Fermenting Acerola (Malpighia emarginata D.C.) and Guava (Psidium guayaba L.) Fruit Processing Co-Products with Probiotic Lactobacilli to Produce Novel Potentially Synbiotic Circular Ingredients
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fruit Processing Co-Products
2.2. Microorganisms and Fermentation Conditions
2.3. Freeze-Drying of Fermented Fruit Co-Products
2.4. Simulated Gastrointestinal Digestion of the Fermented Co-Product Suspensions
2.5. Preparation of Human Fecal Inoculum and In Vitro Fecal Fermentation
2.5.1. Enumeration of the Intestinal Bacterial Populations during In Vitro Fecal Fermentation
2.5.2. Determination of Microbial Metabolism during In Vitro Fecal Fermentation
2.5.3. Determination of the Antioxidant Capacity during In Vitro Fecal Fermentation
2.6. Statistical Analysis
3. Results
3.1. Changes in Relative Abundance of Intestinal Bacterial Populations during In Vitro Fecal Fermentation
3.2. Microbial Metabolic Activity during In Vitro Fecal Fermentation
3.3. Changes in Phenolic Compounds and Antioxidant Capacity during In Vitro Fecal Fermentation
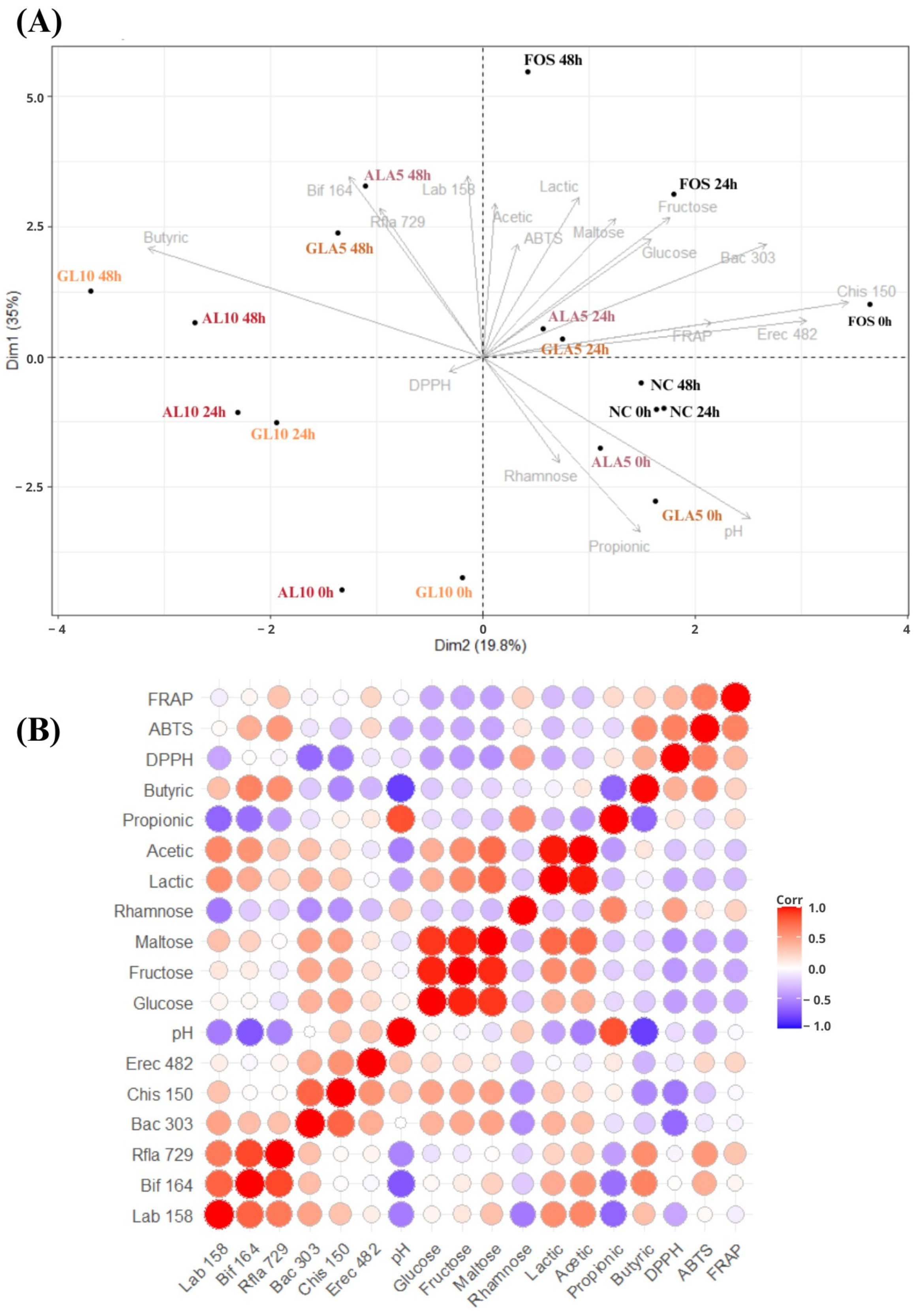
3.4. Chemometric Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sabino, L.B.S.; Gonzaga, M.L.C.; Oliveira, L.S.; Duarte, A.S.G.; Alexandre e Silva, L.M.; de Brito, E.S.; Figuereido, R.W.; Silva, L.M.R.; Sousa, P.H.M. Polysaccharides from acerola, cashew apple, pineapple, mango and passion fruit by-products: Structure, cytotoxicity and gastroprotective effects. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100228. [Google Scholar] [CrossRef]
- Barros, B.R.S.; Nascimento, D.K.D.; Araújo, D.R.C.; Batista, F.R.C.; Lima, A.M.N.O.; Cruz Filho, I.J.; Oliveira, M.L.; de Melo, C.M.L. Phytochemical analysis, nutritional profile and immunostimulatory activity of aqueous extract from Malpighia emarginata DC leaves. Biocatal. Agric. Biotechnol. 2020, 23, 101442. [Google Scholar] [CrossRef]
- Moreira-Araújo, R.S.R.; Barros, N.V.A.; Porto, R.G.C.L.; Brandão, A.C.A.S.; Lima, A.D.; Fett, R. Bioactive compounds and antioxidant activity three fruit species from the Brazilian Cerrado. Rev. Bras. Frutic. 2019, 41, e–011. [Google Scholar]
- Araújo, C.M.; Sampaio, K.B.; Menezes, F.N.D.D.; Almeida, E.T.D.C.; Lima, M.D.S.; Viera, V.B.; Garcia, E.F.; Gómez-Zavaglia, A.; Souza, E.L.; Oliveira, M.E.G. Protective effects of tropical fruit processing coproducts on probiotic Lactobacillus strains during freeze-drying and storage. Microorganisms 2020, 8, 96. [Google Scholar] [CrossRef]
- Lima, R.S.; Ferreira, S.R.S.; Vitali, L.; Block, J.M. May the superfruit red guava and its processing waste be a potential ingredient in functional foods? Food Res. Int. 2019, 115, 451–459. [Google Scholar] [CrossRef]
- Lugo, S.D.R.; Kimita, K.; Nishino, N. Characteristics of decision process towards circular food economy: A review. Clean. Log. Supp. Chain 2023, 7, 100104. [Google Scholar] [CrossRef]
- Albuquerque, M.A.C.; Levit, R.; Beres, C.; Bedani, R.; de LeBlanc, A.M.; Saad, S.M.I.; LeBlanc, J.G. Tropical fruit by-products water extracts as sources of soluble fibres and phenolic compounds with potential antioxidant, anti-inflammatory, and functional properties. J. Funct. Foods 2019, 52, 724–733. [Google Scholar] [CrossRef]
- Gualberto, C.N.; de Oliveira, C.S.; Nogueira, J.P.; de Jesus, M.S.; Araujo, H.C.S.; Rajan, M.; Leite Neta, M.T.S.; Narain, N. Bioactive compounds and antioxidant activities in the agro-industrial residues of acerola (Malpighia emarginata L.), guava (Psidium guajava L.), genipap (Genipa americana L.) and umbu (Spondias tuberosa L.) fruits assisted by ultrasonic or shaker extraction. Food Res. Int. 2021, 147, 110538. [Google Scholar] [CrossRef]
- Miskinis, R.A.S.; do Nascimento, L.Á.; Colussi, R. Bioactive compounds from acerola pomace: A review. Food Chem. 2023, 404, 134613. [Google Scholar] [CrossRef]
- Vega-Baudrit, J.R.; Camacho, M.; Batista-Menezes, D.; Corrales-Ureña, Y.; Zúñiga, J.M.; Chacón, A.M.; Lecot, N.; Henríquez, L.C.; Lopretti, M. Acerola (Malpighia spp.) waste: A sustainable approach to nutraceutical, pharmaceutical, and energy applications. Recycling 2023, 8, 96. [Google Scholar] [CrossRef]
