Reforming Food, Drug, and Nutraceutical Regulations to Improve Public Health and Reduce Healthcare Costs
Abstract
1. Introduction
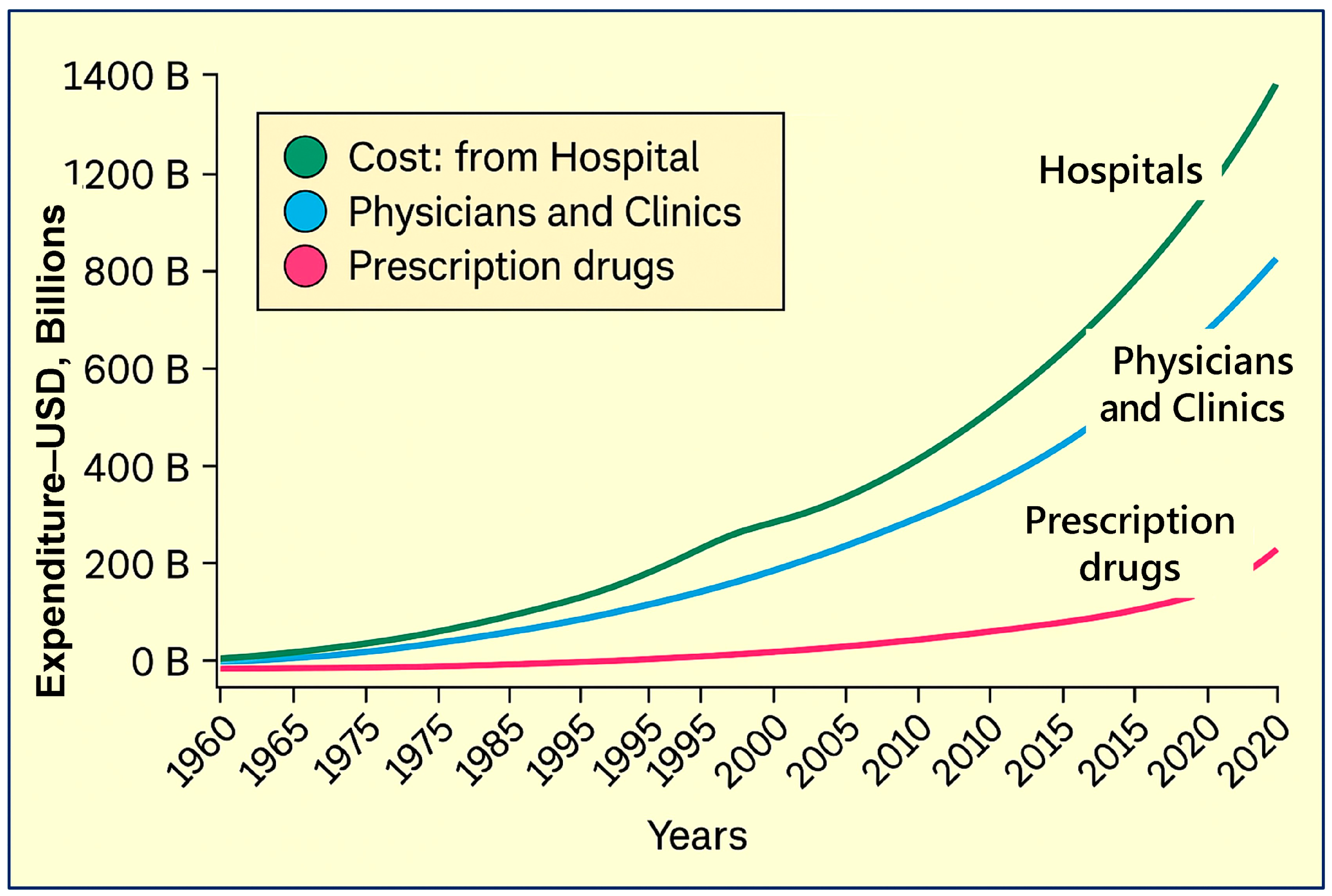
1.1. The Root Causes That Escalate Healthcare Costs
1.2. The Role of Nutrition in Health and Disease
1.3. Preventing Chronic Diseases via Proper Nutrition
1.4. Enhancing Health Through Improved Food Regulation
1.5. Integrating Technology, Artificial Intelligence, and Cybernetics into Healthcare
2. Food and Nutraceuticals for Health and Disease Prevention
2.1. Reevaluating the Safety of Preservatives and Food Additives
2.2. Other Food Additives—FDA Responsibilities to Keep the Nation Safe
2.3. Minimizing Harmful Effects from Smoked and Preserved Meat
3. Evaluations of Food and Nutraceuticals and Evidence-Based Medicine
3.1. Randomized Clinical Trials Are Not the Right Approach to Evaluate Nutrients
3.2. Failures of Evidence-Based Medicine
3.3. EBMs Add to the Escalating Cost of Healthcare
3.4. RCTs and EBMs Are Not Desinged to Evaluate Micro-Nutrients
3.5. Large Vitamin D RCTs and EBM Frameworks Amplify Misleading Conclusions
4. Key Reasons for the Escalation of Chronic Diseases
4.1. Compartmentalized Medicine and the Cost of Excluding Complementary Care
4.2. Root Causes vs. Symptoms: Systemic Failures Fueling Chronic Disease and Costs
4.3. Over-Medicalization Escalates Ill Health and Healthcare Costs
4.4. Early Correction of Micro-Nutrient Deficiencies Reduces the Incidence of Chronic Diseases
4.5. Lack of Actions to Control Chronic Diseases
4.6. Social and Societal Factors Are Not the Main Cause of Rising Healthcare Costs
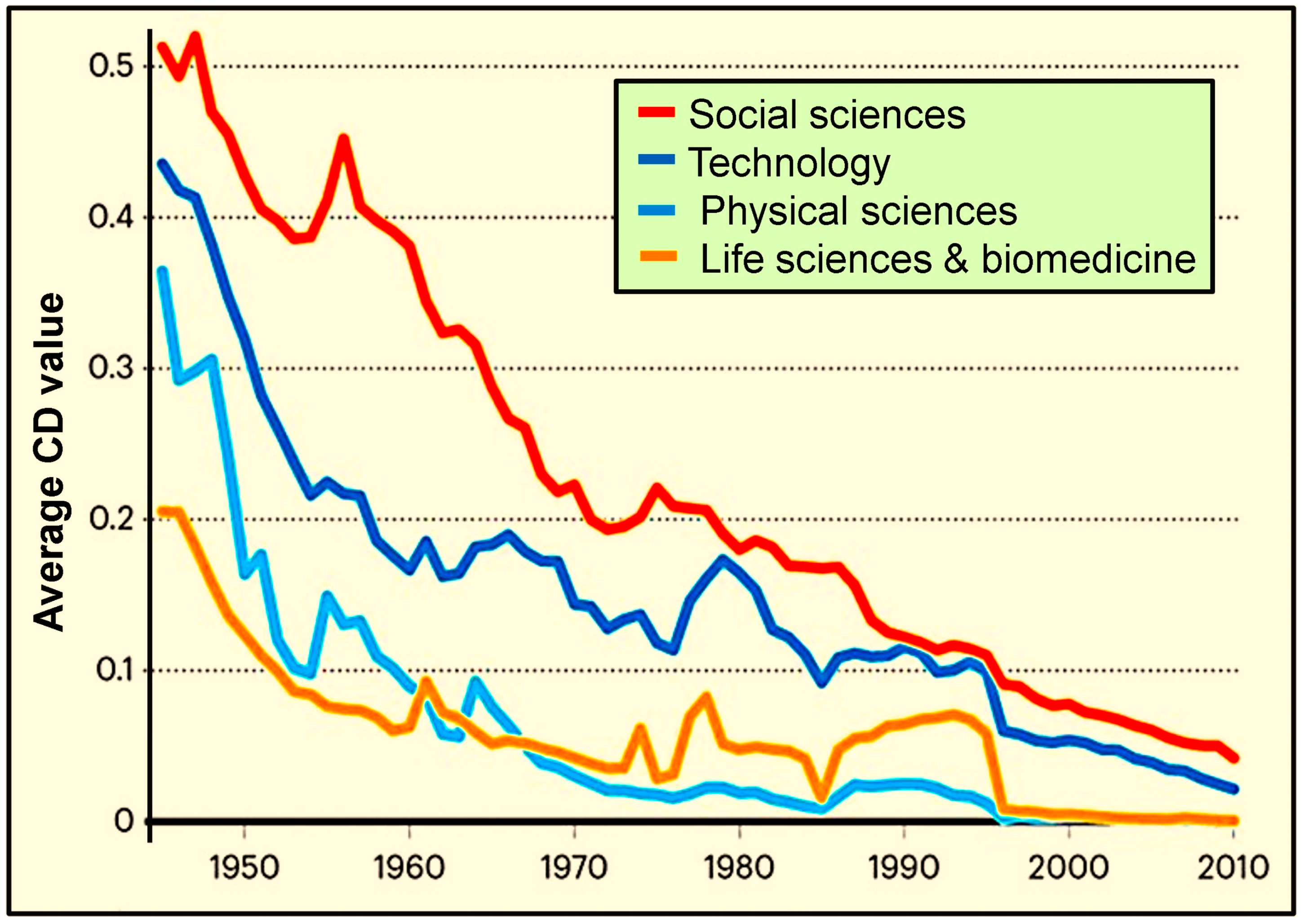
4.7. Declining Scientific Output at the NIH and NSF-Funded Research
4.8. Examples of Ineffective Mega Grants Awarded to Leading Universities
4.9. Misaligned Priorities and Inefficiencies in Federal Research Funding
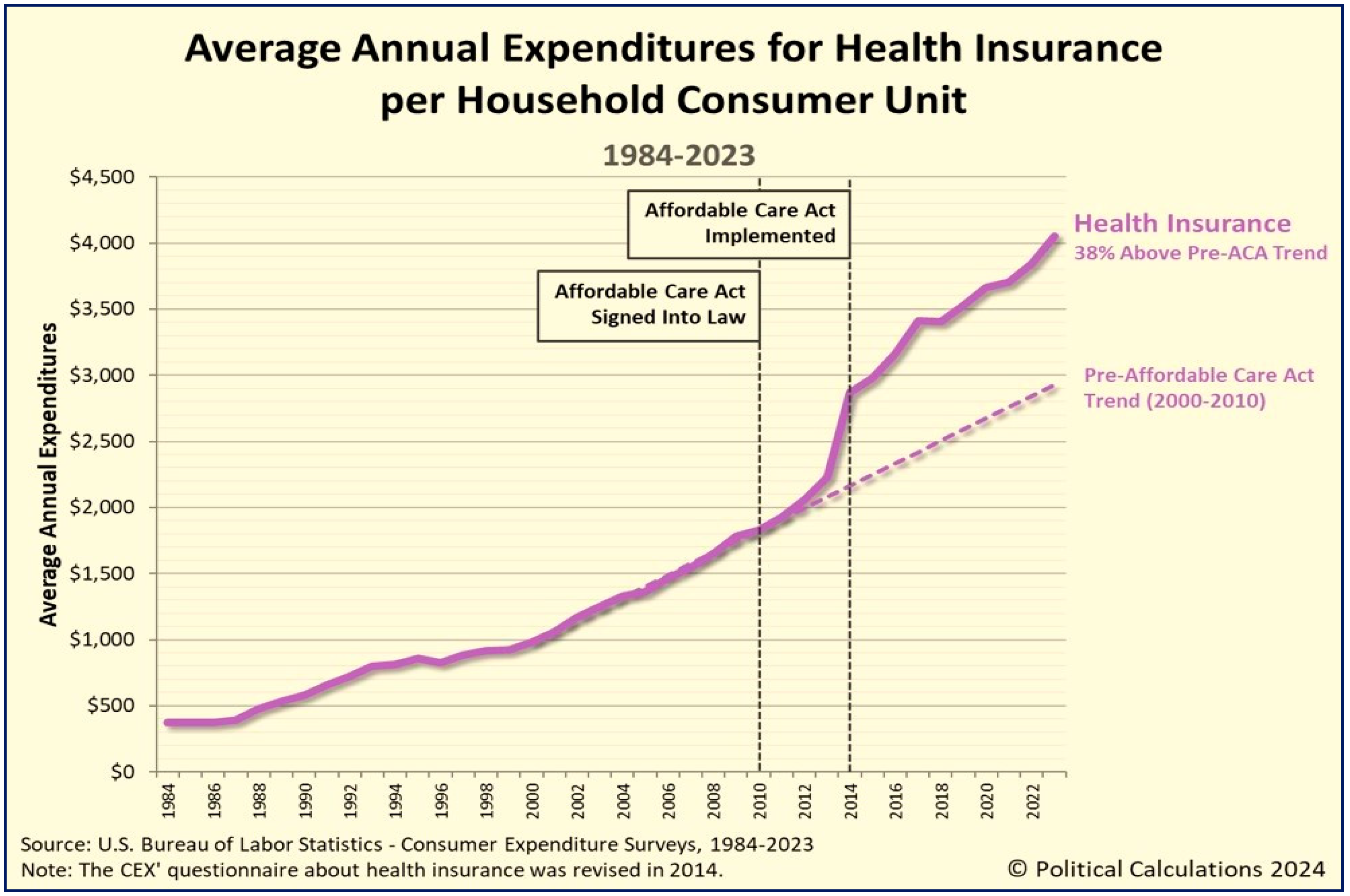
4.10. The Affordable Care Act—Pros and Cons
4.11. The Affordable Care Act—Why Should It Be Replaced
4.12. Sustainable Modification of Lifestyles
5. Wastage, Abuses, and Fraud in the Healthcare System
5.1. The Importance of Early Identifying Major Frauds and Abuses in Healthcare
5.2. Key Programs with the Highest Wastage of Healthcare Funds in the USA
5.3. Federal Healthcare Losses Due to Fraud and Waste
5.4. Taking Decisive Steps to Reverse the Current Negative Trend
5.5. Pharmacy Benefit Manager System—The Need for Abolishing It
5.6. Behind the Pharmacy Counter: How PBMs and GPOs Inflate Drug Prices
6. Strategic Solutions to Curb Escalating Healthcare Costs
6.1. The Lack of Oversight of Healthcare Regulatory Enforcement
6.2. What Needs to Be Achieved
6.3. The Ongoing Conflicts of Interest in Healthcare Agencies
6.4. The Failures of the FDA to Evaluate and Approve Generic, Early Therapies
6.5. Rejecting Generic SARS-CoV-2 Treatments Due to Conflicts of Interest
7. Restructuring of the FDA
7.1. Specific FDA Reforms Are Needed
7.2. Preventing the FDA-Related Wastage of Funds
7.3. Regaining Public Trust in Healthcare Regulation
