Ultrasonic-Assisted Decoloration of Polysaccharides from Seedless Chestnut Rose (Rosa sterilis) Fruit: Insight into the Impact of Different Macroporous Resins on Its Structural Characterization and In Vitro Hypoglycemic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Macroporous Resins and Pretreatment
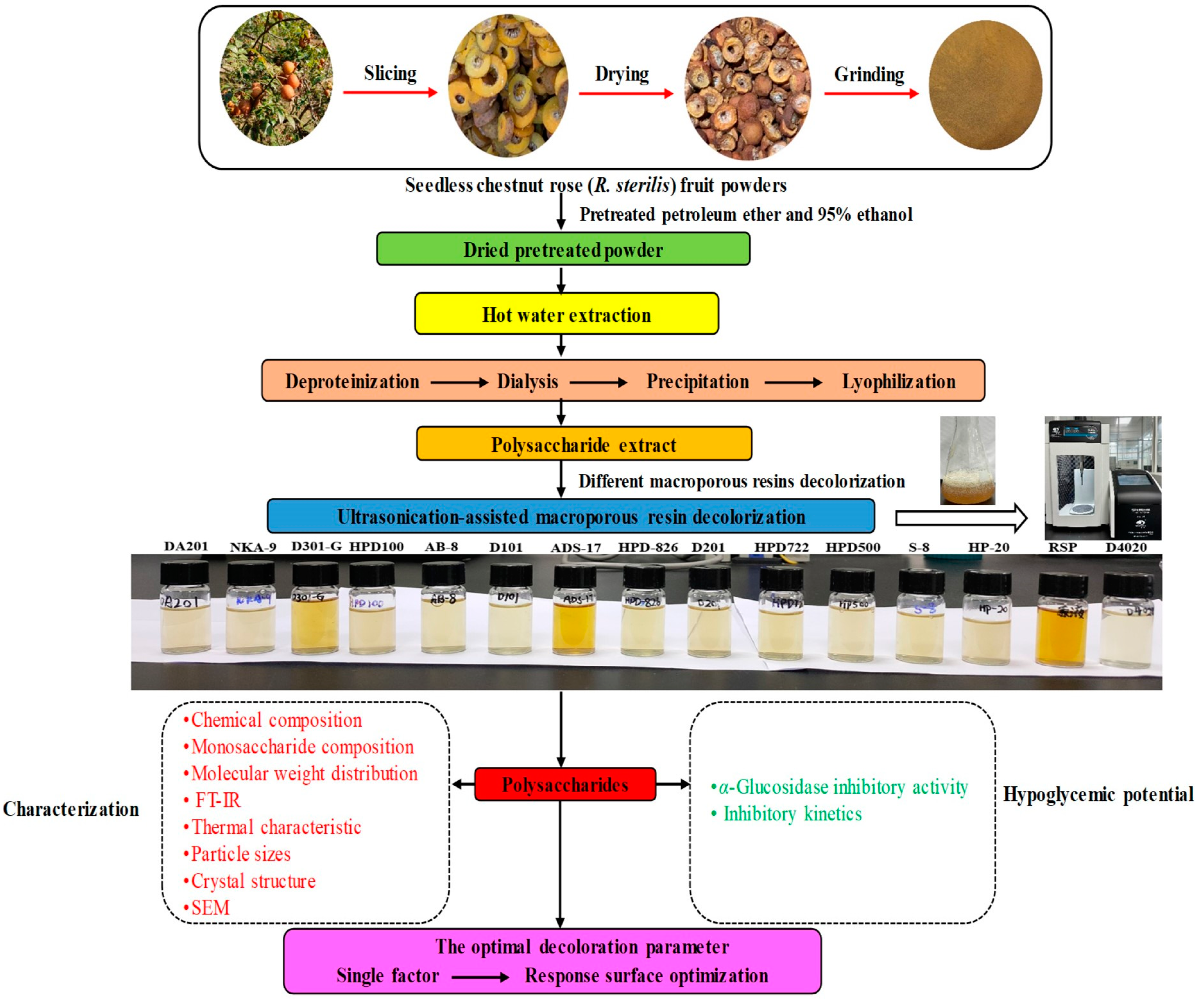
2.3. Preparation of Crude RSP Extract
2.4. Preliminary Screening of Resins Using USMRA
2.5. Chemical Composition of RSP before and after Decoloration with Different Macroporous Resins
2.6. Structural Characterization of RSP before and after Decoloration with Different Macroporous Resins
2.6.1. Molecular Weight Distribution Determination
2.6.2. Fourier Transform Infrared (FT-IR) Spectra
2.6.3. Particle Size
2.6.4. X-ray Diffraction (XRD)
2.6.5. Thermal Stability Properties
2.6.6. Scanning Electron Microscopy (SEM)
2.7. α-Glucosidase Inhibition Assays of RSP before and after Decoloration with Different Macroporous Resins
2.7.1. In Vitro α-Glycosidase Inhibitory Activity Assay
2.7.2. Kinetic Characterization of Inhibition
2.8. Statistical Analysis
3. Results and Discussion
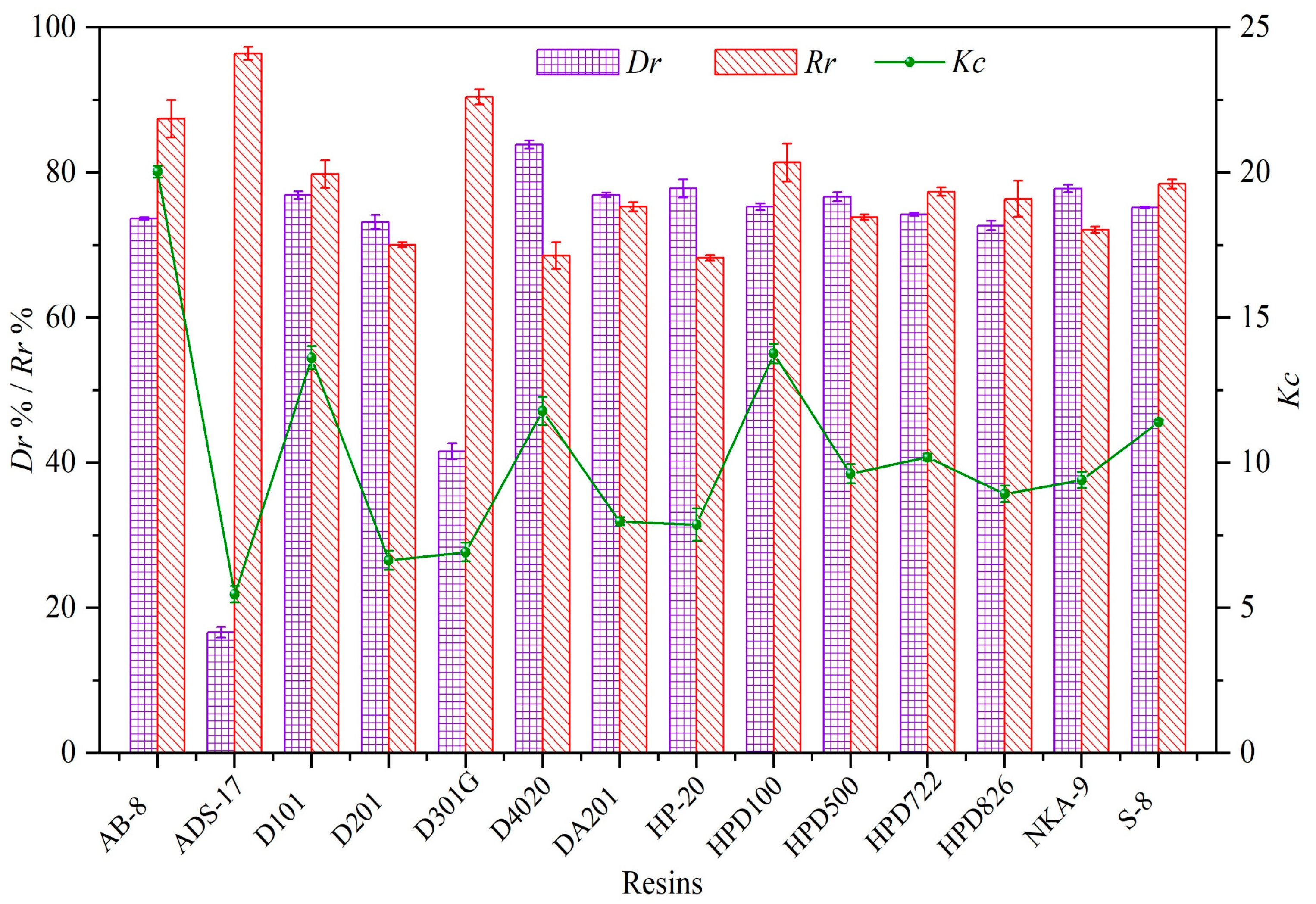
3.1. Selection of Optimal Decoloration Resin
3.2. Changes in Chemical Compositions after Being Treated by Five Resins
3.3. Changes in Structure Characterizations after Being Treated by Five Resins
3.3.1. Monosaccharide Composition
3.3.2. Molecular Weight Distribution
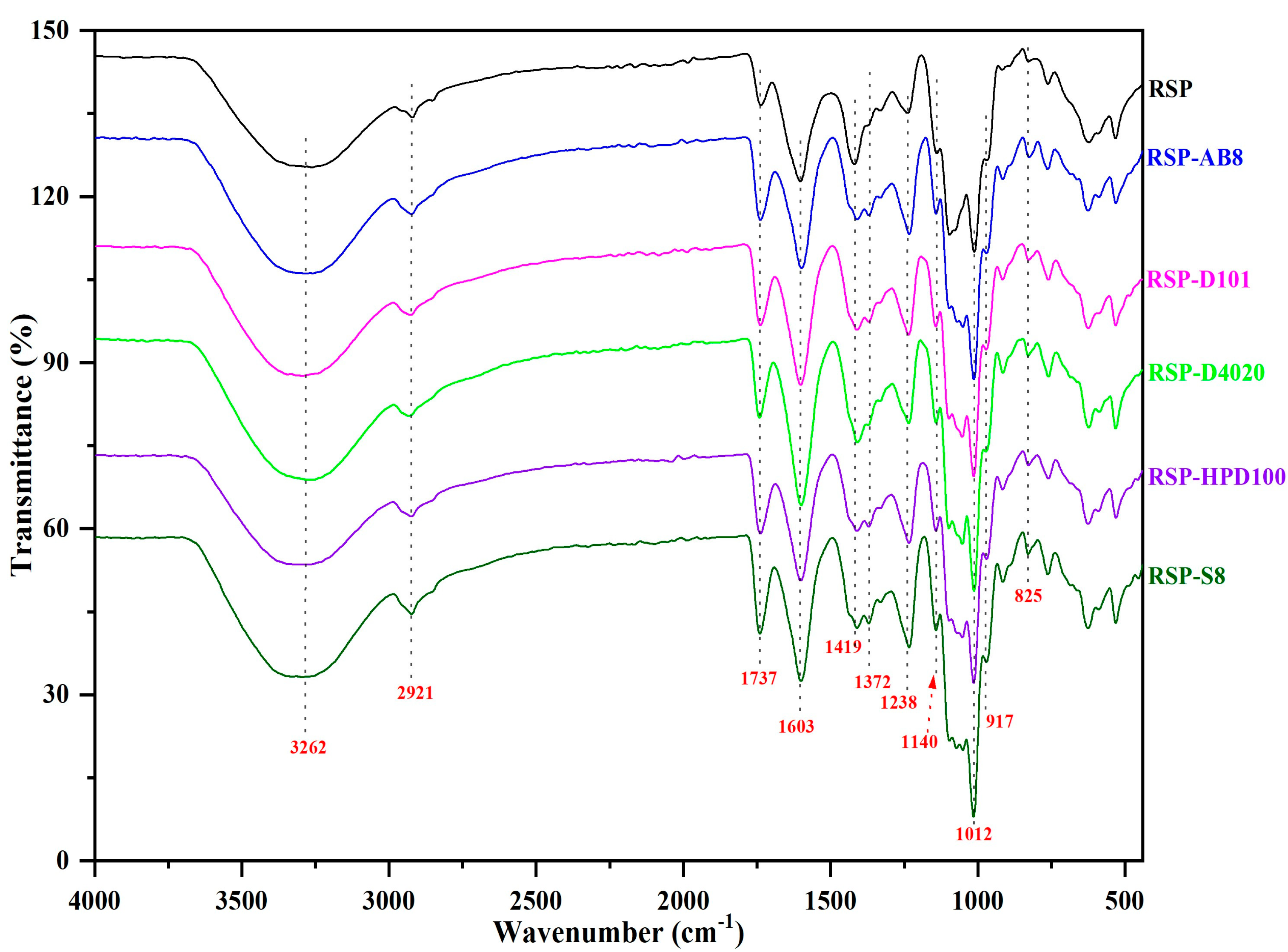
3.3.3. FT-IR Spectra Analysis
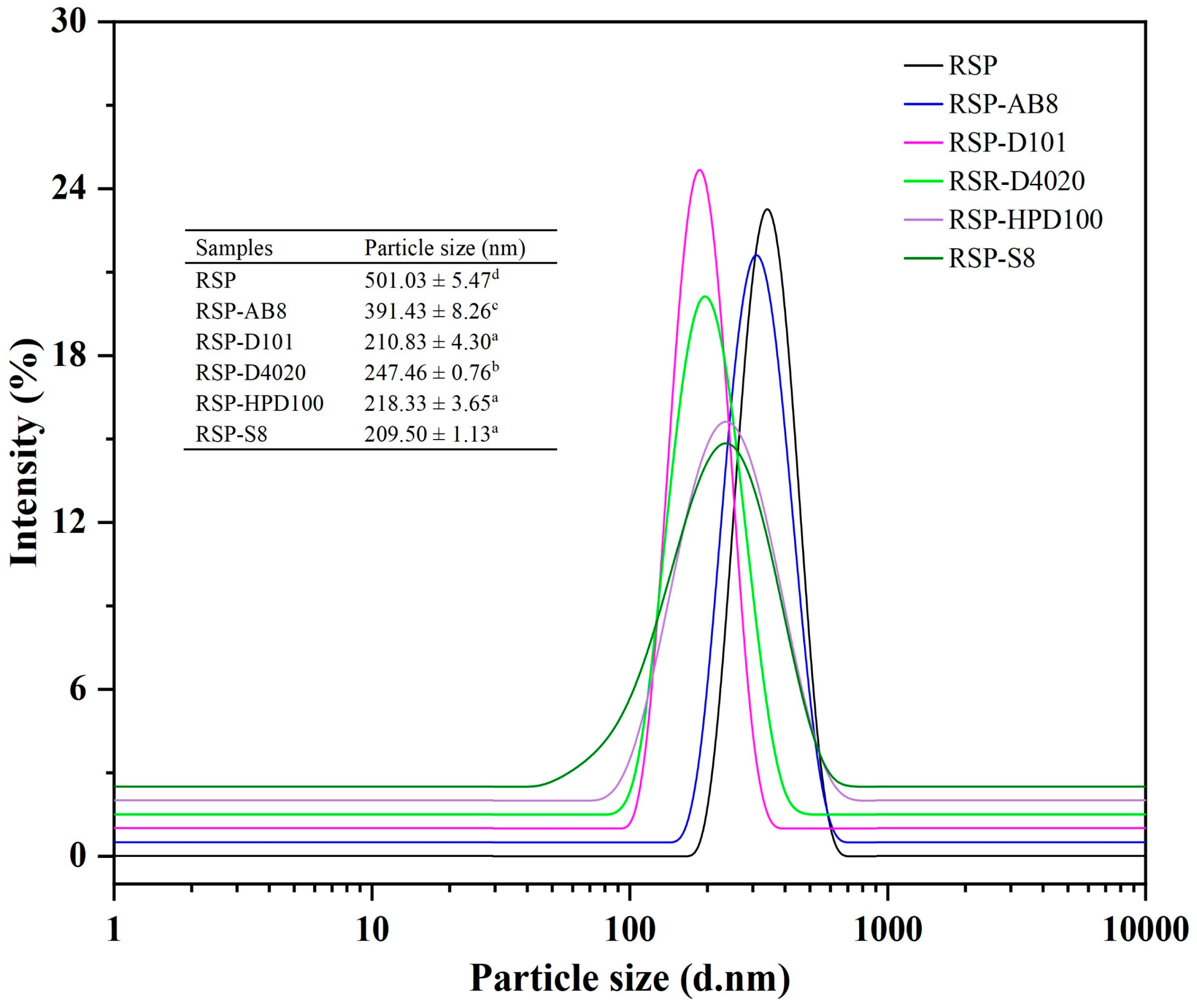
3.3.4. The Average Particle Size and Crystal Structure
3.3.5. Thermal Analysis
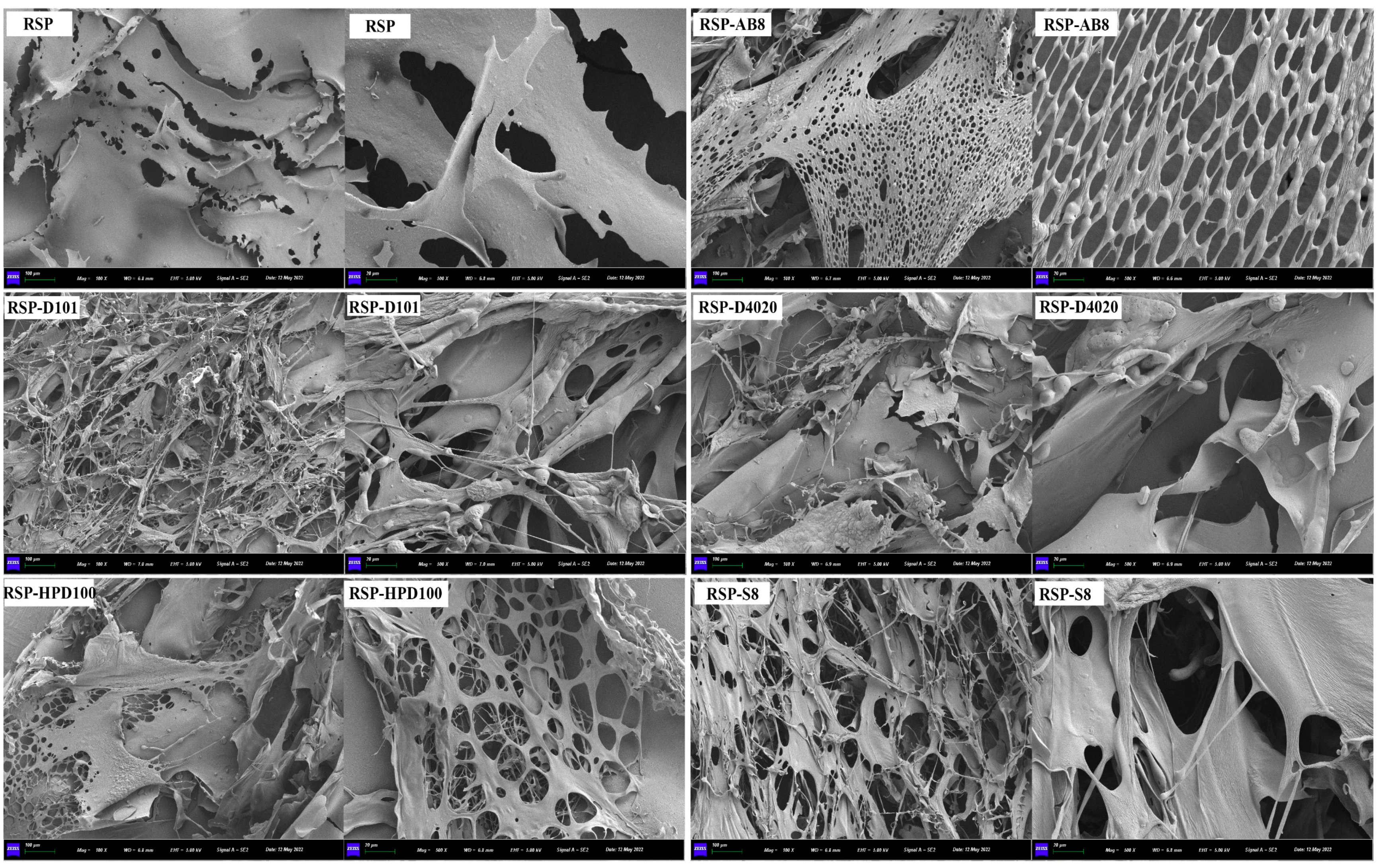
3.3.6. SEM
3.4. In Vitro Hypoglycemic Activity of RSP before and after Decoloration
3.4.1. Inhibitory Effect on α-Glucosidase
3.4.2. Inhibition Kinetics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, Z.; Yan, C.; Jia, C.; Li, Y.; Li, Y.; Li, J.; Yang, X.; Zhan, X.; Ma, C. Structural characterization of chia seed polysaccharides and evaluation of its immunomodulatory and antioxidant activities. Food Chem.-X 2023, 20, 101011. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Dong, S.Y.; Zhou, X.; Zhang, W.; Gu, Y.Z.; Zheng, L.X.; Yang, G.H.; Wang, J.; Zhang, Y. Polysaccharides from waste Zingiber mioga leaves: Ultrasonic-microwave-assisted extraction, characterization, antioxidant and anticoagulant potentials. Ultrason. Sonochem. 2023, 101, 106718. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Chen, G.; Kan, J. Characterization and in vitro simulated gastrointestinal digestion and fermentation of Mentha haplocaly polysaccharide. Int. J. Biol. Macromol. 