- Gibson, R.G.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiao, Y.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Rational use of prebiotics for gut microbiota alterations: Specific bacterial phylotypes and related mechanisms. J. Funct. Foods 2020, 66, 103838. [Google Scholar] [CrossRef]
- Souza, E.L.; Albuquerque, T.M.R.; Santos, A.S.; Massa, N.M.L.; Brito Alves, J.L. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities—A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1645–1659. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, I.O.; Dat-arun, P.; Yupanqui, C.T.; Wichienchot, S. Gut microbiota metabolism of functional carbohydrates and phenolic compounds from soaked and germinated purple rice. J. Funct. Foods 2020, 66, 103787. [Google Scholar] [CrossRef]
- Menezes, F.N.D.D.; Melo, F.H.C.; Vieira, A.R.S.; Almeida, E.T.C.; Lima, M.S.; Aquino, J.S.; Gomez-Zavaglia, A.; Magnani, M.; de Souza, E.L. Acerola (Malpighia glabra L.) and guava (Psidium guayaba L.) industrial processing by-products stimulate probiotic Lactobacillus and Bifidobacterium growth and induce beneficial changes in colonic microbiota. J. Appl. Microbiol. 2021, 130, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Menezes, F.N.D.D.; Cruz Almeid, É.T.; Silva Vieira, A.R.; Souza Aquino, J.; Santos Lima, M.; Magnani, M.; Souza, E.L. Impact of cashew (Anacardium occidentale L.) by-product on composition and metabolic activity of human colonic microbiota in vitro indicates prebiotic properties. Curr. Microbiol. 2021, 78, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- Vaid, S.; Pandey, V.K.; Singh, R.; Dar, A.H.; Shams, R.; Thakur, K.S. A concise review on development of probiotics from Lactobacillus using CRISPR-Cas Technology of Gene Editing. Food Chem. Adv. 2022, 1, 100099. [Google Scholar] [CrossRef]
- Bahrami, G.; Malekshahi, H.; Miraghaee, S.; Madani, H.; Babaei, A. Improving animal model of induced colitis by acetic acid in terms of fibrosis and inflammation incidence in the colon. J. Investig. Surg. 2022, 35, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Marius, F.K.E.; Marie, K.P.; Blandine, M.; Laverdure, T.P.; Daquain, F.T.U.; François, Z.N. Development of a non-dairy probiotic beverage based on sorrel and pineapple juices using Lacticaseibacillus paracasei 62L. J. Agric. Food Res. 2023, 14, 100688. [Google Scholar] [CrossRef]
- Xavier-Santos, D.; Lima, E.D.; Simão, A.N.C.; Bedani, R.; Saad, S.M.I. Effect of the consumption of a synbiotic diet mousse containing Lactobacillus acidophilus La-5 by individuals with metabolic syndrome: A randomized controlled trial. J. Funct. Foods 2018, 41, 55–61. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Rønhave Laursen, R.; Ouwehand, A.C. The production and delivery of probiotics: A review of a practical approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.P.A.; Nascimento, H.M.A.; Sampaio, K.B.; Souza, E.L. A review on bioactive compounds of beet (Beta vulgaris L. subsp. vulgaris) with special emphasis on their beneficial effects on gut microbiota and gastrointestinal health. Crit. Rev. Food Sci. Nutr. 2020, 61, 2022–2033. [Google Scholar] [CrossRef] [PubMed]
- Shori, A.B.; Aljohani, G.S.; Al-zahrani, A.J.; Al-sulbi, O.S.; Baba, A.S. Viability of probiotics and antioxidant activity of cashew milk-based yogurt fermented with selected strains of probiotic Lactobacillus spp. LWT—Food Sci. Technol. 2022, 153, 112482. [Google Scholar] [CrossRef]
- Sanders, M.E.; Marco, M.L. Food formats for effective delivery of probiotics. Annu. Rev. Food Sci. Technol. 2010, 1, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Duarte, F.N.D.; Rodrigues, J.B.; Costa Lima, M.; Lima, M.D.S.; Pacheco, M.T.B.; Pintado, M.M.E.; Aquino, J.S.; de Souza, E.L. Potential prebiotic properties of cashew apple (Anacardium occidentale L.) agro-industrial byproduct on Lactobacillus species. J. Sci. Food Agric. 2017, 97, 3712–3719. [Google Scholar] [CrossRef] [PubMed]
- Nunes, G.L.; Etchepare, M.A.; Cichoski, A.J.; Zepka, L.Q.; Lopes, E.J.; Barin, J.S.; Flores, E.M.M.; Silva, C.B.; Menezes, C.R. Inulin, hi-maize, and trehalose as thermal protectants for increasing viability of Lactobacillus acidophilus encapsulated by spray drying. LWT—Food Sci. Technol. 2018, 89, 128–133. [Google Scholar] [CrossRef]
- Sousa, S.; Pinto, J.; Pereira, C.; Malcata, F.X.; Pacheco, M.T.B.; Gomes, A.M.; Pintado, M. In vitro evaluation of yacon (Smallanthus sonchifolius) tuber flour prebiotic potential. Food Bioprod. Process. 2015, 95, 96–105. [Google Scholar] [CrossRef]