7.4. Lack of Oversight of Government Food Assistance Programs Causing Ill-Health
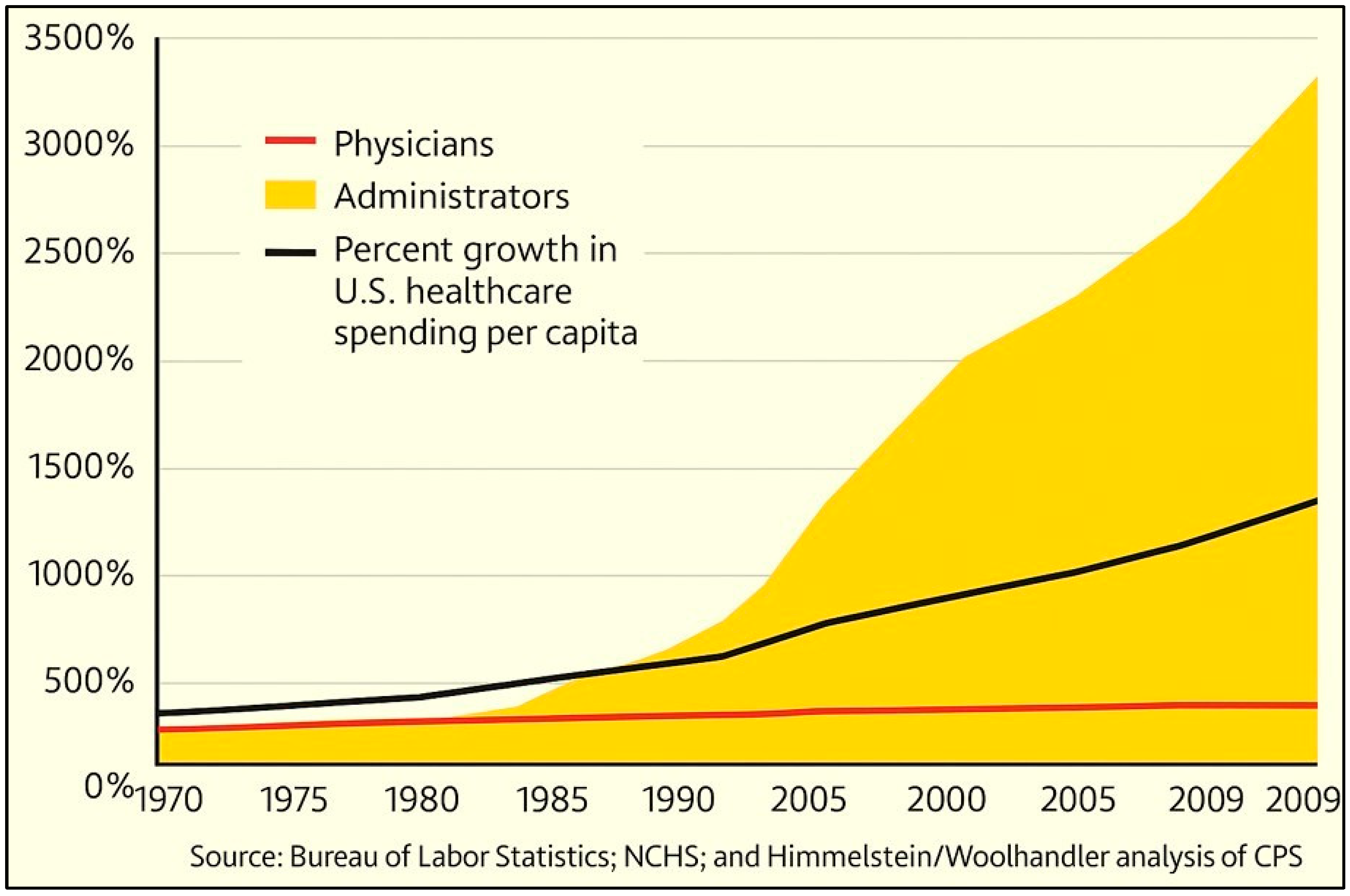
7.5. Administrative Overgrowth in U.S. Government Agencies and Universities
7.6. Streamlining Workforce Inefficiencies in Healthcare and Higher Education
7.7. Key Components Contributing to Escalating Healthcare Costs
8. Establishing the Food and Nutraceutical Agency
8.1. Rationale for Splitting the FDA
8.2. Expected Benefits from the FNA to the Government and the Public
8.3. FNA Should Regain the Lost Credibility of Food and Nutraceutical Governance
8.4. Benefits from the FNA to the Public
8.5. Challenges with the Proposed Legislative and Structural Changes
9. Discussion
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norbeck, T. Drivers of health care costs: A physicians foundation white paper—Second of a three-part series. Mo. Med. 2013, 110, 113–118. [Google Scholar] [PubMed]
- Faggion, C.M., Jr. Evidence-based medicine concepts for patients: Improving shared decision-making. J. Commun. Healthc. 2025, 6, 1–4. [Google Scholar] [CrossRef]
- Ruiz-Nunez, B.; Pruimboom, L.; Dijck-Brouwer, D.A.; Muskiet, F.A. Lifestyle and nutritional imbalances associated with Western diseases: Causes and consequences of chronic systemic low-grade inflammation in an evolutionary context. J. Nutr. Biochem. 2013, 24, 1183–1201. [Google Scholar] [CrossRef]
- Elma, O.; Brain, K.; Dong, H.J. The Importance of nutrition as a lifestyle factor in chronic pain management: A narrative review. J. Clin. Med. 2022, 11, 5950. [Google Scholar] [CrossRef] [PubMed]
- Hacker, K. The burden of chronic disease. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 112–119. [Google Scholar] [CrossRef]
- Amin, K.; Cox, C.; Ortaliza, J.; Wager, E. Health Care Costs and Affordability: Health Policy 101. Available online: https://www.kff.org/health-policy-101-health-care-costs-and-affordability/?entry=table-of-contents-introduction (accessed on 12 December 2024).
- Yong, P.L.; Olsen, L.A. Missed Prevention Opportunities: Roundtable on Evidence-Based Medicine; Institute of Medicine (US); National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Anonymous. Why Healthcare Should Shift from Reactive to Proactive. Available online: https://essentialhealth.health/why-healthcare-should-shift-from-reactive-to-proactive/ (accessed on 10 February 2025).
- Waldman, S.A.; Terzic, A. Health care evolves from reactive to proactive. Clin. Pharmacol. Ther. 2019, 105, 10–13. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Dissanayake, C.B. Nanocrystal-induced chronic tubular-nephropathy in tropical countries: Diagnosis, mitigation, and eradication. Eur. J. Med. Res. 2023, 28, 221. [Google Scholar] [CrossRef] [PubMed]
- Raiten, D.J.; Sakr Ashour, F.A.; Ross, A.C.; Meydani, S.N.; Dawson, H.D.; Stephensen, C.B.; Brabin, B.J.; Suchdev, P.S.; van Ommen, B.; Group, I.C. Inflammation and nutritional science for programs/policies and interpretation of research evidence (INSPIRE). J. Nutr. 2015, 145, 1039S–1108S. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Berni Canani, R.; O’Mahony, L.; Peroni, D.; Sokolowska, M.; Vassilopoulou, E.; Venter, C. Nutrition in chronic inflammatory conditions: Bypassing the mucosal block for micronutrients. Allergy 2024, 79, 353–383. [Google Scholar] [CrossRef]