2022, 222, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Luo, Y.; Wei, X.; Kan, J. Polysaccharide from Hovenia dulcis (Guaizao) improves pancreatic injury and regulates liver glycometabolism to alleviate STZ-induced type 1 diabetes mellitus in rats. Int. J. Biol. Macromol. 2022, 214, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Tudu, M.; Samanta, A. Natural polysaccharides: Chemical properties and application in pharmaceutical formulations. Eur. Polym. J. 2023, 184, 111801. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, H.; Li, Y.; Wu, M.; Yu, M.; Sun, X. Optimized purification process of polysaccharides from Carex meyeriana Kunth by macroporous resin, its characterization and immunomodulatory activity. Int. J. Biol. Macromol. 2019, 132, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yu, R.; Sun, J.; Duan, Y.; Zhou, H.; Zhou, W.; Li, G. Static decolorization of polysaccharides from the leaves of Rhododendro dauricum: Process optimization, characterization and antioxidant activities. Process Biochem. 2022, 121, 113–125. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, J.; Lan, W.; Yu, L.; Bi, Y.; Song, S.; Xiong, B.; Wang, H. Polysaccharide decolorization: Methods, principles of action, structural and functional characterization, and limitations of current research. Trends Food Sci. Technol. 2023, 138, 284–296. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, Y.; Li, C.; Jia, S.; Shi, Y.; Tang, Y.; Li, Y. A preliminary study on preparation, characterization, and prebiotic activity of a polysaccharide from the edible mushroom Ramaria flava. J. Food Biochem. 2022, 46, e14371. [Google Scholar] [CrossRef]
- Du, Q.; Zhou, K.; Zhong, S.L.; Li, C.Y.; Fan, J.B.; Wang, Q.; Hong, Y.; Wen, X.P. Cytological observations in abortion formation and morphological character of pollen in seedless chestnut rose (Rosa sterilis). Flora 2023, 307, 15237. [Google Scholar] [CrossRef]
- Xu, J.W.; Vidyarthi, S.K.; Bai, W.B.; Pan, Z.L. Nutritional constituents, health benefits and processing of Rosa Roxburghii: A review. J. Funct. Foods 2019, 60, 103456. [Google Scholar] [CrossRef]
- Li, H.; Fang, W.Y.; Wang, Z.; Chen, Y. Physicochemical, biological properties, and flavour profile of Rosa roxburghii Tratt, Pyracantha fortuneana, and Rosa laevigata Michx fruits: A comprehensive review. Food Chem. 2022, 366, 130509. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Zhang, Q.; Zhang, Y.H.; Lu, X.Y.; Fu, W.M.; He, J.Y. Chemical analysis of dietary constituents in Rosa roxburghii and Rosa sterilis fruits. Molecules 2016, 21, 1204. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.T.; Lu, M.; Ludlow, R.A.; Wang, D.J.; Zeng, J.W.; An, H.M. Contrastive analysis of trichome distribution, morphology, structure, and associated gene expression reveals the formation factors of different trichome types in two commercial Rosa species. Sci. Hortic. 2021, 285, 110131. [Google Scholar] [CrossRef]