- Garcia, E.F.; Oliveira Araújo, A.; Luciano, W.A.; Albuquerque, T.M.R.; Oliveira Arcanjo, N.M.; Madruga, M.S.; Lima, M.S.; Magnani, M.; Saarela, M.; Souza, E.L. The performance of five fruit-derived and freeze-dried potentially probiotic Lactobacillus strains in apple, orange, and grape juices. J. Sci. Food Agric. 2018, 98, 5000–5010. [Google Scholar] [CrossRef]
- Sampaio, K.B.; Nascimento, Y.M.; Tavares, J.F.; Cavalcanti, M.T.; Brito Alves, J.L.; Garcia, E.F.; Souza, E.L. Development and in vitro evaluation of novel nutraceutical formulations composed of Limosilactobacillus fermentum, quercetin and/or resveratrol. Food Chem. 2021, 342, 128264. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, K.B.; Brito Alves, J.L.; Nascimento, Y.M.; Tavares, J.F.; Silva, M.S.; Nascimento, D.S.; Lima, M.S.; Rodrigues, N.P.A.; Garcia, E.F.; Souza, E.L. Nutraceutical formulations combining Limosilactobacillus fermentum, quercetin, and or resveratrol with beneficial impacts on the abundance of intestinal bacterial populations, metabolite production, and antioxidant capacity during colonic fermentation. Food Res. Int. 2022, 161, 111800. [Google Scholar] [CrossRef]
- Albuquerque, T.M.R.; Magnani, M.; Lima, M.D.S.; Castellano, L.R.C.; Souza, E.L. Effects of digested flours from four different sweet potato (Ipomoea batatas L.) root varieties on the composition and metabolic activity of human colonic microbiota in vitro. J. Food Sci. 2021, 6, 3707–3719. [Google Scholar] [CrossRef]
- Rodrigues, D.; Walton, G.; Sousa, S.; Rocha-Santos, T.A.P.; Duarte, A.C.; Freitas, A.C.; Gomes, A.M.P. In vitro fermentation and prebiotic potential of selected extracts from seaweeds and mushrooms. LWT—Food Sci. Technol. 2016, 73, 131–139. [Google Scholar] [CrossRef]
- Silva, J.Y.P.; Nascimento, H.M.A.; Albuquerque, T.M.R.; Sampaio, K.B.; Lima, M.S.; Monteiro, M.; Leite, I.B.; Silva, E.F.; Nascimento, Y.M.; Silva, M.S.; et al. Revealing the potential impacts of nutraceuticals formulated with freeze-dried jabuticaba peel and Limosilactobacillus fermentum strains candidates for probiotic use on human intestinal microbiota. Probiotics Antimicrob. Proteins 2023. [Google Scholar] [CrossRef]
- Coelho, E.M.; Padilha, C.V.S.; Miskinis, G.A.; Sá, A.G.B.; Pereira, G.E.; Azevedo, L.C.; Lima, M.S. Simultaneous analysis of sugars and organic acids in wine and grape juices by HPLC: Method validation and characterization of products from northeast Brazil. J. Food Compost. Anal. 2018, 66, 160–167. [Google Scholar] [CrossRef]
- Conterno, L.; Martinelli, F.; Tamburini, M.; Fava, F.; Mancini, A.; Sordo, M.; Pindo, M.; Martens, S.; Masuero, D.; Vrhovsek, U.; et al. Measuring the impact of olive pomace enriched biscuits on the gut microbiota and its metabolic activity in mildly hypercholesterolaemic subjects. Eur. J. Nutr. 2017, 58, 63–81. [Google Scholar] [CrossRef]
- Daims, H.; Stoecker, K.; Wagner, M. Fluorescence in situ hybridization for the detection of prokaryotes. In Molecular Microbial Ecology, 1st ed.; Osborn, A.M., Smith, C.J., Eds.; Taylor & Francis: Abingdon, UK, 2005; Volume 6, Chapter 9; pp. 213–239. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 21st ed.; AOAC International: Arlington, TX, USA, 2019; ISBN 978-0935584899. [Google Scholar]
- Padilha, C.V.S.; Miskinis, G.A.; de Souza, M.E.A.O.; Pereira, G.E.; de Oliveira, D.; Bordignon-Luiz, M.T.; dos Santos Lima, M. Rapid determination of flavonoids and phenolic acids in grape juices and wines by RP-HPLC/DAD: Method validation and characterization of commercial products of the new Brazilian varieties of grape. Food Chem. 2017, 228, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.S.; Sampaio, K.B.; Nascimento, Y.M.; Souza, T.A.; Souza, F.S.; Chaves Júnior, J.V.; Tavares, J.F.; Silva, M.S.; Brito Alves, J.L.; Souza, E.L. Evaluating the stability of a novel nutraceutical formulation combining probiotic Limosilactobacillus fermentum 296, quercetin, and resveratrol under different storage conditions. Probiotics Antimicrob. Proteins 2022, 16, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic species in the modulation of gut microbiota: An overview. Biomed. Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef]
