; Hu, X.; Liao, X.; Chen, F.; Ji, J. The In-Vitro Digestion Behaviors of Milk Proteins Acting as Wall Materials in Spray-Dried Microparticles: Effects on the Release of Loaded Blueberry Anthocyanins. Food Hydrocoll. 2021, 115, 106620. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Fu, X. Study on the Bioaccessibility of Phenolic Compounds and Bioactivities of Passion Fruit Juices from Different Regions In Vitro Digestion. J. Food Process Preserv. 2021, 45, 15056. [Google Scholar] [CrossRef]
- Shetty, N.; Cipolla, D.; Park, H.; Zhou, Q.T. Physical Stability of Dry Powder Inhaler Formulations. Expert. Opin. Drug Deliv. 2020, 17, 77–96. [Google Scholar] [CrossRef]












| Sample Label | Coating | (%) | Co-Coating | (%) |
|---|---|---|---|---|
| GW1 | goat whey protein | 100 | - | - |
| T5 | goat whey protein | 95 | trehalose | 5 |
| T10 | goat whey protein | 90 | trehalose | 10 |
| T20 | goat whey protein | 80 | trehalose | 20 |
| T30 | goat whey protein | 70 | trehalose | 30 |
| S5 | goat whey protein | 95 | sucrose | 5 |
| S10 | goat whey protein | 95 | sucrose | 10 |
| S20 | goat whey protein | 90 | sucrose | 20 |
| S30 | goat whey protein | 80 | sucrose | 30 |
| MD2.5 | goat whey protein | 97.5 | maltodextrin (DE 4–7) | 2.5 |
| MD5 | goat whey protein | 95 | maltodextrin (DE 4–7) | 5 |
| MD10 | goat whey protein | 90 | maltodextrin (DE 4–7) | 10 |
| MD15 | goat whey protein | 85 | maltodextrin (DE 4–7) | 15 |
| X5 | goat whey protein | 95 | xylose | 5 |
| X10 | goat whey protein | 90 | xylose | 10 |
| X15 | goat whey protein | 85 | xylose | 15 |
| X30 | goat whey protein | 70 | xylose | 30 |
| Sample 1 | TPC (mgGAE/gdb) 2 | SPC (mgGAE/gdb) 2 |
|---|---|---|
| GW1 | 124.09 ± 1.63 i | 5.62 ± 0.34 bc |
| T5 | 102.63 ± 1.19 e | 9.9 ± 0.61 f |
| T10 | 114.37 ± 0.68 g | 10.89 ± 0.54 g |
| T20 | 113.15 ± 0.66 g | 14.21 ± 0.52 i |
| T30 | 98.84 ± 0.85 d | 22.15 ± 0.15 l |
| S5 | 101.47 ± 1.52 e | 8.98 ± 0.53 e |
| S10 | 102.63 ± 0.85 e | 7.70 ± 0.62 d |
| S20 | 97.50 ± 0.81cd | 10.92 ± 0.27 g |
| S30 | 86.81 ± 1.54 a | 16.66 ± 0.67 k |
| MD2.5 | 113.12 ± 1.18 g | 7.12 ± 0,20 d |
| MD5 | 97.38 ± 0.82 c | 4.54 ± 0.27 a |
| MD10 | 96.89 ± 1.33 c | 5.05 ± 0.59 ab |
| MD15 | 93.30 ± 1.38 b | 5.71 ± 0.26 c |
| X5 | 96.78 ± 1.60 c | 8.93 ± 0.49 e |
| X10 | 117.34 ± 1.28 h | 9.89 ± 0.39 f |
| X15 | 104.10 ± 0.95 f | 12.89 ± 0.46 h |
| X30 | 94.12 ± 1.56 b | 15.78 ± 0.34 j |