- Ma, W.T.; Lu, M.; An, H.M.; Yi, Y. Comparative transcriptomic analysis of Rosa sterili inflorescence branches with different trichome types reveals an R3-MYB transcription factor that negatively regulates trichome formation. J. Integr. Agric. 2022, 21, 2926–2942. [Google Scholar] [CrossRef]
- Luan, L.Q.; Pan, H.B.; Chen, Y.H.; Ye, X.Q.; Hou, Z.Q.; Chen, S.G. Role of extracted phytochemicals from Rosa sterilis S. D. Shi in DSS-induced colitis mice: Potential amelioration of UC. Food Funct. 2023, 14, 8369–8382. [Google Scholar] [PubMed]
- Chen, K.; Zhang, Q.; Yang, S.; Zhang, S.; Chen, G. Comparative study on the impact of different extraction technologies on structural characteristics, physicochemical properties, and biological activities of polysaccharides from seedless chestnut rose (Rosa sterilis) fruit. Foods 2024, 13, 772. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Li, Z.; Gao, R.; Zhao, T.; Luo, D.; Yu, Z.; Zhang, S.; Qi, C.; Wang, Y.; Qiao, H.; et al. Structural characteristics of Rehmannia glutinosa polysaccharides treated using different decolorization processes and their antioxidant effects in intestinal epithelial cells. Foods 2022, 11, 3449. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Sun, Y.; Liang, J.; Li, M.; Li, X. Decolorization affects the structural characteristics and antioxidant activity of polysaccharides from Thesium chinense Turcz: Comparison of activated carbon and hydrogen peroxide decolorization. Int. J. Biol. Macromol. 2020, 155, 1084–1091. [Google Scholar] [CrossRef]
- Yu, B.; Chen, Y.; Zhu, L.; Ban, M.; Yang, L.; Zeng, Y.; Li, S.; Tang, C.; Zhang, D.; Chen, X. An effective and recyclable decolorization method for polysaccharides from Isaria cicadae Miquel by magnetic chitosan microspheres. Rsc Adv. 2022, 12, 3147–3156. [Google Scholar] [CrossRef]
- Yu, X.; Mu, N.; Liu, X.; Shang, Y.; Wang, D.; Li, F. A green method for decolorization of polysaccharides from alfalfa by S-8 macroporous resin and their characterization and antioxidant activity. Rsc Adv. 2023, 13, 9642–9653. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, T.; Han, Y.; Zhu, X.; Zhao, X.; Ma, X.; Jiang, D.; Zhang, Q. An efficient method for decoloration of polysaccharides from the sprouts of Toona sinensis (A. Juss.) Roem by anion exchange macroporous resins. Food Chem. 2017, 217, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Li, S.; Zhu, X.; Yang, J.; Wen, J.; Song, C.; Luo, X.; Ruan, G.; Liu, Y. Purification and the effects on structure and bioactivity for polysaccharide from Actinidia valvata Dunn. using macroporous adsorption resin. Food Sci. Technol. 2022, 42, e99721. [Google Scholar]