- NIEHS. Nutrition, Health, and Your Environment. Available online: https://www.niehs.nih.gov/health/topics/nutrition (accessed on 15 February 2025).
- Narimatsu, H.; Yaguchi, Y.T. The role of diet and nutrition in cancer: Prevention, treatment, and survival. Nutrients 2022, 14, 3329. [Google Scholar] [CrossRef]
- Wiseman, M.J. Nutrition and cancer: Prevention and survival. Br. J. Nutr. 2019, 122, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.; Wimalawansa, S.J.; Pludowski, P.; Cheng, R. Vitamin D: Evidence-based health benefits and recommendations for population guidelines. Nutrients 2025, 17, 277. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J.; Weiss, S.T.; Hollis, B.W. Integrating Endocrine, Genomic, and Extra-Skeletal Benefits of Vitamin D into National and Regional Clinical Guidelines. Nutrients 2024, 16, 3969. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.; Belkaid, Y. Control of immunity via nutritional interventions. Immunity 2022, 55, 210–223. [Google Scholar] [CrossRef]
- Konozy, E.H.E.; Osman, M.E.M. From inflammation to immune regulation: The dual nature of dietary lectins in health and disease. Heliyon 2024, 10, e39471. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Rapidly Increasing Serum 25(OH)D Boosts the Immune System, against Infections-Sepsis and COVID-19. Nutrients 2022, 14, 2997. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Infections and autoimmunity-The immune system and vitamin D: A systematic review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Dissanayake, C.B. Factors Affecting the Environmentally Induced, Chronic Kidney Disease of Unknown Aetiology in Dry Zonal Regions in Tropical Countries—Novel Findings. Environments 2019, 7, 2. [Google Scholar] [CrossRef]
- Razzaque, M.; Wimalawansa, S.J. Minerals and human health: From deficiency to toxicity. Nutrients 2025, 17, 454. [Google Scholar] [CrossRef]
- AbdulRaheem, Y. Unveiling the significance and challenges of integrating prevention Levels in healthcare practice. J. Prim. Care Community Health 2023, 14, 21501319231186500. [Google Scholar] [CrossRef]
- Schwab, U.; Reynolds, A.N.; Sallinen, T.; Rivellese, A.A.; Riserus, U. Dietary fat intakes and cardiovascular disease risk in adults with type 2 diabetes: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 3355–3363. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.L.; Gong, Y.; Qi, Y.J.; Shao, Z.M.; Jiang, Y.Z. Effects of dietary intervention on human diseases: Molecular mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2024, 9, 59. [Google Scholar] [CrossRef]
- IFWH. Primary, Secondary and Tertiary Prevention. Available online: https://www.iwh.on.ca/what-researchers-mean-by/primary-secondary-and-tertiary-prevention (accessed on 19 November 2024).