| Sample | Mean Diameter, d (0.5) (μm) | Specific Surface Area (m2/g) | Span (-) |
|---|---|---|---|
| GW1 | 3.76 | 2.00 | 2.20 |
| T5 | 3.78 | 2.04 | 2.09 |
| T10 | 3.36 | 2.22 | 2.18 |
| T20 | 3.61 | 2.07 | 2.08 |
| T30 | 3.38 | 2.19 | 2.03 |
| S5 | 3.76 | 2.01 | 1.93 |
| S10 | 3.50 | 2.13 | 2.12 |
| S20 | 3.51 | 2.12 | 1.90 |
| S30 | 3.31 | 2.21 | 1.80 |
| MD2.5 | 4.61 | 1.68 | 2.05 |
| MD5 | 3.88 | 1.97 | 2.02 |
| MD10 | 3.37 | 2.21 | 1.99 |
| MD15 | 3.45 | 2.16 | 2.16 |
| X5 | 3.53 | 2.11 | 1.97 |
| X10 | 3.88 | 1.94 | 1.84 |
| X15 | 3.32 | 2.21 | 1.94 |
| X30 | 3.81 | 1.93 | 1.73 |
| Sample | WSI (%) | WAI (-) | SP (-) | Polarity (%) |
|---|---|---|---|---|
| GW1 | 57.47 ± 0.54 f | 4.37 ± 0.01 ab | 10.28 ± 0.10 fghi | 46.47 ± 0.32 |
| T5 | 51.40 ± 0.97 cd | 4.82 ± 0.24 efg | 9.92 ± 0.28 defg | 43.93 ± 2.71 |
| T10 | 55.25 ± 0.26 e | 4.68 ± 0.01cde | 10.45 ± 0.03 ghi | 45.99 ± 0.89 |
| T20 | 56.46 ± 0.69 ef | 4.67 ± 0.13 cde | 10.74 ± 0.14 hi | 44.80 ± 1.23 |
| T30 | 55.63 ± 0.22 e | 4.24 ± 0.07 a | 9.56 ± 0.10 cdef | 44.78 ± 1.17 |
| S5 | 50.78 ± 0.80 bcd | 4.64 ± 0.37 bcde | 9.44 ± 0.90 bcde | 40.06 ± 1.19 |
| S10 | 50.83 ± 1.46 bcd | 4.96 ± 0.44 fgh | 10.07 ± 0.59 efgh | 44.01 ± 0.36 |
| S20 | 49.55 ± 1.36 b | 4.62 ± 0.01 bcde | 9.15 ± 0.23 bc | 48.39 ± 1.25 |
| S30 | 57.68 ± 2.48 f | 4.57 ± 0.13 bcde | 10.80 ± 0.33 i | 48.25 ± 0.93 |
| MD2.5 | 49.63 ± 2.21bc | 4.41 ± 0.04 abc | 8.77 ± 0.46 ab | 36.46 ± 1.74 |
| MD5 | 51.17 ± 1.86 bcd | 4.7 ± 0.04 def | 9.64 ± 0.46 cdef | 47.82 ± 1.62 |
| MD10 | 50.79 ± 0.15 bcd | 4.55 ± 0.12 bcde | 9.25 ± 0.28 bcd | 41.50 ± 1.72 |
| MD15 | 43.91 ± 0.21 a | 4.55 ± 0.04 bcd | 8.11 ± 0.04 a | 41.24 ± 0.28 |
| X5 | 51.80 ± 0.19 d | 5.18 ± 0.63 h | 10.75 ± 1.27 hi | 40.65 ± 0.18 |
| X10 | 62.62 ± 4.20 g | 5.08 ± 0.00 gh | 13.68 ± 1.52 k | 43.85 ± 0.51 |
| X15 | 62.59 ± 0.40 g | 4.60 ± 0.13 bcde | 12.31 ± 0.49 j | 43.42 ± 0.63 |
| X30 | 63.15 ± 0.07 g | 5.13 ± 0.22 h | 13.93 ± 0.58 k | 47.42 ± 2.36 |
| Phenols (μg/gdb) | Samples | ||||
|---|---|---|---|---|---|
| GW1 | T10 | S10 | MD5 | X10 | |
| Procyanidin B1 | 1121.04 ± 48.47 | 1054.90 ± 26.64 | 923.51 ± 40.55 | 1329.88 ± 61.03 | 987.87 ± 90.96 |
| Caffeic acid | 23.97 ± 0.28 | 21.89 ± 0.13 | 19.78 ± 0.29 | 21.49 ± 0.25 | 17.21 ± 0.50 |
| Chlorogenic acid | 81.32 ± 1.73 | 67.99 ± 4.58 | 77.79 ± 1.30 | 89.30 ± 2.33 | 70.14 ± 1.01 |