- Belwal, T.; Li, L.; Xu, Y.; Cravotto, G.; Luo, Z. Ultrasonic-assisted modifications of macroporous resin to improve anthocyanin purification from a Pyrus communis var. Starkrimson extract. Ultrason. Sonochem. 2020, 62, 104853. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Boussetta, N.; Lebovka, N.; Vorobiev, E. Ultrasound assisted purification of polyphenols of apple skins by adsorption/desorption procedure. Ultrason. Sonochem. 2019, 55, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Dil, E.A.; Ghaedi, M.; Asfaram, A.; Mehrabi, F.; Bazrafshan, A.A.; Tayebi, L. Synthesis and application of Ce-doped TiO2 nanoparticles loaded on activated carbon for ultrasound-assisted adsorption of Basic Red 46 dye. Ultrason. Sonochem. 2019, 58, 104702. [Google Scholar]
- Luo, Z.; Wang, X.; Gao, Q.; Guo, Z.; Li, W.; Zhao, Y. Application of ultrasound-assisted processing in improving the purification effect and bioactivity of licorice flavonoids from licorice residues. Ind. Crops Prod. 2021, 167, 113544. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, P.; Dai, Y.; Luo, X.; Manickam, S.; Li, D.; Han, Y.; Show, P.L. Bridge between mass transfer behavior and properties of bubbles under two-stage ultrasound-assisted physisorption of polyphenols using macroporous resin. Chem. Eng. J. 2022, 436, 135158. [Google Scholar] [CrossRef]
- Liang, L.; Liu, G.; Yu, G.; Song, Y.; Li, Q. Simultaneous decoloration and purification of crude oligosaccharides from pumpkin (Cucurbita moschata Duch) by macroporous adsorbent resin. Food Chem. 2019, 277, 744–752. [Google Scholar] [CrossRef]
- Sevag, M.G.; Lackman, D.B.; Smolens, J. The isolation of the components of streptococcal nucleoproteins in serologically active form. J. Biol. Chem. 1938, 24, 42–49. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkr, N.; Asboehan, G. New method for quantitative-determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Bai, J.W.; Bu, X.Y.; Yin, Y.T.; Wang, L.B.; Yang, Y.; Xu, Y.Q. Characterization of selenized polysaccharides from Ribes nigrum L. and its inhibitory effects on α-amylase and α-glucosidase. Carbohydr. Polym. 2021, 259, 117729. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.Q.; Cao, J.J.; Liu, R.; Chen, H.Q. Structural characterization, α-amylase and α-glucosidase inhibitory activities of polysaccharides from wheat bran. Food Chem. 2021, 341, 128218. [Google Scholar] [CrossRef] [PubMed]
- Cornishb, A.; Eisenthal, R. Statistical considerations in estimation of enzyme kinetic-parameters by direct linear plot and other methods. Biochem. J. 1974, 139, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zu, Y.; Liu, W.; Hou, C.; Chen, L.; Li, S.; Shi, X.; Tong, M. Preparative separation of vitexin and isovitexin from pigeonpea extracts with macroporous resins. J. Chromatogr. A 2007, 1139, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Coca, M.; Teresa Garcia, M.; Mato, S.; Carton, A.; Gonzalez, G. Evolution of colorants in sugarbeet juices during decolorization using styrenic resins. J. Food Eng. 2008, 89, 429–434. [Google Scholar] [CrossRef]