- Jeurissen, P.P.T.; Kruse, F.M.; Busse, R.; Himmelstein, D.U.; Mossialos, E.; Woolhandler, S. For-profit hospitals have thrived because of generous public reimbursement schemes, Not greater efficiency: A multi-country case study. Int. J. Health Serv. Plan. Adm. Eval. 2021, 51, 67–89. [Google Scholar] [CrossRef]
- Diseases, G.B.D.; Injuries, C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Remington, R.; Axelrod, D.; Bingham, E.; Boyle, J.; Breslow, L.; Citrin, T.; Evans, J.R.; Grumbach, M.M.; Haggerty, R.J.; Harmon, R.; et al. The Future of Public Health: Public Health as a Problem-Solving Activity: Barriers to Effective Action; National Academies Press (US): Washington, DC, USA; Institute of Medicine (US): Washington, DC, USA, 1988. [Google Scholar]
- Benavidez, G.A.; Zahand, W.; Hung, P.; Eberth, J. Chronic disease prevalence in the US: Sociodemographic and geographic variations by Zip code tabulation area. Prev. Chronic Dis. 2024, 21, 230267. [Google Scholar] [CrossRef]
- Zittermann, A. Vitamin D supplements appropriate for preventing osteoporosis?—Vitamin D supplements only in case of deficiency and as a partial aspect of osteoporosis prevention. Dtsch. Med. Wochenschr. 2014, 139, 416. [Google Scholar] [CrossRef]
- Wimalawansa, S. Part 1-5: Vitamin D: Everything You Need Know… and More. Available online: https://www.grassrootshealth.net/blog/vitamin-d-everything-need-know/ (accessed on 5 June 2024).
- German, J.B.; Zivkovic, A.M.; Dallas, D.C.; Smilowitz, J.T. Nutrigenomics and personalized diets: What will they mean for food? Annu. Rev. Food Sci. Technol. 2011, 2, 97–123. [Google Scholar] [CrossRef] [PubMed]
- Hilzenrath, D. Drug Money: FDA Depends on Industry Funding; Money Comes with “Strings Attached”. Available online: https://www.pogo.org/investigations/fda-depends-on-industry-funding-money-comes-with-strings-attached (accessed on 15 February 2025).
- Matthews, E.D.; Kurnat-Thoma, E.L. U.S. food policy to address diet-related chronic disease. Front. Public Health 2024, 12, 1339859. [Google Scholar] [CrossRef]
- Reddy, S.; Fox, J.; Purohit, M.P. Artificial intelligence-enabled healthcare delivery. J. R. Soc. Med. 2019, 112, 22–28. [Google Scholar] [CrossRef]
- Maleki Varnosfaderani, S.; Forouzanfar, M. The role of AI in hospitals and clinics: Transforming tealthcare in the 21st century. Bioengineering 2024, 11, 337. [Google Scholar] [CrossRef]
- Lee, H.-F. COVID-19 Will Accelerate the AI Health Care Revolution. Available online: https://www.wired.com/story/covid-19-will-accelerate-ai-health-care-revolution/?utm_source=chatgpt.com (accessed on 15 January 2025).
- Gawande, M.S.; Zade, N.; Kumar, P.; Gundewar, S.; Weerarathna, I.N.; Verma, P. The role of artificial intelligence in pandemic responses: From epidemiological modeling to vaccine development. Mol. Biomed. 2025, 6, 1. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Salas, S.; Gonzalez-Arias, M. Nutrition: Macronutrient intake, imbalances, and interventions. In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Singar, S.; Nagpal, R.; Arjmandi, B.H.; Akhavan, N.S. Personalized nutrition: Tailoring dietary recommendations through genetic insights. Nutrients 2024, 16, 2673. [Google Scholar] [CrossRef] [PubMed]
- Noah, L. Enhancing the Regulatory Decision-Making Approval Process for Direct Food Ingredient Technologies: Workshop Summary [Appendix A-Legal Aspects of the Food Additive Approval Process]; Institute of Medicine (US) Food Forum: Washington, DC, USA, 1999. [Google Scholar]
- United States Congress. The Delaney Clause Is Incorporated into the Food and Color Additive Provisions of the Food, Drug, and Cosmetic Act (FDCA or the Act): FDCA § 409(c)(3)(A), 21 U.S.C. § 348(c)(3)(A) (Food Additives); FDCA § 721(b)(5)(B), 21 U.S.C. § 379e(b)(5)(B) (Color Additives). Available online: https://uscode.house.gov/view.xhtml?req=(title:21%20section:348%20edition:prelim)#:~:text=(A)%20by%20order%20establish%20a,reasons%20for%20such%20action;%20or (accessed on 29 March 2025).
- Armstrong, S.; Dunaif, G.E. Food Additive Reform: Time to Repeal the Delaney Clause? Available online: https://www.fdli.org/2019/02/food-additive-reform-time-to-repeal-the-delaney-clause/ (accessed on 18 November 2024).
- FDA. FDA to Revoke Authorization for the Use of Red No. 3 in Food and Ingested Drugs. Available online: https://www.fda.gov/food/hfp-constituent-updates/fda-revoke-authorization-use-red-no-3-food-and-ingested-drugs#:~:text=The%20FDA%20is%20amending%20its,a%202022%20color%20additive%20petition (accessed on 4 February 2025).
- Goldstein, Z. Titanium Dioxide: Why FDA Should Ban This Harmful Additive. Available online: https://www.cspinet.org/cspi-news/titanium-dioxide-why-fda-should-ban-harmful-additive (accessed on 10 February 2025).
- Knisely, A. Governor Signs Bill Banning SOME artificial Dyes in Food Sold in West Virginia. Available online: https://scdailygazette.com/2025/03/31/governor-signs-bill-banning-some-artificial-dyes-in-food-sold-in-west-virginia/#:~:text=West%20Virginia%20Gov.%20Patrick%20Morrisey,dyes%20are%20unnecessary%20and%20harmful (accessed on 31 March 2025).
- Barreau, F.; Tisseyre, C.; Menard, S.; Ferrand, A.; Carriere, M. Titanium dioxide particles from the diet: Involvement in the genesis of inflammatory bowel diseases and colorectal cancer. Part. Fibre Toxicol. 2021, 18, 26. [Google Scholar] [CrossRef]
- Pinget, G.; Tan, J.; Janac, B.; Kaakoush, N.O.; Angelatos, A.S.; O’Sullivan, J.; Koay, Y.C.; Sierro, F.; Davis, J.; Divakarla, S.K.; et al. Impact of the food additive titanium gioxide (E171) on gut microbiota-host interaction. Front. Nutr. 2019, 6, 57. [Google Scholar] [CrossRef]
- Sheikh, Z. What Is Titanium Dioxide? Available online: https://www.webmd.com/diet/titanium-dioxide-in-food (accessed on 9 November 2024).
- FDA. Parabens in Cosmetics. Available online: https://www.fda.gov/cosmetics/cosmetic-ingredients/parabens-cosmetics#:~:text=Parabens%20are%20a%20family%20of,both%20the%20products%20and%20consumers (accessed on 8 November 2014).
- Minassian, L. 12 Banned Foods Americans Should Stop Eating. Available online: https://foodrevolution.org/blog/banned-ingredients-in-other-countries/ (accessed on 5 March 2025).
- Chatterjee, S.; Adhikary, S.; Bhattacharya, S.; Chakraborty, A.; Dutta, S.; Roy, D.; Ganguly, A.; Nanda, S.; Rajak, P. Parabens as the double-edged sword: Understanding the benefits and potential health risks. Sci. Total Environ. 2024, 954, 176547. [Google Scholar] [CrossRef]
- Tong, J.H.; Elmore, S.; Huang, S.S.; Tachachartvanich, P.; Manz, K.; Pennell, K.; Wilson, M.D.; Borowsky, A.; La Merrill, M.A. Chronic exposure to low levels of parabens increases mammary cancer growth and metastasis in mice. Endocrinology 2023, 164, bqad007. [Google Scholar] [CrossRef] [PubMed]
- Moreschio, A.; Aaron, N. Concern Over Food Additives Banned in Europe but Not US. Available online: https://cbsaustin.com/news/nation-world/concern-over-food-additives-banned-in-europe-but-not-us-health-fitness-eating-drinking-diet-ingredients-cancer-treatments-illness-sickness-chemicals-candy-groceries-shopping (accessed on 10 March 2025).
- Conley, M. Potassium Bromate: 50 Years of Research Shows Serious Health Risks. Available online: https://usrtk.org/chemicals/potassium-bromate/ (accessed on 27 November 2024).
- Kurokawa, Y.; Maekawa, A.; Takahashi, M.; Hayashi, Y. Toxicity and carcinogenicity of potassium bromate—A new renal carcinogen. Environ. Health Perspect. 1990, 87, 309–335. [Google Scholar] [CrossRef]
- Anderson, E.; Zagorski, J. Trending Brominated Vegetable Oil (BVO). Available online: https://www.canr.msu.edu/news/trending-brominated-vegetable-oil-bvo#:~:text=Bromine%20is%20added%20to%20vegetable%20oil%20to,bromine)%20can%20accumulate%20in%20the%20body%20tissues (accessed on 9 November 2014).