| Syringic acid | 122.55 ± 1.83 | 204.17 ± 4.07 | 182.78 ± 0.80 | 199.24 ± 1.55 | 158.92 ± 1.30 |
| p-coumaric acid | 11.55 ± 0.08 | 10.61 ± 0.17 | 9.82 ± 0.09 | 14.13 ± 0.11 | 6.61 ± 0.59 |
| Ellagic acid | 84.07 ± 0.86 | 100.32 ± 2.25 | 90.88 ± 2.30 | 87.67 ± 2.97 | 83.06 ± 1.90 |
| Rutin | 159.54 ± 2.11 | 189.07 ± 5.20 | 152.52 ± 0.73 | 196.83 ± 0.92 | 218.63 ± 9.55 |
| Resveratrol | 19.93 ± 0.03 | 26.89 ± 0.49 | 23.54 ± 0.29 | 26.02 ± 0.70 | 20.26 ± 1.38 |
| Kaempferol | 21.13 ± 0.55 | 20.98 ± 0.20 | 23.69 ± 0.42 | 25.44 ± 0.15 | 20.21 ± 0.38 |
| Quercetin | 317.88 ± 2.76 | 395.91 ± 0.58 | 351.92 ± 4.97 | 359.10 ± 0.23 | 303.78 ± 2.72 |
| Phenols/Sample | Before Digestion | Oral Phase | Gastric Phase | Intestinal Phase | ||
|---|---|---|---|---|---|---|
| OP3 | GP63 | GP123 | IP183 | IP243 | ||
| Gallic acid (μg/gdb) | ||||||
| GW1 | 732.71 ± 0.41 | nd | nd | nd | 229.21 ± 39.59 | 244.16 ± 29.77 |
| T10 | 827.66 ± 8.45 | 5.28 ± 0.15 | 9.19 ± 0.15 | 9.72 ± 0.30 | 268.69 ± 7.17 | 230.88 ± 7.47 |
| S10 | 754.16 ± 4.09 | 4.93 ± 0.22 | 9.11 ± 0.90 | 8.37 ± 0.15 | 371.44 ± 3.90 | 216.55 ± 3.00 |
| MD5 | 959.15 ± 3.04 | 5.36 ± 0.23 | 10.41 ± 0.30 | 11.15 ± 0.15 | 288.51 ± 1.80 | 161.04 ± 2.40 |
| X10 | 854.20 ± 10.44 | 6.33 ± 0.60 | 7.60 ± 0.30 | 7.81 ± 0.00 | 301.31 ± 8.06 | 177.58 ± 5.08 |
| 3,4-dihidroxybenzoic acid (μg/gdb) | ||||||
| GW1 | 60.22 ± 0.21 | nd | nd | nd | 136.60 ± 5.66 | 149.23 ± 9.23 |
| T10 | 66.10 ± 9.22 | nd | nd | nd | 106.89 ± 1.79 | 143.64 ± 2.99 |
| S10 | 71.63 ± 0.48 | nd | nd | nd | 65.90 ± 7.49 | 51.06 ± 0.90 |
| MD5 | 79.60 ± 0.29 | nd | nd | nd | 61.82 ± 3.30 | 33.99 ± 3.00 |
| X10 | 69.38 ± 2.24 | nd | nd | nd | 53.21 ± 4.18 | 52.15 ± 3.28 |
| p-hidroxybenzoic acid (μg/gdb) | ||||||
| GW1 | 18.39 ± 1.23 | 5.00 ± 7.07 | 6.42 ± 9.08 | 3.89 ± 5.51 | 10.73 ± 15.18 | 8.84 ± 12.50 |
| T10 | 27.76 ± 2.99 | 18.75 ± 3.51 | 25.45 ± 2.54 | 20.91 ± 2.09 | 31.47 ± 3.29 | 27.67 ± 0.30 |
| S10 | 32.50 ± 0.72 | 18.86 ± 0.15 | 24.05 ± 1.35 | 16.32 ± 0.90 | 46.40 ± 1.50 | 37.72 ± 5.99 |
| MD5 | 28.67 ± 1.25 | 22.15 ± 2.03 | 16.68 ± 3.46 | 15.51 ± 0.60 | 55.66 ± 3.61 | 52.05 ± 3.91 |
| X10 | 14.80 ± 0.32 | 19.74 ± 2.09 | 27.13 ± 0.75 | 22.59 ± 0.30 | 61.23 ± 4.18 | 58.49 ± 4.48 |
| Catechin (μg/gdb) | ||||||
| GW1 | 4675.02 ± 99.35 | nd | nd | nd | 601.56 ± 35.72 | 701.54 ± 29.47 |
| T10 | 5928.03 ± 65.47 | nd | nd | nd | 495.78 ± 1.49 | 591.47 ± 22.11 |