- Allen, S.J.; Gan, Q.; Matthews, R.; Johnson, P.A. Comparison of optimised isotherm models for basic dye adsorption by kudzu. Bioresour. Technol. 2003, 88, 143–152. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Cao, T.; Zhang, T.; Liu, Y.; Yan, Y. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Hum. Wellness 2023, 12, 1969–1980. [Google Scholar] [CrossRef]
- Zheng, M.; Ma, M.; Yang, Y.; Liu, Z.; Liu, S.; Hong, T.; Ni, H.; Jiang, Z. Structural characterization and antioxidant activity of polysaccharides extracted from Porphyra haitanensis by different methods. Int. J. Biol. Macromol. 2023, 242, 125003. [Google Scholar] [CrossRef] [PubMed]
- Kacuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds:: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, M.; Liu, M.; Bi, J. Physicochemical, rheological properties and in vitro hypoglycemic activities of polysaccharide fractions from peach gum. Carbohydr. Polym. 2022, 296, 119954. [Google Scholar] [CrossRef]
- You, G.; Liu, X.L.; Zhao, M.M. Preparation and characterization of hsian-tsao gum and chitosan complex coacervates. Food Hydrocoll. 2018, 74, 255–266. [Google Scholar] [CrossRef]
- Jiao, X.; Li, F.; Zhao, J.; Wei, Y.; Zhang, L.; Wang, H.; Yu, W.; Li, Q. Structural diversity and physicochemical properties of polysaccharides isolated from pumpkin (Cucurbita moschata) by different methods. Food Res. Int. 2023, 163, 112157. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Liu, R.; Chen, M.; Li, Z.; Qin, T.; Qian, Y.; Zhao, S.; Liu, M.; Zeng, Q.; Shen, J. Removal of copper ions from water using polysaccharide-constructed hydrogels. Carbohydr. Polym. 2019, 209, 101–110. [Google Scholar] [CrossRef]
- Chen, C.; Wang, P.-P.; Huang, Q.; You, L.-J.; Liu, R.H.; Zhao, M.-M.; Fu, X.; Luo, Z.-G. A comparison study on polysaccharides extracted from Fructus Mori using different methods: Structural characterization and glucose entrapment. Food Funct. 2019, 10, 3684–3695. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Feng, K.-L.; Wei, S.-Y.; Xiang, X.-R.; Ding, Y.; Li, H.-Y.; Zhao, L.; Qin, W.; Gan, R.-Y.; Wu, D.-T. Comparison of structural characteristics and bioactivities of polysaccharides from loquat leaves prepared by different drying techniques. Int. J. Biol. Macromol. 2020, 145, 611–619. [Google Scholar] [CrossRef]