- FDA. FDA Revokes Regulation Allowing the Use of Brominated Vegetable Oil (BVO) in Food. Available online: https://www.fda.gov/food/hfp-constituent-updates/fda-revokes-regulation-allowing-use-brominated-vegetable-oil-bvo-food (accessed on 12 November 2024).
- Lijinsky, W.; Keefer, L.; Conrad, E.; Van de Bogart, R. Nitrosation of tertiary amines and some biologic implications. J. Natl. Cancer Inst. 1972, 49, 1239–1249. [Google Scholar]
- Rywotycki, R. Meat nitrosamine contamination level depending on animal breeding factors. Meat Sci. 2003, 65, 669–676. [Google Scholar] [CrossRef]
- Lijinsky, W.; Epstein, S.S. Nitrosamines as environmental carcinogens. Nature 1970, 225, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Mirvish, S.S. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995, 93, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Sinharoy, P.; McAllister, S.L.; Vasu, M.; Gross, E.R. Environmental aldehyde sources and the health implications of exposure. Adv. Exp. Med. Biol. 2019, 1193, 35–52. [Google Scholar] [CrossRef]
- Gerhards, P. Unveiling the Aadvanced Workflow for PAH Analysis in Smoke Flavored Foods. Available online: https://www.thermofisher.com/blog/analyteguru/unveiling-the-advanced-workflow-for-pah-analysis-in-smoke-flavored-foods/ (accessed on 15 March 2025).
- Hikisz, P.; Jacenik, D. The tobacco smoke component, acrolein, as a major culprit in lung diseases and respiratory cancers: Molecular mechanisms of acrolein cytotoxic activity. Cells 2023, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Unlocking insights: Navigating COVID-19 challenges and Emulating future pandemic Resilience strategies with strengthening natural immunity. Heliyon 2024, 10, e34691. [Google Scholar] [CrossRef]
- Polonowita, A.; Wimalawansa, S.J. The impact of withholding cost-effective early treatments, such as vitamin D, on COVID-19: An analysis using an innovative logical paradigm. World J. Adv. Pharma Life Sci. 2023, 5, 013–034. [Google Scholar] [CrossRef]
- Polonowita, A.; Wimalawansa, S.J. Molecular quantum and logic process of consciousness—Vitamin D big-data in COVID-19—A case for incorporating machine learning in medicine. Eur. J. Parenter. Pharm. Sci. 2023, 10, 24–43. [Google Scholar] [CrossRef]
- Deaton, A.; Cartwright, N. Understanding and misunderstanding randomized controlled trials. Soc. Sci. Med. 2018, 210, 2–21. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Physiology of Vitamin D-Focusing on Disease Prevention. Nutrients 2024, 16, 1666. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Enhancing the design of nutrient clinical trials for disease prevention—A focus on vitamin D: A systematic review. Nutr. Rev. 2025, 83, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K.; La Caze, A.; Kelly, M.P.; Parkkinen, V.P.; Williamson, J. The use of mechanistic evidence in drug approval. J. Eval. Clin. Pract. 2018, 24, 1166–1176. [Google Scholar] [CrossRef]
- Pereza, N.; Hauser, G.; Devic Pavlic, S.; Maric, I.; Sotosek, V.; Grgasovic, T.; Mrsic-Pelcic, J. How to create a faculty development program that transforms medical education according to actual institutional needs: Evidence-based approach and experience at the University of Rijeka, Faculty of Medicine, Croatia. Front. Med. 2025, 12, 1513119. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D deficiency meets Hill’s criteria for causation in SARS-CoV-2 susceptibility, complications, and mortality: A systematic review. Nutrients 2025, 17, 599. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Physiological Basis for Using Vitamin D to Improve Health. Biomedicines 2023, 11, 1542. [Google Scholar] [CrossRef]
- Sertkaya, A.; Birkenbach, A.; Berlind, A.; Eyraud, J. Examination of Clinical Trial Costs and Barriers for Drug Development. Available online: https://aspe.hhs.gov/reports/examination-clinical-trial-costs-barriers-drug-development-0 (accessed on 15 February 2025).
- Sears, R. How Evidence Based Medicine Became an Illusion. Available online: https://www.madinamerica.com/2022/03/evidence-based-medicine-became-illusion/ (accessed on 7 January 2025).
- Wimalawansa, S.J. Decoding the paradox: Understanding elevated hospitalization and reduced mortality in SARS-CoV-2 variants. Int. J. Front. Sci. Technol. Res. 2024, 6, 1–20. [Google Scholar] [CrossRef]
- Guni, A.; Whiting, P.; Darzi, A.; Ashrafian, H. The next generation of evidence synthesis for diagnostic accuracy studies in artificial intelligence. Lancet Digit. Health 2024, 6, e541–e542. [Google Scholar] [CrossRef]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef]
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grubler, M.R.; Marz, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, R27–R43. [Google Scholar] [CrossRef]
- Grant, W.B.; Bhattoa, H.P.; Boucher, B.J. Seasonal variations of U.S. mortality rates: Roles of solar ultraviolet-B doses, vitamin D, gene expression, and infections. J. Steroid Biochem. Mol. Biol. 2017, 173, 5–12. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.; Avenell, A. Effects of vitamin D supplementation on musculoskeletal health: A systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018, 6, 847–858. [Google Scholar] [CrossRef]
- Scragg, R. Emerging evidence of thresholds for beneficial effects from vitamin D supplementation. Nutrients 2018, 10, 561. [Google Scholar] [CrossRef]
- Sackett, D.L.; Rosenberg, W.M.; Gray, J.A.; Haynes, R.B.; Richardson, W.S. Evidence-based medicine: What it is and what it isn’t. 1996. Clin. Orthop. Relat. Res. 2007, 455, 3–5. [Google Scholar]
- Metzdorff, M.T. Evidence-based medicine: What it is, what it isn’t, and are we practicing it? J. Trauma Acute Care Surg. 2013, 75, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Every-Palmer, S.; Howick, J. How evidence-based medicine is failing due to biased trials and selective publication. J. Eval. Clin. Pract. 2014, 20, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; De Ponti, F. Drug repurposing in the COVID-19 era: Insights from case studies showing pharmaceutical peculiarities. Pharmaceutics 2021, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Song, M.; Eliassen, A.H.; Wang, M.; Fung, T.T.; Clinton, S.K.; Rimm, E.B.; Hu, F.B.; Willett, W.C.; Tabung, F.K.; et al. Optimal dietary patterns for prevention of chronic disease. Nat. Med. 2023, 29, 719–728. [Google Scholar] [CrossRef]
- Tan, Y.W.B.; Lau, J.H.; AshaRani, P.V.; Roystonn, K.; Devi, F.; Lee, Y.Y.; Whitton, C.; Wang, P.; Shafie, S.; Chang, S.; et al. Dietary patterns of persons with chronic conditions within a multi-ethnic population: Results from the nationwide Knowledge, Attitudes and Practices survey on diabetes in Singapore. Arch. Public Health 2022, 80, 62. [Google Scholar] [CrossRef]
- Allman-Farinelli, M.; Partridge, S.R.; Roy, R. Weight-related dietary behaviors in young adults. Curr. Obes. Rep. 2016, 5, 23–29. [Google Scholar] [CrossRef]
- Wilmot, E.G.; Edwardson, C.L.; Achana, F.A.; Davies, M.J.; Gorely, T.; Gray, L.J.; Khunti, K.; Yates, T.; Biddle, S.J. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: Systematic review and meta-analysis. Diabetologia 2012, 55, 2895–2905. [Google Scholar] [CrossRef]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.M.; Lancet Physical Activity Series 2 Executive, C.; Lancet Sedentary Behaviour Working, G. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef]
- Balakrishna, R.; Bjornerud, T.; Bemanian, M.; Aune, D.; Fadnes, L.T. Consumption of nuts and needs and health outcomes including cardiovascular disease, diabetes and metabolic disease, cancer, and mortality: An umbrella review. Adv. Nutr. 2022, 13, 2136–2148. [Google Scholar] [CrossRef] [PubMed]
- Beaglehole, R.; Horton, R. Chronic diseases: Global action must match global evidence. Lancet 2010, 376, 1619–1621. [Google Scholar] [CrossRef]
- Blackburn, G.L. Medicalizing obesity: Individual, economic, and medical consequences. Virtual Mentor 2011, 13, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suarez, V.J.; Beltran-Velasco, A.I.; Redondo-Florez, L.; Martin-Rodriguez, A.; Tornero-Aguilera, J.F. Global impacts of Western diet and its effects on metabolism and health: A narrative review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Scragg, R.; Jackson, R.; Holdaway, I.M.; Lim, T.; Beaglehole, R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: A community-based study. Int. J. Epidemiol. 1990, 19, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Beaglehole, R.; Bonita, R.; Horton, R.; Adams, C.; Alleyne, G.; Asaria, P.; Baugh, V.; Bekedam, H.; Billo, N.; Casswell, S.; et al. Priority actions for the non-communicable disease crisis. Lancet 2011, 377, 1438–1447. [Google Scholar] [CrossRef]
- Lemstra, M.; Nwankwo, C.; Bird, Y.; Moraros, J. Primary nonadherence to chronic disease medications: A meta-analysis. Patient Prefer. Adherence 2018, 12, 721–731. [Google Scholar] [CrossRef]
- Thorpe, K.E.; Allen, L.; Joski, P. The role of chronic disease, obesity, and Improved treatment and detection in accounting for the rise in healthcare spending between 1987 and 2011. Appl. Health Econ. Health Policy 2015, 13, 381–387. [Google Scholar] [CrossRef]
- Atieh, O.; Daher, J.; Durieux, J.C.; Abboud, M.; Labbato, D.; Baissary, J.; Koberssy, Z.; Ailstock, K.; Cummings, M.; Funderburg, N.T.; et al. Vitamins K2 and D3 improve long COVID, fungal translocation, and inflammation: Randomized controlled trial. Nutrients 2025, 17, 304. [Google Scholar] [CrossRef]
- Doaei, S.; Mardi, A.; Zare, M. Role of micronutrients in the modulation of immune system and platelet activating factor in patients with COVID-19; a narrative review. Front. Nutr. 2023, 10, 1207237. [Google Scholar] [CrossRef] [PubMed]
- Terzic, A.; Waldman, S. Chronic diseases: The emerging pandemic. Clin. Transl. Sci. 2011, 4, 225–226. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, R.; He, J.; Wang, L.; Chen, H.; Niu, X.; Sun, Y.; Guan, Y.; Gong, Y.; Zhang, L.; et al. Prevalence of cardiovascular-kidney-metabolic syndrome stages by social determinants of health. JAMA Netw. Open 2024, 7, e2445309. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Observatory 2011. Available online: https://www.who.int/data/gho (accessed on 25 October 2024).