- Gomes, I.A.; Venâncio, A.; Lima, J.P.; Freitas-Silva, O. Fruit-based non-dairy beverage: A new approach for probiotics. Adv. Biol. Chem. 2021, 11, 302–330. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Milagro, F.I.; Guruceaga, E.; Cuervo, M.; Goni, L.; García-Granero, M.; Martinez, J.A.; Riezu-Boj, J.I. A weight-loss model based on baseline microbiota and genetic scores for selection of dietary treatments in overweight and obese population. Clin. Nutr. 2022, 41, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, J.; Yeom, Z.; Heo, D.; Lim, Y.H. Neuroprotective effect of Ruminococcus albus on oxidatively stressed SH-SY5Y cells and animals. Sci. Rep. 2017, 7, 14520. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, S.; Chen, M.; Cui, Y.; Shi, C.; Pu, X.; Gao, W.; Li, Q.; Han, J.; Zhang, A. Effects of buckwheat milk co-fermented with two probiotics and two commercial yoghurt strains on gut microbiota and production of short-chain fatty acids. Food Biosci. 2023, 53, 102537. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Getselter, D.; Ziv, O.; Oron, O.; Reuveni, E.; Koren, O.; Elliott, E. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry 2019, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhang, Y.; Zheng, L.; Rong, N.; Yang, Y.; Gong, P.; Yang, Y.; Siwu, X.; Zhang, C.; Zhu, L.; et al. Bifidobacterium and Lactobacillus improve inflammatory bowel disease in zebrafish of different ages by regulating the intestinal mucosal barrier and microbiota. Life Sci. 2023, 324, 121699. [Google Scholar] [CrossRef]
- Diotallevi, C.; Gaudioso, G.; Fava, F.; Angeli, A.; Lotti, C.; Vrhovsek, U.; Rinott, E.; Shai, I.; Gobbetti, M.; Tuohy, K. Measuring the effect of Mankai® (Wolffia globosa) on the gut microbiota and its metabolic output using an in vitro colon model. J. Funct. Foods 2021, 84, 104597. [Google Scholar] [CrossRef]
- Iljazovic, A.; Amend, L.; Galvez, E.J.C.; Oliveira, R.; Strowing, T. Modulation of inflammatory responses by gastrointestinal Prevotella spp.—From associations to functional studies. J. Med. Microbiol. 2021, 311, 151472. [Google Scholar] [CrossRef]
- Liu, C.; Kolida, S.; Charalampopoulos, D.; Rastall, R.A. An evaluation of the prebiotic potential of microbial levans from Erwinia sp. 10119. J. Funct. Foods. 2020, 64, 103668. [Google Scholar] [CrossRef]
- Oliveira, S.P.A.; Albuquerque, T.M.R.; Massa, N.M.L.; Rodrigues, N.P.A.; Sampaio, K.B.; Nascimento, H.M.A.; Lima, M.S.; Conceição, M.L.; Souza, E.L. Investigating the effects of conventional and unconventional edible parts of red beet (Beta vulgaris L.) on target bacterial groups and metabolic activity of human colonic microbiota to produce novel and sustainable prebiotic ingredients. Food Res. Int. 2023, 171, 112998. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Massa, N.M.L.; de Oliveira, S.P.A.; Rodrigues, N.P.A.; Menezes, F.N.D.D.; dos Santos Lima, M.; Magnani, M.; de Souza, E.L. In vitro colonic fermentation and potential prebiotic properties of pre-digested jabuticaba (Myrciaria jaboticaba (Vell.) Berg) by-products. Food Chem. 2022, 388, 133003. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Maciejewska, D.; Kulpa, D.; Celewicz, Z.; Ziętek, M. The associations of SCFA with anthropometric parameters and carbohydrate metabolism in pregnant women. Int. J. Mol. Sci. 2020, 21, 9212. [Google Scholar] [CrossRef]
- Hsiao, Y.P.; Chen, H.L.; Tsai, J.N.; Lin, M.Y.; Liao, J.W.; Wei, M.S.; Ko, J.L.; Ou, C.C. Administration of Lactobacillus reuteri combined with Clostridium butyricum attenuates cisplatin-induced renal damage by gut microbiota reconstitution, increasing butyric acid production, and suppressing renal inflammation. Nutrients 2021, 13, 2792. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.M.; Bast, A.; Vanhoutvin, S.A.; Fischer, M.A.; Kodde, A.; Troost, F.J.; Venema, K.; Brummer, R.M. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nutr. 2009, 28, 88–93. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A double-edged sword for health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Evdokimova, S.A.; Karetkin, B.A.; Guseva, E.V.; Gordienko, M.G.; Khabibulina, N.V.; Panfilov, V.I.; Menshutina, N.V.; Gradova, N.B. A study and modeling of Bifidobacterium and Bacillus coculture continuous fermentation under distal intestine simulated conditions. Microorganisms 2022, 10, 929. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, L.; Zhao, L.; Yan, F.; Zhu, X.; Lu, Q.; Liu, R. Metabolomic profiles of A-type procyanidin dimer and trimer with gut microbiota in vitro. J. Funct. Foods 2021, 85, 104637. [Google Scholar] [CrossRef]