- Jia, Y.; Xue, Z.; Wang, Y.; Lu, Y.; Li, R.; Li, N.; Wang, Q.; Zhang, M.; Chen, H. Chemical structure and inhibition on α-glucosidase of polysaccharides from corn silk by fractional precipitation. Carbohydr. Polym. 2021, 252, 117185. [Google Scholar] [CrossRef]
- Sohretoglu, D.; Sari, S.; Barut, B.; Ozel, A. Discovery of potent α-glucosidase inhibitor flavonols: Insights into mechanism of action through inhibition kinetics and docking simulations. Bioorg. Chem. 2018, 79, 257–264. [Google Scholar] [CrossRef]
- Cardullo, N.; Muccilli, V.; Pulvirenti, L.; Cornu, A.; Pouysegu, L.; Deffieux, D.; Quideau, S.; Tringali, C. C-glucosidic ellagitannins and galloylated glucoses as potential functional food ingredients with anti-diabetic properties: A study of α-glucosidase and α-amylase inhibition. Food Chem. 2020, 313, 126099. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Chen, C.R.; Wu, W.H.; Wen, C.L.; Chang, C.I.; Hou, W.C. Anti-α-glucosidase and anti-dipeptidyl peptidase-IV activities of extracts and purified compounds from Vitis thunbergii var. taiwaniana. J. Agric. Food Chem. 2015, 63, 6393–6401. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wu, X.; Pan, J.; Hu, X.; Gong, D.; Zhang, G. New Insights into the inhibition mechanism of betulinic acid on α-glucosidase. J. Agric. Food Chem. 2018, 66, 7065–7075. [Google Scholar] [CrossRef] [PubMed]



| Resin | Structure | Mode of Adsorption | Appearance | Surface Area (m2/g) | Wet True Density (g/mL) | Sized Bead Content after Grinding (%) | Particle Size (mm) |
|---|---|---|---|---|---|---|---|
| AB-8 | Cross-linked polystyrene | Non-ionic weak-polar | Milky white opaque spherical particles | 480–520 | 1.00–1.10 | ≥95 | 0.3–1.25 |
| ADS-17 | Cross-linked polystyrene | Non-ionic hydrogen bond | White opaque spherical particles | 90–150 | 1.03–1.07 | ≥90 | 0.3–1.25 |
| D101 | Cross-linked polystyrene | Non-ionic non-polar | Milky white opaque spherical particles | 550–600 | 1.10–1.18 | ≥90 | 0.3–1.25 |
| D201 | Cross-linked polystyrene | Anionic-polar | Milky white opaque spherical particles | 500–550 | 1.06–1.10 | ≥90 | 0.315–1.25 |
| D301-G | Cross-linked polystyrene | Non-ionic weak-polar | Canary yellow opaque spherical particles | 500–550 | 1.03–1.07 | ≥95 | 0.315–1.25 |
| D4020 | Cross-linked polystyrene | Non-ionic non-polar | White opaque spherical particles | 540–580 | 1.01–1.05 | ≥90 | 0.3–1.25 |
| DA201 | Cross-linked polystyrene | Anionic-polar | White opaque spherical particles | 500–550 | 1.03–1.07 | ≥90 | 0.3–1.25 |
| HP-20 | Cross-linked polystyrene | Non-ionic non-polar | White opaque spherical particles | 500–600 | 1.03–1.07 | ≥90 | 0.3–1.25 |
| HPD100 | Cross-linked polystyrene | Non-ionic non-polar | White opaque spherical particles | 650–700 | 1.03–1.07 | ≥90 | 0.3–1.25 |
| HPD500 | Cross-linked polystyrene | Anionic-polar | White opaque spherical particles | 500–550 | 1.03–1.07 | ≥90 | 0.3–1.25 |