- Yong, P.L.; Saunders, R.S.; Olsen, L.A. (Eds.) The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary; National Academies Press (US): Washington, DC, USA, 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK53914/ (accessed on 25 October 2024).
- Burke, L.A.; Ryan, A.M. The complex relationship between cost and quality in US health care. Virtual Mentor 2014, 16, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. National Institutes of Health. Available online: https://en.wikipedia.org/wiki/National_Institutes_of_Health#:~:text=In%201901%2C%20the%20Division%20of,to%20National%20Institutes%20of%20Health (accessed on 11 March 2025).[Green Version]
- Anonymous. National Science Foundation: FY 2025 Budget Request to Congress. Available online: https://nsf-gov-resources.nsf.gov/files/00_NSF_FY25_CJ_Entire%20Rollup_web.pdf?VersionId=cbkdqD_UMweHEIsZwPjtVgcQRwMccgvu (accessed on 25 March 2025).
- Kozlov, M.; Mallapaty, S. Exclusive: NIH to terminate hundreds of active research grants. Nature 2025, 639, 281–282. [Google Scholar] [CrossRef]
- Gilpin, N.W. The NIH is a sound investment for the US taxpayer. eLife 2025, 14, 106710. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M. ‘Disruptive’ science has declined—And no one knows why. Nature 2023, 613, 225. [Google Scholar] [CrossRef]
- Infante, M.; Ricordi, C.; Baidal, D.A.; Alejandro, R.; Lanzoni, G.; Sears, B.; Caprio, M.; Fabbri, A. VITAL study: An incomplete picture? Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3142–3147. [Google Scholar] [CrossRef]
- Grant, W.B.; Wimalawansa, S.J.; Holick, M.F. Vitamin D supplements and reasonable solar UVB should be recommended to prevent escalating incidence of chronic diseases. Br. Med. J. 2015, 350, h321. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. IOM recommendations vs. vitamin D guidelines applicable to the rest of the world. In Proceedings of the 5th International Conference on Vitamin D, Abu Dhabi, United Arab Emirates, 24–25 March 2017. [Google Scholar]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Manson, J.E.; Bassuk, S.S.; Lee, I.M.; Cook, N.R.; Albert, M.A.; Gordon, D.; Zaharris, E.; Macfadyen, J.G.; Danielson, E.; Lin, J.; et al. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp. Clin. Trials 2012, 33, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, M.; Ebeling, P.R.; McLeod, D.S.A.; English, D.; Romero, B.D.; Baxter, C.; Armstrong, B.K.; Hartel, G.; Kimlin, M.; O’Connell, R.L.; et al. The effect of monthly vitamin D supplementation on fractures: A tertiary outcome from the population-based, double-blind, randomised, placebo-controlled D-Health trial. Lancet Diabetes Endocrinol. 2023, 11, 324–332. [Google Scholar] [CrossRef]
- Neale, R.E.; Armstrong, B.K.; Baxter, C.; Duarte Romero, B.; Ebeling, P.; English, D.R.; Kimlin, M.G.; McLeod, D.S.; RL, O.C.; van der Pols, J.C.; et al. The D-Health Trial: A randomized trial of vitamin D for prevention of mortality and cancer. Contemp. Clin. Trials 2016, 48, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Leahey, E.; Funk, R.J. Papers and patents are becoming less disruptive over time. Nature 2023, 613, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Carter, J. DIEing Academic Research Budgets. Available online: https://barsoom.substack.com/p/dieing-academic-research-budgets (accessed on 25 March 2025).
- Anonymous. Visualizing Forty Years of Health Insurance Cost Inflation. Available online: https://politicalcalculations.blogspot.com/2024/10/visualizing-forty-years-of-health.html#google_vignette (accessed on 24 March 2025).
- Bailey, S.R.; O’Malley, J.P.; Gold, R.; Heintzman, J.; Marino, M.; DeVoe, J.E. Receipt of diabetes preventive services differs by insurance status at visit. Am. J. Prev. Med. 2015, 48, 229–233. [Google Scholar] [CrossRef]
- Adler-Milstein, J.; Everson, J.; Lee, S.Y. EHR adoption and hospital performance: Time-related effects. Health Serv. Res. 2015, 50, 1751–1771. [Google Scholar] [CrossRef]
- Sloan, F.; Hsieh, C.-R. Health Economics, 2nd ed.; Mit Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Zhou, Y.Y.; Kanter, M.H.; Wang, J.J.; Garrido, T. Improved quality at Kaiser Permanente through e-mail between physicians and patients. Health Aff. 2010, 29, 1370–1375. [Google Scholar] [CrossRef]
- Bodenheimer, T.; Sinsky, C. From triple to quadruple aim: Care of the patient requires care of the provider. Ann. Fam. Med. 2014, 12, 573–576. [Google Scholar] [CrossRef]
- Anonymous. Alert and Special Legislative Update—Pharmacy Benefits Managers. Available online: https://aapsonline.org/alert-and-special-legislative-update-pharmacy-benefits-managers/ (accessed on 20 March 2025).
- Saidu, S.F.; Danielson, R.A. Social determinants of health associated with increased prevalence of childhood malnutrition in Africa. Front. Nutr. 2024, 11, 1456089. [Google Scholar] [CrossRef]
- Hirsch, B.R.; Balu, S.; Schulman, K.A. The impact of specialty pharmaceuticals as drivers of health care costs. Health Aff. 2014, 33, 1714–1720. [Google Scholar] [CrossRef]
- Mercado, C.I.; Bullard, K.M.; Bolduc, M.L.F.; Andrews, C.A.; Freggens, Z.R.F.; Liggett, G.; Banks, D.; Johnson, S.B.; Penman-Aguilar, A.; Njai, R. A shift in approach to addressing public health Inequities and the effect of societal structural and systemic drivers on social determinants of health. Public. Health Rep. 2024, 333549241283586. [Google Scholar] [CrossRef]
- Anonymous. America’s Hospitals and Health Systems Continue to Face Escalating Operational Costs and Economic Pressures as they Care for Patients and Communities. Available online: https://www.aha.org/costsofcaring (accessed on 20 February 2025).
- Anonymous. The Dangers of Polypharmacy and the Case for Deprescribing in Older Adults. Available online: https://www.nia.nih.gov/news/dangers-polypharmacy-and-case-deprescribing-older-adults (accessed on 20 December 2024).