- Chen, G.J.; Hong, Q.Y.; Ji, N.; Wu, W.N.; Ma, L.Z. Influences of different drying methods on the structural characteristics and prebiotic activity of polysaccharides from bamboo shoot (Chimonobambusa quadrangularis) residues. Int. J. Biol. Macromol. 2020, 155, 674–684. [Google Scholar] [CrossRef]
- Chen, T.; Long, W.; Zhang, C.; Liu, S.; Zhao, L.; Hamaker, B.R. Fiber-utilizing capacity varies in Prevotella—versus Bacteroides—dominated gut microbiota. Sci. Rep. 2017, 7, 2594. [Google Scholar] [CrossRef]
- Kim, H.K.; Kostidis, S.; Choi, Y.H. NMR analysis of fecal samples. Methods Mol. Biol. 2018, 1730, 317–328. [Google Scholar] [CrossRef]
- Kaczmarczyk, O.; Dąbek-Drobny, A.; Woźniakiewicz, M.; Paśko, P.; Dobrowolska-Iwanek, J.; Woźniakiewicz, A.; Piątek-Guziewicz, A.; Zagrodzki, P.; Mach, T.; Zwolińska-Wcisło, M. Fecal levels of lactic, succinic and short-chain fatty acids in patients with ulcerative colitis and crohn disease: A pilot study. J. Clin. Med. 2021, 10, 4701. [Google Scholar] [CrossRef]
- Bianchi, F.; Lopes, N.P.; Adorno, M.A.T.; Sakamoto, I.K.; Genovese, M.I.; Saad, S.M.I.; Sivieri, K. Impact of combining acerola by-product with a probiotic strain on a gut microbiome model. Int. J. Food Sci. Nutr. 2019, 70, 182–194. [Google Scholar] [CrossRef]
- Duan, H.; Yu, Q.; Ni, Y.; Li, J.; Fan, L. Effect of Agaricus bisporus Polysaccharides on human gut microbiota during in vitro fermentation: An integrative analysis of microbiome and metabolome. Foods 2023, 12, 859. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.H.; Zabed, H.M.; Yun, J.; Zhang, G.; Qi, X. An insight into the roles of dietary tryptophan and its metabolites in intestinal inflammation and inflammatory bowel disease. Mol. Nutr. Food Res. 2021, 65, 2000461. [Google Scholar] [CrossRef]
- Jaafar, M.H.; Xu, P.; Mageswaran, U.-M.; Balasubramaniam, S.-D.; Solayappan, M.; Woon, J.-J.; The, C.S.-J.; Todorov, S.D.; Park, Y.-H.; Liu, G.; et al. Constipation anti-aging effects by dairy-based lactic acid bacteria. J. Anim. Sci. Technol. 2024, 66, 178–203. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.L.; Arruda, T.Y.P.; Morzelle, M.C.; Pereira, A.P.A.; Casarotti, S.N. Fruit by-products as potential prebiotics and promising functional ingredients to produce fermented milk. Food Res. Int. 2022, 161, 111841. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Sela, D.A.; Xiao, J.B.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 36. [Google Scholar] [CrossRef]
- Liu, Z.; de Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.P. Microbial metabolism of theaflavin-3,3′-digallate and its gut microbiota composition modulatory effects. J. Agric. Food Chem. 2021, 69, 232–245. [Google Scholar] [CrossRef]
- Stoupi, S.; Williamson, G.; Drynan, J.W.; Barron, D.; Clifford, M.N. A comparison of the in vitro biotransformation of (-)-epicatechin and procyanidin B2 by human faecal microbiota. Mol. Nutr. Food Res. 2010, 54, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Bazzocco, S.; Mattila, I.; Guyot, S.; Renard, C.M.; Aura, A.M. Factors affecting the conversion of apple polyphenols to phenolic acids and fruit matrix to short-chain fatty acids by human faecal microbiota in vitro. Eur. J. Nutr. 2008, 47, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Vilas Boas, P.B.; Lepercq, P.; Comtet-Marre, S.; Auffret, P.; Ruiz, P.; Bott, R.; Renard, C.M.G.C.; Dufour, C.; Chatel, J.M.; et al. Procyanidin-cell wall interactions within apple matrices decrease the metabolization of procyanidins by the human gut microbiota and the anti-inflammatory effect of the resulting microbial metabolome in vitro. Nutrients 2019, 11, 664. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Pérez-Jiménez, J.; Touriño, S.; Serrano, J.; Fuguet, E.; Torres, J.L.; Goñi, I. Proanthocyanidin metabolites associated with dietary fibre from in vitro colonic fermentation and proanthocyanidin metabolites in human plasma. Mol. Nutr. Food Res. 2010, 54, 939–946. [Google Scholar] [CrossRef]