| HPD722 | Cross-linked polystyrene | Non-ionic weak-polar | Milky white opaque spherical particles | 480–520 | 1.05–1.09 | ≥90 | 0.3–1.25 |
| HPD826 | Cross-linked polystyrene | Non-ionic hydrogen bond | White opaque spherical particles | 500–600 | 1.03–1.07 | ≥90 | 0.3–1.25 |
| NKA-9 | Cross-linked polystyrene | Anionic-polar | Light yellow opaque spherical particles | 500–550 | 1.03–1.07 | ≥90 | 0.3–1.25 |
| S-8 | Cross-linked polystyrene | Anionic-polar | Milky white opaque spherical particles | 100–120 | 1.03–1.07 | ≥90 | 0.3–1.25 |
| RSP | RSP-AB8 | RSP-D101 | RSR-D4020 | RSP-HPD100 | RSP-S8 | |
|---|---|---|---|---|---|---|
| Chemical composition (%, w/w) | ||||||
| Total sugar | 61.80 ± 2.62 a | 68.41 ± 2.82 b | 72.60 ± 1.18 c | 77.82 ± 4.90 d | 72.73 ± 5.67 c | 73.41 ± 3.92 c |
| Protein | 2.04 ± 0.07 a | 2.24 ± 0.09 a | 2.32 ± 0.09 a | 2.20 ± 0.07 a | 2.25 ± 0.09 a | 2.22 ± 0.08 a |
| Uronic acid | 14.26 ± 1.28 a | 18.66 ± 1.42 b | 22.05 ± 2.79 c | 22.39 ± 1.28 c | 26.51 ± 1.95 d | 19.08 ± 1.07 b |
| Monosaccharide composition (molar ratio, %) | ||||||
| Fuc | 0.53 | 0.31 | 0.29 | 0.25 | 0.22 | 0.23 |
| Rha | 2.63 | 3.09 | 3.38 | 1.89 | 4.07 | 3.41 |
| Ara | 9.42 | 9.21 | 9.86 | 7.29 | 11.81 | 11.47 |
| Gal | 38.16 | 29.34 | 36.96 | 29.06 | 39.15 | 42.50 |
| Glc | 23.95 | 30.97 | 11.76 | 25.82 | 6.84 | 15.73 |
| Xyl | 3.22 | 1.42 | 1.72 | 1.42 | 2.03 | 1.90 |
| Man | 1.64 | 0.84 | 0.75 | 0.78 | 0.69 | 0.73 |
| GalA | 20.45 | 24.82 | 35.28 | 33.49 | 35.19 | 24.03 |
| Molecular weight distribution | ||||||
| Mw (kDa) | 307.54 ± 2.31 a | 341.06 ± 17.96 b | 338.19 ± 22.28 b | 326.52 ± 6.37 a | 323.84 ± 7.58 a | 361.88 ± 5.75 c |
| Mn (kDa) | 40.70 ± 0.40 a | 58.19 ± 1.35 c | 49.86 ± 1.03 b | 57.45 ± 1.22 c | 42.33 ± 0.07 a | 46.45 ± 0.18 b |
| Mw/Mn | 7.56 ± 0.21 c | 5.86 ± 0.26 a | 6.79 ± 0.59 b | 5.68 ± 0.05 a | 7.65 ± 0.19 c | 7.79 ± 0.15 c |
| Sample | Stage 1 | Stage 2 | Stage 3 | Total Mass Loss (%) | T50 (°C) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mass Loss (%) | Start Temperature (°C) | Maximum Decomposition Rate (%/min) | Tmax (°C) | Mass Loss (%) | Start Temperature (°C) | Mass Loss (%) | |||
| RSP | 9.0 ± 0.3 b | 129.4 ± 4.3 a | 4.72 ± 0.24 a | 224.4 ± 5.3 a | 60.4 ± 2.1 a | 574.4 ± 6.9 a | 4.4 ± 0.2 bc | 73.8 ± 2.1 a | 296.3 ± 5.7 a |
| RSP-AB8 | 7.9 ± 0.4 c | 125.5 ± 3.9 b | 4.44 ± 0.39 a | 215.5 ± 4.2 b | 58.5 ± 2.6 ab | 575.4 ± 6.1 a | 4.9 ± 0.1 b | 71.3 ± 0.8 a | 295.9 ± 3.6 a |
| RSP-D101 | 10.0 ± 0.4 a | 129.1 ± 5.2 a | 3.78 ± 0.37 b | 219.1 ± 6.0 a | 59.4 ± 2.6 a | 574.4 ± 4.5 a | 4.0 ± 0.2 c | 73.4 ± 1.3 a | 295.1 ± 5.0 a |
| RSP-D4020 | 10.6 ± 0.5 a | 124.2 ± 2.8 b | 3.92 ± 0.42 b | 214.2 ± 5.1 b | 55.9 ± 1.4 b | 574.2 ± 5.9 a | 7.3 ± 0.4 a | 73.8 ± 1.6 a | 294.9 ± 4.5 a |