- Hobbs, L.; Holtz-Eakin, D. The Future of America’s Entitlements: What You Need to Know About the Medicare and Social Security Trustees Reports. Available online: https://www.americanactionforum.org/research/2024-medicare-social-security-report/#:~:text=At%20its%20current%20pace%2C%20Medicare%20Part%20A,disability%20benefits%20will%20become%20exhausted%20by%202035.&text=The%20report%20estimates%20that%20the%20combined%20(retirement,one%20year%20later%20than%20last%20year’s%20estimate (accessed on 10 February 2025).
- Dodaro, G. Medicare and Medicaid: Additional Actions Needed to Enhance Program Integrity and Save Billions. Available online: https://www.gao.gov/assets/gao-24-107487.pdf (accessed on 3 March 2025).
- Anonymous. Budget of the U.S. Government: Fiscal Year 2025. Available online: https://www.whitehouse.gov/wp-content/uploads/2024/03/budget_fy2025.pdf (accessed on 22 March 2025).
- Anonymous. Fact Sheet: How Much Waste, Fraud, and Abuse Is There in Social Security? Available online: https://www.crfb.org/press-releases/fact-sheet-how-much-waste-fraud-and-abuse-there-social-security (accessed on 17 March 2025).
- Goss, J. How Much Fraud, Waste and Abuse Is There in the Social Security Administration. Available online: https://gossandfentress.com/how-much-fraud-waste-and-abuse-is-there-in-the-social-security-administration-part-two/ (accessed on 20 March 2025).
- Rasmussen, P.; Farmer, C.M. The Promise and challenges of VA community care: Veterans’ issues in focus. Rand Health Q. 2023, 10, 9. [Google Scholar] [PubMed]
- McCarthy, K. Health Chairwoman Dr. Miller-Meeks at Hearing on Mental Health Care, Substance Abuse, and Rehab Access: “VA Does Not Have a Resource Problem—It Has an Access and Process Problem”. Available online: https://veterans.house.gov/news/documentsingle.aspx?DocumentID=6674 (accessed on 16 April 2025).
- Maestas, N. Identifying work capacity and promoting work: A strategy for modernizing the SSDI program. Ann. Am. Acad. Pol. Soc. Sci. 2019, 686, 93–120. [Google Scholar] [CrossRef] [PubMed]
- Dal Bianco, C. Disability insurance and the effects of return-to-work policies. Rev. Economic Dynamics 2023, 49, 351–373. [Google Scholar] [CrossRef]
- Himmelstein, D.; Woolhandler, S. Administrative waste in the US health care system in comparison to other nations. Health Aff. 2021, 40, 89–98. [Google Scholar]
- Resnick, M.J. Waste in the US health care system: Estimated costs and potential for savings. J. Urol. 2020, 203, 872. [Google Scholar] [CrossRef]
- Freed, M.; Cubanski, J.; Neuman, T. Medicare Program Integrity and Efforts to Root out Improper Payments, Fraud, Waste and Abuse. Available online: https://www.kff.org/medicare/issue-brief/medicare-program-integrity-and-efforts-to-root-out-improper-payments-fraud-waste-and-abuse/#:~:text=The%20Trump%20administration%20recently%20cited,same%20as%20estimates%20of%20fraud. (accessed on 2 April 2025).
- Fiscal Year 2023 Improper Payments Fact Sheet. Available online: https://www.cms.gov/newsroom/fact-sheets/fiscal-year-2023-improper-payments-fact-sheet#:~:text=The%20Medicaid%20improper%20payment%20rate%20(comprised%20of,from%20the%202022%20reported%20rate%20of%2015.62% (accessed on 20 March 2025).
- Anonymous. Fact Check: President Trump Will Always Protect Social Security, Medicare. Available online: https://www.whitehouse.gov/articles/2025/03/fact-check-president-trump-will-always-protect-social-security-medicare/ (accessed on 20 March 2025).
- Himmelstein, D.U.; Jun, M.; Busse, R.; Chevreul, K.; Geissler, A.; Jeurissen, P.; Thomson, S.; Vinet, M.A.; Woolhandler, S. A comparison of hospital administrative costs in eight nations: US costs exceed all others by far. Health Aff. 2014, 33, 1586–1594. [Google Scholar] [CrossRef]
- Shrank, W.H.; Rogstad, T.L.; Parekh, N. Waste in the US health care system: Estimated costs and potential for savings. JAMA 2019, 322, 1501–1509. [Google Scholar] [CrossRef]
- Kesselheim, A.; Avorn, J.; Sarpatwari, A. The high cost of prescription drugs in the United States: Origins and prospects for reform. JAMA 2016, 316, 858–871. [Google Scholar] [CrossRef]
- Fleming, T.R.; Demets, D.L.; McShane, L.M. Discussion: The role, position, and function of the FDA-The past, present, and future. Biostatistics 2017, 18, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Lupkin, S. One-Third of New Drugs Had Safety Problems After FDA Approval. Available online: https://www.npr.org/sections/health-shots/2017/05/09/527575055/one-third-of-new-drugs-had-safety-problems-after-fda-approval (accessed on 9 November 2024).
- Congress. H. Rept. 112-495—Food and Drug Administration Reform Act of 2012. Available online: https://www.congress.gov/congressional-report/112th-congress/house-report/495#:~:text=Section%20602%2D%2DConflicts%20of%20interest%20The%20Food%20and,provision%20has%20discouraged%20the%20use%20of%20the (accessed on 10 November 2024).
- Dunn, A.M.; Florence, M.P.; Turow, R.; Grimm, N.L. Gauging the Likelihood of Trump Administration FDA Reforms. Available online: https://www.skadden.com/insights/publications/2025/01/gauging-the-likelihood-of-trump-administration-fda-reforms (accessed on 10 February 2025).
- Bottomiller, H. The FDA’s Food Failure. Available online: https://www.politico.com/interactives/2022/fda-fails-regulate-food-health-safety-hazards/ (accessed on 20 December 2024).
- Rapfogel, N. 5 Things to Know About Pharmacy Benefit Managers. Available online: https://www.americanprogress.org/article/5-things-to-know-about-pharmacy-benefit-managers/#:~:text=Requiring%20PBM%20transparency%20is%20not,that%20might%20better%20serve%20them (accessed on 15 November 2024).
- Ginder-Vogel, K. The Evolution and Future of Pharmacy Benefits Managers. Available online: https://pharmacy.wisc.edu/2024/03/13/the-evolution-and-future-of-pharmacy-benefits-managers/#:~:text=By%20Katie%20Ginder%2DVogel,their%20discussion’s%20most%20salient%20points (accessed on 10 January 2025).
- Conti, R.M.; Frandsen, B.; Rebitzer, J.B. Pharmacy Benefit Managers and the U.S. Pharmaceutical Market. Available online: https://hmpi.org/2024/04/12/pharmacy-benefit-managers-and-the-us-pharmaceutical-market/ (accessed on 10 March 2025).
- Burns, J. Pharmacy Benefit Managers Raised Prices by over 1,000% on Specialty Drugs. Available online: https://healthjournalism.org/blog/2025/02/pharmacy-benefit-managers-raised-prices-by-over-1000-on-specialty-drugs/ (accessed on 12 March 2025).
- Gale, A. If pharmacy benefit managers raise drug prices, Then why are they needed? Mo. Med. 2023, 120, 243–244. [Google Scholar]
- Cox, C.; Ortaliza, J.; Wagner, E.; Amin, K. Health Care Costs and Affordability: How has U.S. Health Care Spending Changed Over Time? Available online: https://www.kff.org/health-policy-101-health-care-costs-and-affordability?entry=table-of-contents-how-has-u-s-health-care-spending-changed-over-time (accessed on 20 March 2025).
- Anonymous. Food Safety: Status of Foodborne Illness in the US. Available online: https://www.gao.gov/assets/gao-25-107606.pdf (accessed on 3 March 2025).
- Firm, K.L. FDA Chiefs’ Ties to Big Pharma Raise Conflict of Interest Concerns. Available online: https://www.linkedin.com/pulse/fda-chiefs-ties-big-pharma-raise-conflict-interest-concerns-eg2se/ (accessed on 11 November 2024).
- Diu, S. Slowing down accelerated approval: Examining the role of industry influence, patient advocacy organizations, and political pressure on FDA drug approval. Foedham Law. Review 2022, 90, 2303. [Google Scholar]
- Johnston, J.L.; Ross, J.S.; Ramachandran, R. US Food and Drug Administration approval of drugs not meeting pivotal trial Primary end points, 2018–2021. JAMA Intern. Med. 2023, 183, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Karas, L. FDA’s Revolving Door: Reckoning and Reform. Available online: https://law.stanford.edu/publications/fdas-revolving-door-reckoning-and-reform/ (accessed on 12 November 2024).