- Jiménez-Girón, A.; Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Muñoz-González, I.; Sánchez-Patán, F.; Monagas, M.; Martín-Álvarez, P.J.; Murri, M.; Tinahones, F.J.; Andrés-Lacueva, C.; et al. Comparative study of microbial-derived phenolic metabolites in human feces after intake of gin, red wine, and dealcoholized red wine. J. Agric. Food Chem. 2013, 61, 3909–3915. [Google Scholar] [CrossRef]
- Pacheco-Ordaz, R.; Wall-Medrano, A.; Goñi, M.G.; Ramos-Clamont-Montfort, G.; Ayala-Zavala, J.F.; González-Aguilar, G.A. Effect of phenolic compounds on the growth of selected probiotic and pathogenic bacteria. Lett. Appl. Microbiol. 2018, 66, 25–31. [Google Scholar] [CrossRef]
- Nayeem, N.; Asdaq, S.M.B.; Salem, E.; AHEl-Alfqy, S. Gallic Acid: A promising lead molecule for drug development. J. Appl. Pharm. Sci. 2016, 8, 213. [Google Scholar] [CrossRef]
- Sağdıçoğlu Celep, G.; Demirkaya, A.; Solak, E. Antioxidant and anticancer activities of gallic acid loaded sodium alginate microspheres on colon cancer. Curr. Appl. Phys. 2020, 40, 30–42. [Google Scholar] [CrossRef]
- Fan, S.; Li, J.; Zhang, X.; Xu, D.; Liu, X.; Dias, A.C.P.; Zhang, X.; Chen, C. A study on the identification, quantification, and biological activity of compounds from Cornus officinalis before and after in vitro gastrointestinal digestion and simulated colonic fermentation. J. Funct. Foods 2022, 98, 105272. [Google Scholar] [CrossRef]
- Fidelis, M.; Santos, J.S.; Escher, G.B.; Rocha, R.S.; Cruz, A.G.; Cruz, T.M.; Marques, M.B.; Nunes, J.B.; Carmo, M.A.V.; Almeida, L.A.; et al. Polyphenols of jabuticaba [Myrciaria jaboticaba (Vell.) O. Berg] seeds incorporated in a yogurt model exert antioxidant activity and modulate gut microbiota of 1,2-dimethylhydrazine-induced colon cancer in rats. Food Chem. 2021, 334, 127565. [Google Scholar] [CrossRef]
- Pang, J.; Liu, Y.; Kang, L.; Ye, H.; Zang, J.; Wang, J.; Han, D. Bifidobacterium animalis promotes the growth of weaning piglets by improving intestinal development, enhancing antioxidant capacity, and modulating gut microbiota. Appl. Environ. Microbiol. 2022, 88, e0129622. [Google Scholar] [CrossRef]
- Rizzo, G. The antioxidant role of soy and soy foods in human health. Antioxidants 2020, 9, 635. [Google Scholar] [CrossRef]
- Aiello, A.; Pizzolongo, F.; Luca, L.; Blaiotta, G.; Aponte, M.; Addeo, F.; Romano, R. Production of butyric acid by different strains of Lactobacillus plantarum (Lactiplantibacillus plantarum). Int. Dairy J. 2023, 140, 105589. [Google Scholar] [CrossRef]
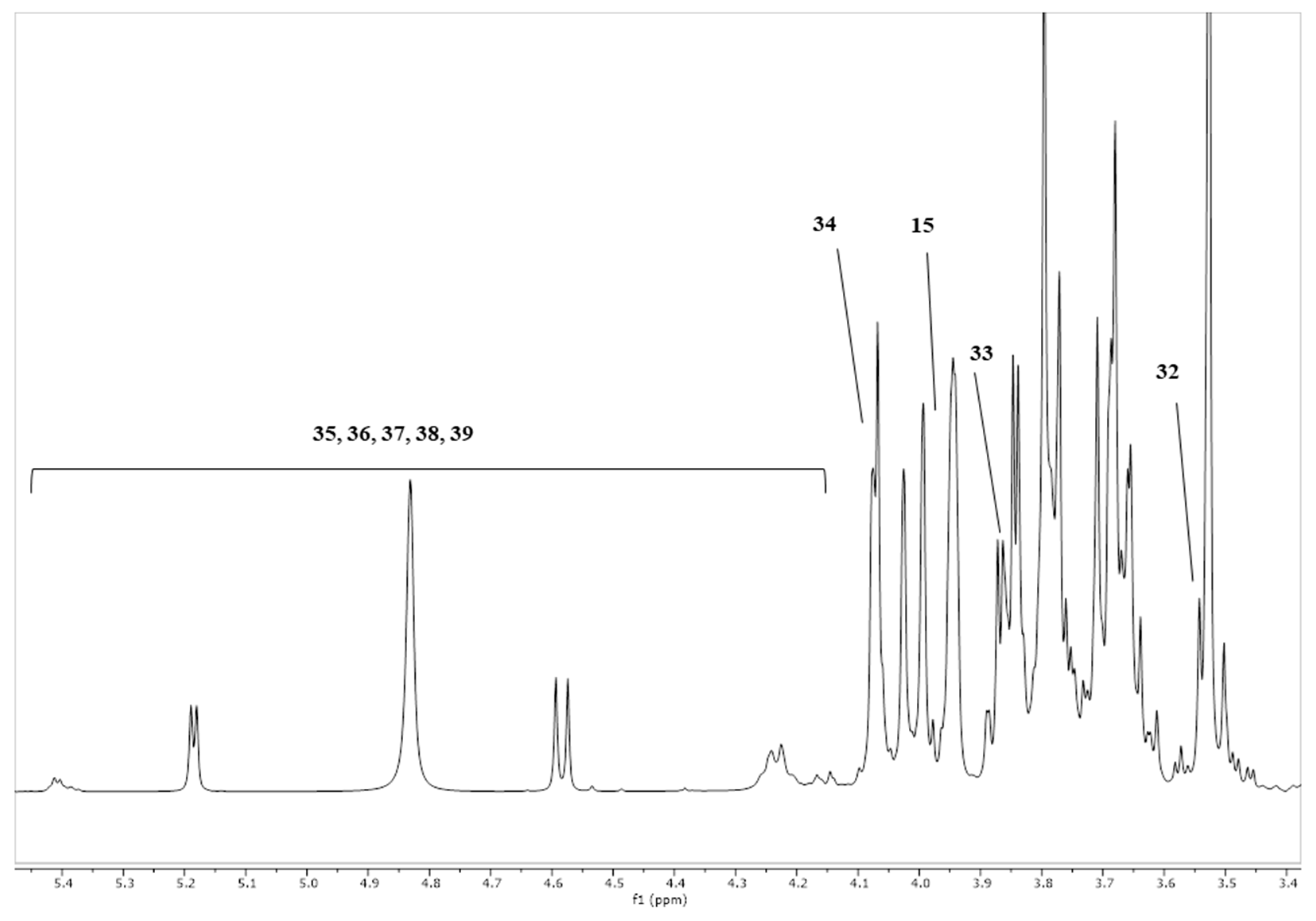
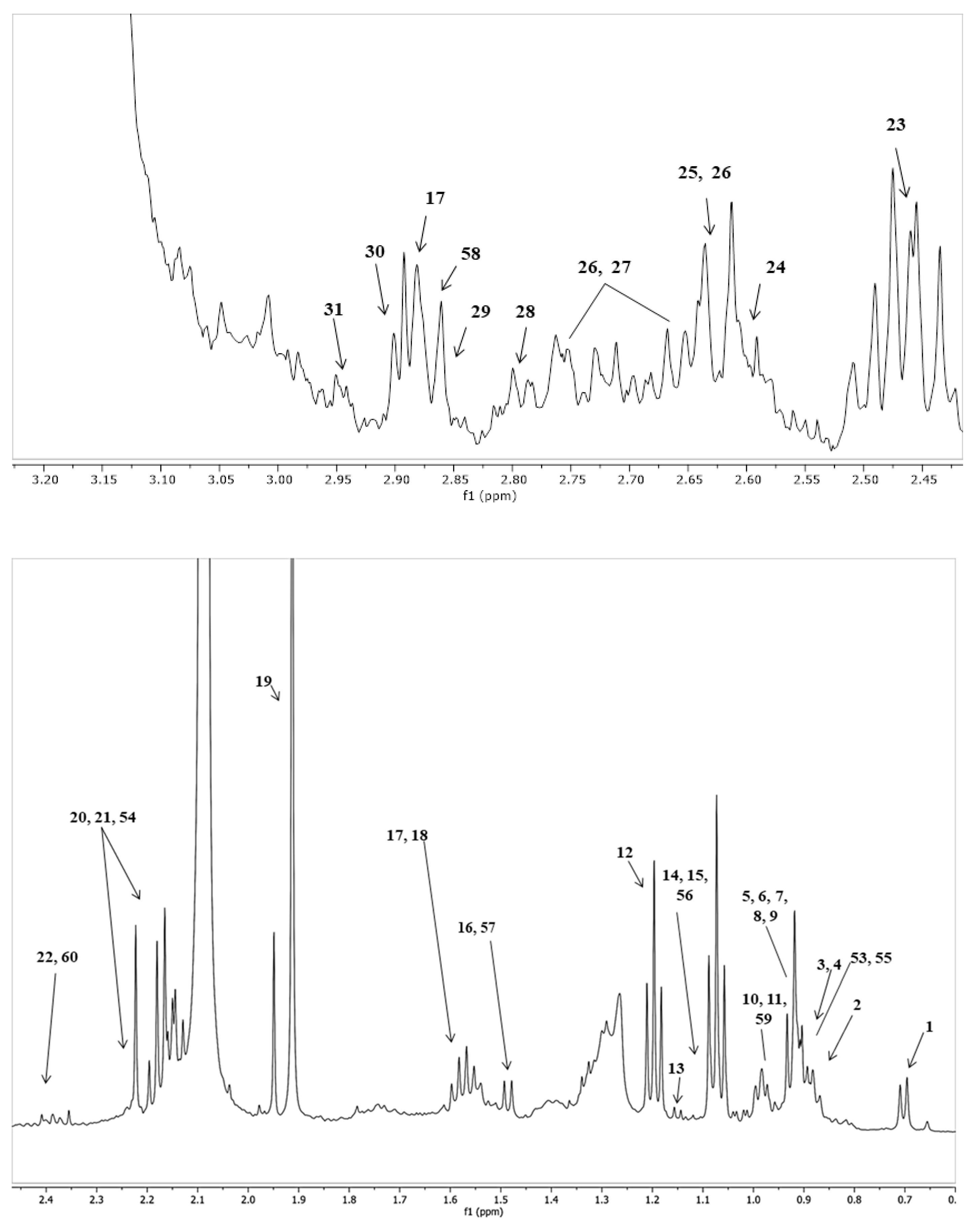
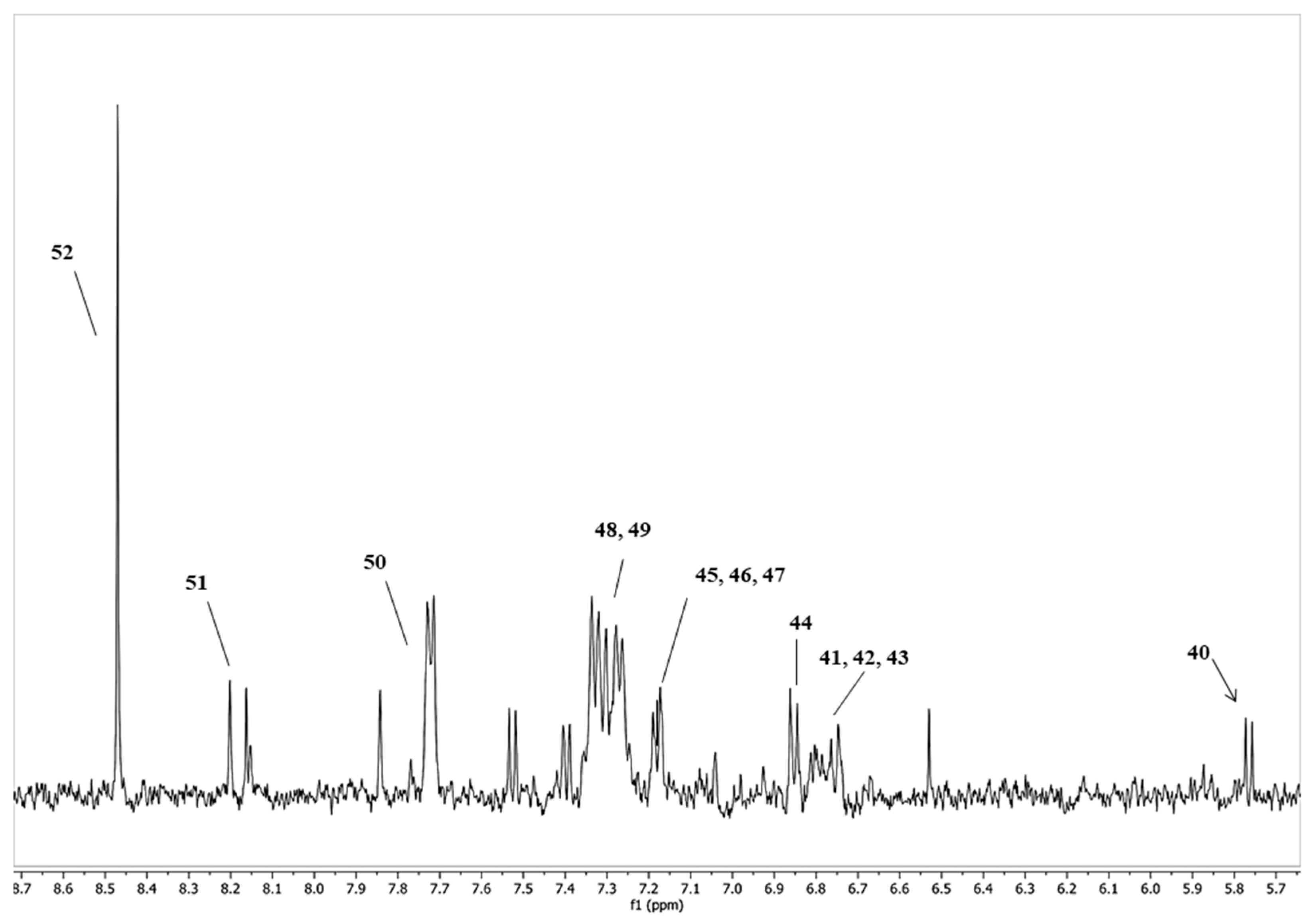
| Bacterial Groups | Fermentation Medium | Fermentation Period | ||
|---|---|---|---|---|
| 0 h | 24 h | 48 h | ||
| Lactobacillus spp./Enterococcus spp. | NC | 5.89 ± 0.31 Ba | 6.23 ± 0.35 ABb | 6.68 ± 0.41 Ac |
| FOS | 4.81 ± 0.16 Cb | 5.76 ± 0.28 Bb | 12.18 ± 0.39 Aa | |
| AL10 | 3.06 ± 0.22 Cc | 3.88 ± 0.17 Bc | 6.10 ± 0.34 Ac | |
| ALA5 | 6.20 ± 0.28 Ca | 7.00 ± 0.36 Ba | 7.84 ± 0.26 Ab | |
| GL10 | 2.70 ± 0.14 Cc | 4.34 ± 0.25 Bc | 7.90 ± 0.28 Ab | |
| GLA5 | 2.65 ± 0.20 Bc | 6.93 ± 0.31 Aa | 6.40 ± 0.42 Ac | |
| Bifidobacterium spp. | NC | 2.43 ± 0.22 Abc | 2.70 ± 0.26 Ac | 1.84 ± 0.16 Be |
| FOS | 2.71 ± 0.20 Cb | 4.09 ± 0.18 Ba | 9.35 ± 0.68 Aa | |
| AL10 | 1.53 ± 0.19 Cd | 3.38 ± 0.28 Bb | 4.45 ± 0.51 Ad | |
| ALA5 | 3.60 ± 0.27 Ca | 4.15 ± 0.25 Ba | 9.20 ± 0.56 Aa | |
| GL10 | 1.44 ± 0.16 Bd | 1.12 ± 0.18 Bd | 6.62 ± 0.44 Ac | |
| GLA5 | 2.14 ± 0.18 Cc | 2.86 ± 0.22 Bc | 7.65 ± 0.47 Ab | |
| Ruminococcus albus/R. flavefaciens | NC | 5.38 ± 0.27 Aa | 5.07 ± 0.24 Ab | 1.77 ± 0.20 Bd |
| FOS | 3.0 ± 0.21 Cb | 4.05 ± 0.29 Bc | 9.75 ± 0.63 Ab | |
| AL10 | 1.57 ± 0.18 Bd | 1.73 ± 0.12 Bd | 4.08 ± 0.52 Ac | |
| ALA5 | 5.39 ± 0.30 Ca | 6.96 ± 0.39 Ba | 12.79 ± 0.88 Aa | |
| GL10 | 2.03 ± 0.26 Cc | 4.79 ± 0.23 Bbc | 9.14 ± 0.65 Ab | |
| GLA5 | 4.98 ± 0.39 Ba | 4.26 ± 0.35 Bc | 9.68 ± 0.74 Ab | |
| Bacteroides spp./Prevotella spp. | NC | 6.33 ± 0.51 Bb | 9.50 ± 0.64 Aa | 8.40 ± 0.55 Ab |
| FOS | 8.14 ± 0.47 Ba | 9.61 ± 0.69 Aa | 9.23 ± 0.62 ABab | |
| AL10 | 2.26 ± 0.19 Bd | 1.62 ± 0.28 Cc | 3.11 ± 0.33 Ad | |
| ALA5 | 8.83 ± 0.46 Aa | 9.16 ± 0.71 Aa | 9.83 ± 0.60 Aa | |
| GL10 | 0.42 ± 0.15 Ae | 0.43 ± 0.13 Ad | 0.67 ± 0.13 Aa | |
| GLA5 | 5.0 ± 0.32 Ac | 2.33 ± 0.37 Bb | 4.33 ± 0.38 Ac | |