| RSP-HPD100 | 9.2 ± 0.3 b | 130.6 ± 5.4 a | 4.55 ± 0.26 a | 220.6 ± 7.1 a | 58.1 ± 2.0 ab | 575.6 ± 5.1 a | 5.2 ± 0.3 b | 73.3 ± 2.2 a | 295.0 ± 3.3 a |
| RSP-S8 | 9.8 ± 0.2 ab | 131.1 ± 4.0 a | 4.49 ± 0.34 a | 212.7 ± 4.9 b | 56.9 ± 2.8 b | 574.7 ± 4.3 a | 5.6 ± 0.2 b | 72.3 ± 0.9 a | 296.7 ± 4.2 a |
| Sample | Concentration (mg/mL) | Inhibition Type | Km (mM) | Vmax (∆A405/min) | Ki (mg/mL) | Kii (mg/mL) | Ki/Kii |
|---|---|---|---|---|---|---|---|
| RSP | 0 | Mixed inhibition | 3.726 | 0.134 | 3.710 | 1.965 | 1.888 |
| 3 | 2.991 | 0.0853 | |||||
| 5 | 2.556 | 0.0477 | |||||
| RSP-AB8 | 0 | Mixed inhibition | 3.601 | 0.132 | 3.257 | 1.577 | 2.065 |
| 3 | 2.751 | 0.0720 | |||||
| 5 | 2.368 | 0.0403 | |||||
| RSP-D4020 | 0 | Mixed inhibition | 3.607 | 0.134 | 2.832 | 1.439 | 1.968 |
| 3 | 2.773 | 0.0633 | |||||
| 5 | 2.382 | 0.0372 | |||||
| RSP-S8 | 0 | Mixed inhibition | 3.743 | 0.130 | 3.329 | 1.568 | 2.123 |
| 3 | 2.775 | 0.0623 | |||||
| 5 | 2.378 | 0.0378 | |||||
| RSP-HPD100 | 0 | Mixed inhibition | 3.646 | 0.134 | 2.174 | 1.036 | 2.098 |
| 3 | 2.467 | 0.0521 | |||||
| 5 | 2.195 | 0.0292 | |||||
| RSP-D101 | 0 | Mixed inhibition | 3.599 | 0.135 | 3.036 | 1.459 | 2.081 |
| 3 | 2.769 | 0.0689 | |||||
| 5 | 2.338 | 0.0388 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Sun, M.; Chen, K.; Wang, L.; Sun, J. Ultrasonic-Assisted Decoloration of Polysaccharides from Seedless Chestnut Rose (Rosa sterilis) Fruit: Insight into the Impact of Different Macroporous Resins on Its Structural Characterization and In Vitro Hypoglycemic Activity. Foods 2024, 13, 1349. https://doi.org/10.3390/foods13091349
Chen G, Sun M, Chen K, Wang L, Sun J. Ultrasonic-Assisted Decoloration of Polysaccharides from Seedless Chestnut Rose (Rosa sterilis) Fruit: Insight into the Impact of Different Macroporous Resins on Its Structural Characterization and In Vitro Hypoglycemic Activity. Foods. 2024; 13(9):1349. https://doi.org/10.3390/foods13091349
Chicago/Turabian StyleChen, Guangjing, Meiwen Sun, Kaiwen Chen, Lisha Wang, and Juyan Sun. 2024. "Ultrasonic-Assisted Decoloration of Polysaccharides from Seedless Chestnut Rose (Rosa sterilis) Fruit: Insight into the Impact of Different Macroporous Resins on Its Structural Characterization and In Vitro Hypoglycemic Activity" Foods 13, no. 9: 1349. https://doi.org/10.3390/foods13091349
APA StyleChen, G., Sun, M., Chen, K., Wang, L., & Sun, J. (2024). Ultrasonic-Assisted Decoloration of Polysaccharides from Seedless Chestnut Rose (Rosa sterilis) Fruit: Insight into the Impact of Different Macroporous Resins on Its Structural Characterization and In Vitro Hypoglycemic Activity. Foods, 13(9), 1349. https://doi.org/10.3390/foods13091349