| S10 | 4861.45 ± 80.73 | nd | nd | nd | 593.28 ± 47.35 | 630.58 ± 47.35 |
| MD5 | 5325.62 ± 14.85 | nd | nd | nd | 496.92 ± 12.32 | 249.84 ± 2.40 |
| X10 | 4359.75 ± 12.53 | nd | nd | nd | 459.25 ± 2.09 | 488.60 ± 37.62 |
| Vanillic acid (μg/gdb) | ||||||
| GW1 | 41.55 ± 0.54 | nd | nd | nd | 18.94 ± 2.38 | 22.73 ± 0.00 |
| T10 | 45.35 ± 0.41 | nd | nd | nd | 18.80 ± 0.30 | 18.17 ± 0.60 |
| S10 | 42.66 ± 0.66 | nd | nd | nd | 15.26 ± 2.40 | 16.53 ± 0.00 |
| MD5 | 47.55 ± 0.24 | nd | nd | nd | 16.57± 0.00 | 12.53 ± 0.30 |
| X10 | 35.28 ± 0.66 | nd | nd | nd | 17.53 ± 0.30 | 14.36 ± 0.00 |
| Procyanindin B2 (μg/gdb) | ||||||
| GW1 | 1034.65 ± 105.81 | 117.08 ± 1.71 | nd | 24.31 ± 0.74 | 348.77 ± 2.08 | 466.01 ± 45.84 |
| T10 | 1060.26 ± 49.77 | 110.95 ± 5.60 | 135.51 ± 2.54 | 30.84 ± 1.79 | 57.03 ± 13.74 | 91.47 ± 8.66 |
| S10 | 1401.46 ± 31.99 | 172.48 ± 4.49 | 130.73 ± 7.19 | 73.84 ± 12.44 | 83.91 ± 8.99 | 85.39 ± 3.90 |
| MD5 | 1158.63 ± 54.88 | 131.82 ± 6.16 | 55.66 ± 12.62 | nd | 58.85 ± 14.12 | 37.18 ± 2.70 |
| X10 | 883.58 ± 41.05 | 186.97 ± 13.44 | 119.72 ± 9.26 | 50.68 ± 3.88 | 82.98 ± 10.45 | 61.87 ± 11.05 |
| Epicatechin (μg/gdb) | ||||||
| GW1 | 3284.86 ± 10.78 | 1228.00 ± 28.58 | 335.93 ± 5.66 | 360.35 ± 3.57 | 152.39 ± 0.60 | 97.03 ± 4.46 |
| T10 | 4001.84 ± 38.51 | 1122.73 ± 107.10 | 1074.57 ± 44.21 | 572.88 ± 16.13 | 336.92 ± 2.09 | 335.87 ± 27.48 |
| S10 | 3238.02 ± 50.81 | 1361.43 ± 23.60 | 824.03 ± 27.57 | 570.72 ± 11.84 | 263.59 ± 10.19 | 223.96 ± 11.69 |
| MD5 | 3650.18 ± 5.68 | 1092.79 ± 74.44 | 504.14 ± 36.65 | 334.93 ± 9.16 | 215.42 ± 14.42 | 193.97 ± 2.10 |
| X10 | 2012.11 ± 49.61 | 1154.77 ± 8.96 | 657.73 ± 5.37 | 467.91 ± 0.00 | 255.70 ± 8.06 | 266.89 ± 1.79 |
| Galocatechin gallate (μg/gdb) | ||||||
| GW1 | 1229.93 ± 25.65 | nd | 680.38 ± 16.52 | 659.44 ± 3.57 | 2117.66 ± 24.11 | 2144.18 ± 10.42 |
| T10 | 1403.32 ± 57.59 | nd | 812.21 ± 22.11 | nd | 2050.27 ± 1.79 | 2188.42 ± 23.90 |
| S10 | 1475.91 ± 9.08 | nd | 781.33 ± 10.04 | 766.82 ± 26.97 | 2095.56 ± 40.15 | 2137.09 ± 14.98 |
| MD5 | 1393.36 ± 7.50 | nd | 735.29 ± 3.00 | 779.80 ± 23.28 | 1951.14 ± 20.43 | 1840.88 ± 19.53 |
| X10 | 1259.73 ± 12.22 | nd | nd | 768.79 ± 24.78 | 1969.17 ± 53.15 | 1893.16 ± 38.82 |
| Ferulic acid (μg/gdb) | ||||||
| GW1 | 3.47 ± 0.06 | nd | nd | nd | 4.21 ± 1.79 | 11.79 ± 0.00 |
| T10 | 3.80 ± 0.11 | nd | nd | nd | 12.04 ± 2.09 | 16.69 ± 2.09 |
| S10 | 3.73 ± 0.04 | nd | nd | nd | 11.23 ± 0.30 | 14.83 ± 2.40 |