| Clostridium histolyticum | NC | 5.71 ± 0.47 Bc | 9.73 ± 0.64 Aa | 6.21 ± 0.29 Bb |
| FOS | 9.11 ± 0.52 Ab | 6.20 ± 0.51 Bb | 7.66 ± 0.45 Ca | |
| AL10 | 1.81 ± 0.14 Ae | 1.92 ± 0.28 Ac | 1.16 ± 0.16 Bd | |
| ALA5 | 10.66 ± 0.98 Aa | 6.16 ± 0.57 Bb | 3.33 ± 0.42 Cc | |
| GL10 | 1.89 ± 0.22 Ae | 1.85 ± 0.31 Ac | 0.88 ± 0.16 Bd | |
| GLA5 | 4.60 ± 0.37 Ad | 5.60 ± 0.68 Ab | 3.70 ± 0.27 Bc | |
| Eubacterium rectale/C. coccoides | NC | 9.83 ± 0.47 Aa | 8.96 ± 0.44 ABb | 8.20 ± 0.49 Bb |
| FOS | 9.60 ± 0.59 Aa | 7.0 ± 0.48 Bc | 5.60 ± 0.32 Cd | |
| AL10 | 1.92 ± 0.14 Cd | 2.85 ± 0.26 Bd | 3.89 ± 0.28 Ae | |
| ALA5 | 5.83 ± 0.40 Ab | 6.33 ± 0.35 Ac | 6.66 ± 0.49 Ac | |
| GL10 | 4.71 ± 0.52 Ac | 1.47 ± 0.29 Be | 0.30 ± 0.11 Ca | |
| GLA5 | 9.33 ± 0.81 Ba | 12.71 ± 0.89 Aa | 10.20 ± 0.76 Aba | |
| Parameters | Samples | Fermentation Period | ||
|---|---|---|---|---|
| 0 h | 24 h | 48 h | ||
| pH values | NC | 7.07 ± 0.01 Aa | 6.81 ± 0.02 Ba | 5.63 ± 0.02 Ca |
| FOS | 7.06 ± 0.01 Aa | 3.56 ± 0.01 Be | 2.60 ± 0.02 Cd | |
| AL10 | 7.00 ± 0.02 Ab | 3.38 ± 0.03 Bf | 3.17 ± 0.01 Cb | |
| ALA5 | 6.96 ± 0.00 Ac | 4.33 ± 0.02 Bc | 3.13 ± 0.00 Cc | |
| GL10 | 6.93 ± 0.01 Ad | 4.24 ± 0.00 Bd | 3.12 ± 0.01 Cc | |
| GLA5 | 6.93 ± 0.01 Ad | 4.99 ± 0.00 Bb | 3.12 ± 0.01 Cc | |
| Sugars (g/L) | ||||
| Glucose | NC | <LOD | <LOD | <LOD |
| FOS | 8.47 ± 0.03 Aa | 5.31 ± 0.04 Ba | 2.94 ± 0.01 Ca | |
| AL10 | 0.43 ± 0.04 Ac | <LOD | <LOD | |
| ALA5 | 0.31 ± 0.04 Ad | <LOD | <LOD | |
| GL10 | 0.48 ± 0.01 Ab | <LOD | <LOD | |
| GLA5 | 0.47 ± 0.01 Ab | <LOD | <LOD | |
| Fructose | NC | <LOD | <LOD | <LOD |
| FOS | 7.28 ± 0.01 Ba | 7.55 ± 0.03 Aa | 3.59 ± 0.04 Ca | |
| AL10 | 0.18 ± 0.01 Ac | <LOD | <LOD | |
| ALA5 | 0.12 ± 0.05 Ad | <LOD | <LOD | |
| GL10 | 0.25 ± 0.04 Ab | 0.07 ± 0.01 Bb | <LOD | |
| GLA5 | 0.24 ± 0.02 Ab | <LOD | <LOD | |
| Maltose | NC | <LOD | <LOD | <LOD |
| FOS | 0.16 ± 0.04 Aa | 0.15 ± 0.03 Aa | 0.15 ± 0.01 Aa | |
| AL10 | 0.02 ± 0.01 Ab | <LOD | <LOD | |
| ALA5 | 0.02 ± 0.01 Ab | <LOD | <LOD | |
| GL10 | <LOD | <LOD | <LOD | |
| GLA5 | <LOD | <LOD | <LOD | |
| Rhamnose | NC | <LOD | <LOD | <LOD |
| FOS | <LOD | <LOD | <LOD | |
| AL10 | 0.40 ± 0.01 Ac | 0.22 ± 0.04 Ba | 0.17 ± 0.01 Bb | |
| ALA5 | 0.13 ± 0.04 Bd | <LOD | 0.23 ± 0.04 Aa | |
| GL10 | 0.84 ± 0.02 Aa | 0.15 ± 0.03 Cb | 0.28 ± 0.04 Ba | |
| GLA5 | 0.61 ± 0.02 Ab | 0.06 ± 0.01 Cc | 0.16 ± 0.01 Bb | |
| Acids (g/L) | ||||
| Lactic | NC | <LOD | <LOD | <LOD |
| FOS | <LOD | 5.35 ± 0.05 Ba | 7.83 ± 0.05 Aa | |
| AL10 | 0.13 ± 0.01 Ba | 0.17 ± 0.01 Ad | <LOD | |
| ALA5 | 0.13 ± 0.01 Ba | 0.24 ± 0.02 Ac | <LOD | |
| GL10 | 0.11 ± 0.02 Ba | 0.42 ± 0.04 Ab | <LOD | |
| GLA5 | <LOD | 0.39 ± 0.02 Ab | <LOD | |
| Acetic | NC | 0.22 ± 0.02 Bc | 0.25 ± 0.00 Ac | 0.21 ± 0.00 Bd |
| FOS | 0.36 ± 0.03 Ca | 1.59 ± 0.01 Ba | 2.13 ± 0.02 Aa | |
| AL10 | 0.30 ± 0.05 Ca | 0.45 ± 0.06 Bb | 0.58 ± 0.01 Ab | |
| ALA5 | 0.32 ± 0.03 Ba | 0.39 ± 0.05 Bb | 0.49 ± 0.01 Ac | |
| GL10 | 0.34 ± 0.02 Ca | 0.45 ± 0.01 Ab | 0.48 ± 0.05 Bc | |
| GLA5 | 0.26 ± 0.01 Bb | 0.41 ± 0.01 Ab | 0.51 ± 0.08 Ac | |
| Propionic | NC | 1.09 ± 0.00 Ae | 0.99 ± 0.02 Ba | 0.40 ± 0.00 Ca |
| FOS | 0.68 ± 0.06 Af | 0.39 ± 0.00 Bd | 0.31 ± 0.01 Cb | |
| AL10 | 1.32 ± 0.02 Ac | 0.62 ± 0.01 Bb | 0.34 ± 0.04 Cb | |
| ALA5 | 1.16 ± 0.02 Ad | 0.65 ± 0.03 Bb | 0.41 ± 0.06 Ca | |
| GL10 | 1.40 ± 0.01 Ab | 0.61 ± 0.02 Bb | 0.20 ± 0.01 Cd | |
| GLA5 | 1.74 ± 0.08 Aa | 0.56 ± 0.02 Bc | 0.24 ± 0.00 Cc | |
| Butyric | NC | 0.23 ± 0.00 Bc | 0.97 ± 0.01 Ad | <LOD |
| FOS | 0.29 ± 0.01 Cb | 0.68 ± 0.00 Be | 0.79 ± 0.00 Ad | |
| AL10 | 0.23 ± 0.00 Cc | 1.34 ± 0.01 Ba | 2.03 ± 0.01 Ab | |
| ALA5 | 0.28 ± 0.03 Cb | 1.27 ± 0.00 Bb | 1.86 ± 0.05 Ac | |
| GL10 | 0.22 ± 0.02 Cc | 1.09 ± 0.00 Bc | 2.11 ± 0.06 Aa | |
| GLA5 | 0.33 ± 0.01 Ca | 1.08 ± 0.05 Bc | 1.77 ± 0.09 Ac | |
| Parameters | Samples | Fermentation Period | ||
|---|---|---|---|---|
| 0 h | 24 h | 48 h | ||
| Phenolic acids | ||||
| Gallic acid | AL10 | <LOD | 4.68 ± 0.00 Ad | 3.48 ± 0.02 Bd |
| ALA5 | <LOD | 5.94 ± 0.01 Ac | 5.19 ± 0.03 Bc | |
| GL10 | <LOD | 7.05 ± 0.01 Ab | 6.83 ± 0.00 Bb | |
| GLA5 | <LOD | 9.69 ± 0.04 Aa | 8.95 ± 0.03 Ba | |
| Flavanols | ||||
| Procyanidin A2 | AL10 | 8.21 ± 0.02 Ab | 1.44 ± 0.01 Bc | 1.07 ± 0.01 Cc |
| ALA5 | 7.09 ± 0.01 Ad | 1.92 ± 0.01 Ba | 1.31 ± 0.05 Ca | |
| GL10 | 7.44 ± 0.00 Ac | 1.21 ± 0.01 Bd | 1.03 ± 0.03 Cc | |
| GLA5 | 9.16 ± 0.01 Aa | 1.57 ± 0.02 Bb | 1.18 ± 0.01 Cb | |
| Procyanidin B1 | AL10 | 1.46 ± 0.01 Ad | <LOD | <LOD |
| ALA5 | 2.25 ± 0.02 Ac | <LOD | <LOD | |
| GL10 | 7.85 ± 0.04 Aa | 2.71 ± 0.01 Bc | <LOD | |
| GLA5 | 7.41 ± 0.03 Ab | 3.11 ± 0.04 Bb | <LOD | |
| Procyanidin B2 | AL10 | 9.88 ± 0.01 Ad | 1.25 ± 0.03 Bd | <LOD |
| ALA5 | 13.23 ± 0.05 Ab | 2.33 ± 0.03 Bc | 1.47 ± 0.02 Cc | |
| GL10 | 11.51 ± 0.01 Ac | 9.76 ± 0.08 Bb | 9.43 ± 0.10 Ca | |
| GLA5 | 15.69 ± 0.02 Aa | 11.58 ± 0.05 Ba | 6.59 ± 0.05 Cb | |
| Catechin | AL10 | <LOD | <LOD | <LOD |
| ALA5 | <LOD | 6.61 ± 0.03 Aa | 1.03 ± 0.02 Ba | |
| GL10 | <LOD | <LOD | <LOD | |
| GLA5 | <LOD | <LOD | <LOD | |
| Epigallocatechin gallate | AL10 | 2.32 ± 0.02 Cc | 6.58 ± 0.01 Ba | 7.20 ± 0.03 Aa |
| ALA5 | 5.38 ± 0.05 Ba | 5.43 ± 0.06 Bb | 5.83 ± 0.03 Ab | |
| GL10 | 1.69 ± 0.01 Cd | 4.44 ± 0.03 Bd | 4.84 ± 0.01 Ac | |
| GLA5 | 3.37 ± 0.01 Bb | 4.71 ± 0.06 Ac | 4.78 ± 0.05 Ad | |
| Epicatechin | AL10 | <LOD | 3.21 ± 0.01 Aa | <LOD |
| ALA5 | 1.25 ± 0.02 Aa | <LOD | <LOD | |
| GL10 | <LOD | <LOD | <LOD | |
| GLA5 | <LOD | <LOD | <LOD | |
| Flavonols | ||||
| Quercetin 3-Glucoside | AL10 | <LOD | <LOD | <LOD |
| ALA5 | 1.28 ± 0.03 Aa | 1.20 ± 0.01 Ba | <LOD | |
| GL10 | <LOD | <LOD | <LOD | |
| GLA5 | <LOD | <LOD | <LOD | |
| Isorhamnetin | AL10 | 5.64 ± 0.05 Aa | <LOD | <LOD |
| ALA5 | 5.71 ± 0.03 Aa | <LOD | <LOD | |
| GL10 | <LOD | <LOD | <LOD | |
| GLA5 | <LOD | <LOD | <LOD | |
| Antioxidant capacity | ||||
| DPPH• (μmol/g) 1 | AL10 | 14.19 ± 0.52 Ab | 13.51 ± 0.20 Bb | 14.10 ± 0.52 Ab |
| ALA5 | 8.29 ± 0.43 Bd | 5.70 ± 0.16 Cd | 9.87 ± 0.41 Ad | |
| GL10 | 10.59 ± 0.25 Ac | 9.14 ± 0.32 Bc | 10.77 ± 0.11 Ac | |
| GLA5 | 15.47 ± 0.26 Aa | 14.50 ± 0.21 Ba | 15.51 ± 0.28 Aa | |
| ABTS•+ (μmol/g) 1 | AL10 | 16.89 ± 0.18 Bd | 19.97 ± 0.46 Ac | 22.08 ± 0.35 Ab |
| ALA5 | 20.96 ± 0.49 Bb | 18.24 ± 0.37 Cd | 28.79 ± 0.22 Aa | |
| GL10 | 18.50 ± 0.51 Cc | 21.05 ± 0.26 Ab | 19.43 ± 0.28 Bc | |
| GLA5 | 23.94 ± 0.22 Ca | 24.69 ± 0.33 Ba | 28.50 ± 0.15 Aa | |
| FRAP (µmol FeSO4/g) | AL10 | 13.60 ± 0.36 Ad | 11.60 ± 0.19 Bc | 13.40 ± 0.20 Ad |
| ALA5 | 19.70 ± 0.44 Cb | 28.40 ± 0.27 Aa | 24.30 ± 0.61 Ba | |
| GL10 | 18.50 ± 0.15 Bc | 20.70 ± 0.18 Ab | 15.30 ± 0.52 Cc | |
| GLA5 | 27.70 ± 0.23 Ba | 28.40 ± 0.15 Aa | 19.80 ± 0.45 Cb | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, C.M.; de Albuquerque, T.M.R.; Sampaio, K.B.; de Oliveira, J.N.; da Silva, J.Y.P.; Lima, M.d.S.; Nascimento, Y.M.d.; da Silva, E.F.; da Silva, M.S.; Tavares, J.F.; et al. Fermenting Acerola (Malpighia emarginata D.C.) and Guava (Psidium guayaba L.) Fruit Processing Co-Products with Probiotic Lactobacilli to Produce Novel Potentially Synbiotic Circular Ingredients. Foods 2024, 13, 1375. https://doi.org/10.3390/foods13091375
Araújo CM, de Albuquerque TMR, Sampaio KB, de Oliveira JN, da Silva JYP, Lima MdS, Nascimento YMd, da Silva EF, da Silva MS, Tavares JF, et al. Fermenting Acerola (Malpighia emarginata D.C.) and Guava (Psidium guayaba L.) Fruit Processing Co-Products with Probiotic Lactobacilli to Produce Novel Potentially Synbiotic Circular Ingredients. Foods. 2024; 13(9):1375. https://doi.org/10.3390/foods13091375
Chicago/Turabian StyleAraújo, Caroliny M., Thatyane Mariano R. de Albuquerque, Karoliny B. Sampaio, Jordana N. de Oliveira, Jaielison Yandro P. da Silva, Marcos dos S. Lima, Yuri M. do Nascimento, Evandro F. da Silva, Marcelo S. da Silva, Josean F. Tavares, and et al. 2024. "Fermenting Acerola (Malpighia emarginata D.C.) and Guava (Psidium guayaba L.) Fruit Processing Co-Products with Probiotic Lactobacilli to Produce Novel Potentially Synbiotic Circular Ingredients" Foods 13, no. 9: 1375. https://doi.org/10.3390/foods13091375
APA StyleAraújo, C. M., de Albuquerque, T. M. R., Sampaio, K. B., de Oliveira, J. N., da Silva, J. Y. P., Lima, M. d. S., Nascimento, Y. M. d., da Silva, E. F., da Silva, M. S., Tavares, J. F., de Souza, E. L., & de Oliveira, M. E. G. (2024). Fermenting Acerola (Malpighia emarginata D.C.) and Guava (Psidium guayaba L.) Fruit Processing Co-Products with Probiotic Lactobacilli to Produce Novel Potentially Synbiotic Circular Ingredients. Foods, 13(9), 1375. https://doi.org/10.3390/foods13091375