| MD5 | 4.95 ± 0.15 | nd | nd | nd | 11.90 ± 0.00 | 7.65 ± 0.00 |
| X10 | 4.12 ± 0.58 | nd | nd | nd | 15.84 ± 0.30 | 10.77 ± 0.30 |
| Epicatechin gallate (μg/gdb) | ||||||
| GW1 | 168.75 ± 3.24 | nd | nd | nd | 384.76 ± 4.17 | 491.69 ± 16.07 |
| T10 | 221.77 ± 16.62 | nd | nd | nd | 566.12 ± 2.99 | 628.64 ± 5.38 |
| S10 | 184.63 ± 7.28 | nd | nd | nd | 380.97 ± 28.77 | 660.24 ± 32.96 |
| MD5 | 260.80 ± 10.43 | nd | nd | nd | 217.55 ± 13.82 | 370.51 ± 12.62 |
| X10 | 181.83 ± 0.81 | nd | nd | nd | 553.63 ± 33.44 | 572.00 ± 19.41 |
| o-coumaric acid (μg/gdb) | ||||||
| GW1 | 13.49 ± 0.17 | 58.62 ± 2.68 | 15.05 ± 0.74 | 21.36 ± 0.15 | 49.67 ± 1.19 | 98.72 ± 6.85 |
| T10 | 15.19 ± 0.89 | 93.42 ± 4.85 | 161.70 ± 2.54 | 88.61 ± 1.05 | 48.16 ± 4.78 | 71.61 ± 2.69 |
| S10 | 12.78 ± 0.58 | 116.91 ± 0.22 | 102.77 ± 0.30 | 34.54 ± 2.70 | 63.14 ± 0.60 | 50.01 ± 4.79 |
| MD5 | 14.87 ± 0.39 | 75.69 ± 4.73 | 18.91 ± 3.00 | 7.75 ± 1.95 | 42.28 ± 0.30 | 38.03 ± 0.30 |
| X10 | 12.64 ± 1.57 | 80.82 ± 3.06 | 44.76 ± 3.88 | 36.95 ± 0.00 | 59.12 ± 2.99 | 50.46 ± 2.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perković, G.; Martinović, J.; Šelo, G.; Bucić-Kojić, A.; Planinić, M.; Ambrus, R. Characterization of Grape Pomace Extract Microcapsules: The Influence of Carbohydrate Co-Coating on the Stabilization of Goat Whey Protein as a Primary Coating. Foods 2024, 13, 1346. https://doi.org/10.3390/foods13091346
Perković G, Martinović J, Šelo G, Bucić-Kojić A, Planinić M, Ambrus R. Characterization of Grape Pomace Extract Microcapsules: The Influence of Carbohydrate Co-Coating on the Stabilization of Goat Whey Protein as a Primary Coating. Foods. 2024; 13(9):1346. https://doi.org/10.3390/foods13091346
Chicago/Turabian StylePerković, Gabriela, Josipa Martinović, Gordana Šelo, Ana Bucić-Kojić, Mirela Planinić, and Rita Ambrus. 2024. "Characterization of Grape Pomace Extract Microcapsules: The Influence of Carbohydrate Co-Coating on the Stabilization of Goat Whey Protein as a Primary Coating" Foods 13, no. 9: 1346. https://doi.org/10.3390/foods13091346
APA StylePerković, G., Martinović, J., Šelo, G., Bucić-Kojić, A., Planinić, M., & Ambrus, R. (2024). Characterization of Grape Pomace Extract Microcapsules: The Influence of Carbohydrate Co-Coating on the Stabilization of Goat Whey Protein as a Primary Coating. Foods, 13(9), 1346. https://doi.org/10.3390/foods13091346