- Fernandez Lynch, H.; Joffe, S.; McCoy, M.S. The limits of acceptable political influence over the FDA. Nat. Med. 2021, 27, 188–190. [Google Scholar] [CrossRef] [PubMed]
- CDC. Clinical Data Summary Pilot Program. Available online: https://www.fda.gov/drugs/development-approval-process-drugs/clinical-data-summary-pilot-program#:~:text=The%20Center%20for%20Drug%20Evaluation,medical%20researchers%2C%20and%20the%20public (accessed on 11 November 2024).
- FDA. Information for Consumers on Using Dietary Supplements. Available online: https://www.fda.gov/food/dietary-supplements/information-consumers-using-dietary-supplements#:~:text=Under%20DSHEA%2C%20FDA%20is%20not,your%20family%20about%20dietary%20supplements (accessed on 10 November 2024).
- Baell, J.; Jennings, M.; Parker, M. Ivermectin Shows Us How Hard It Is to Use Old Drugs for COVID. Here’s How to Do Better Next Time. Available online: https://theconversation.com/ivermectin-shows-us-how-hard-it-is-to-use-old-drugs-for-covid-heres-how-to-do-better-next-time-168192 (accessed on 15 November 2024).
- Group-C19.com. Vitamin D for COVID-19: Real-Time Analysis of All 300 Studies. Available online: https://c19early.org/d (accessed on 25 March 2024).
- Zuckerman, D.M. Emergency Use Authorizations (EUAs) versus FDA approval: Implications for COVID-19 and public health. Am. J. Public Health 2021, 111, 1065–1069. [Google Scholar] [CrossRef]
- Surgeon General, F. Updated Guidance for COVID-19 Boosters for the Fall and Winter 2024–2025 Season. Available online: https://www.floridahealth.gov/newsroom/2024/09/20210912-UpdatedGuidanceCOVID-19.html (accessed on 10 January 2025).
- Wimalawansa, S.J. Unveiling the interplay-vitamin D and ACE-2 molecular interactions in mitigating complications and deaths from SARS-CoV-2. Biology 2024, 13, 831. [Google Scholar] [CrossRef]
- HRSA. 340B Drug Pricing Program. Available online: https://www.hrsa.gov/opa#:~:text=The%20340B%20Program%20enables%20covered,entities%20at%20significantly%20reduced%20prices (accessed on 5 January 2025).
- Anshida, V.P.; Kumari, R.A.; Murthy, C.S.; Samuel, A. Extracellular matrix degradation by host matrix metalloproteinases in restorative dentistry and endodontics: An overview. J. Oral. Maxillofac. Pathol. 2020, 24, 352–360. [Google Scholar] [CrossRef]
- Aboy, M.; Crespo, C.; Stern, A. Beyond the 510(k): The Regulation of Novel Moderate-Risk Medical Devices, Intellectual Property Considerations, and Innovation Incentives in the FDA’s De Novo Pathway. Available online: https://www.nature.com/articles/s41746-024-01021-y#:~:text=Accordingly%2C%20the%20510(k),of%20future%20device%20safety6 (accessed on 10 November 2024).
- FDA. FDA Continues to Take Steps to Strengthen the Premarket Notification [510(k)] Program—Program Updates. Available online: https://www.fda.gov/medical-devices/510k-clearances/fda-continues-take-steps-strengthen-premarket-notification-510k-program-program-updates#:~:text=The%20510(k)%20Program%20accounts,changes%20to%20the%20device%20marketplace (accessed on 10 November 2024).
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef]
- Jones, J.L. One Culprit in Rising College Costs: Administrative Expenses. Available online: https://www.usnews.com/education/articles/one-culprit-in-rising-college-costs (accessed on 15 February 2025).
- Hultman, J. The High Cost of Waste in Healthcare. Available online: https://www.podiatrym.com/pdf/2019/8/HultmanFeature819web.pdf (accessed on 10 February 2025).
- Mulhern, C.; Spies, R.R.; Wu, D.D. The Effects of Rising Student Costs in Higher Education. Available online: https://sr.ithaka.org/publications/the-effects-of-rising-student-costs-in-higher-education/ (accessed on 17 March 2025).
- Cooper, P. How Many Administrators Do Colleges Have? Available online: https://www.aei.org/education/how-many-administrators-do-colleges-have/ (accessed on 2 April 2025).
- Green, J. Administrative Bloat at Universities RAISES Costs Without Helping Students. Available online: https://www.heritage.org/education/commentary/administrative-bloat-universities-raises-costs-without-helping-students (accessed on 10 March 2025).
- Ellen, N. Are Rising Education Costs a Necessary Evolution or Excessive Expenditure? Available online: https://academichelp.net/blog/higher-education-news/are-rising-education-costs-a-necessary-evolution-or-excessive-expenditure.html (accessed on 20 December 2024).
- Picchi, A. One-Third of Graduate Schools Leave THEIR Alums Drowning in Debt. Available online: https://www.cbsnews.com/news/student-loan-graduate-school-drowning-in-debt/ (accessed on 15 February 2025).
- Twomey, J. Recent Grads High in Student LOAN Debt, Low in Financial Literacy. Available online: https://www.earnest.com/blog/student-debt-research/ (accessed on 10 February 2025).
- Aboulenein, A.U.S. Healthcare Spending Rises to $4.8 Trillion in 2023, Outpacing GDP. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/us-healthcare-spending-rises-48-trillion-2023-outpacing-gdp-2024-06-12/ (accessed on 20 January 2025).
- Release, P. CMS Office of the Actuary Releases 2021–2030 Projections of National Health Expenditures. Available online: https://www.cms.gov/newsroom/press-releases/cms-office-actuary-releases-2021-2030-projections-national-health-expenditures (accessed on 10 June 2025).
- Kakemam, E.; Arab-Zozani, M.; Raeissi, P.; Albelbeisi, A.H. The occurrence, types, reasons, and mitigation strategies of defensive medicine among physicians: A scoping review. BMC Health Serv. Res. 2022, 22, 800. [Google Scholar] [CrossRef]
- Long, J. Congress Boosts Funding for FDA Dietary Supplement Office. Available online: https://www.naturalproductsinsider.com/regulatory/congress-boosts-funding-fda-dietary-supplement-office (accessed on 20 January 2025).
- Rudolph, L.; Caplan, J.; Mitchell, C.; Ben-Moshe, K.; Dillon, L. Health in All Policies: Improving Health Through Intersectoral Collaboration. Available online: https://nam.edu/perspectives-2013-health-in-all-policies-improving-health-through-intersectoral-collaboration/ (accessed on 21 November 2024).
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A comprehensive review on nutraceuticals: Therapy support and formulation challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef] [PubMed]
- AlAli, M.; Alqubaisy, M.; Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.S.; Lim, S.E. Nutraceuticals: Transformation of Conventional Foods into Health Promoters/Disease Preventers and Safety Considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, A.; Cheeran, A.T.; Slvalingam, A.; Vasanth, K. A review on the influence of nutraceuticals and functional foods on health. Funct. Chem. Adv. 2024, 5, 100749. [Google Scholar] [CrossRef]
- Anonymous. Foods: Some Foods Contain Chemicals on the Proposition 65 List (Fact Sheets). Available online: https://www.p65warnings.ca.gov/fact-sheets/foods#:~:text=BPA%20can%20leach%20into%20food,beverages%20from%20plastic%20food%20packaging (accessed on 15 March 2025).
- Mamun, A.A.; Prasetya, T.A.E.; Dewi, I.R.; Ahmad, M. Microplastics in human food chains: Food becoming a threat to health safety. Sci. Total Environ. 2023, 858, 159834. [Google Scholar] [CrossRef]
- Hovav, K. Which Types of Cookware Should You Avoid? The Best and Worst Pots and Pans for Your Health. Available online: https://www.goodrx.com/health-topic/environmental/dangerous-cookware-to-avoid (accessed on 20 March 2025).
- Califf, R.M. Benefit-risk assessments at the US food and drug administration: Finding the balance. JAMA 2017, 317, 693–694. [Google Scholar] [CrossRef]
- Deubberly, H. How Cybernetics Connects Computing, Counterculture, and Design. Available online: https://www.dubberly.com/articles/cybernetics-and-counterculture.html (accessed on 25 March 2025).
- Ficher, T.; Herr, C. Design Cybernetics: Navigating the New; Design Research Foundations; Springer Nature: New York, NY, USA, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wimalawansa, S.J. Reforming Food, Drug, and Nutraceutical Regulations to Improve Public Health and Reduce Healthcare Costs. Foods 2025, 14, 2328. https://doi.org/10.3390/foods14132328
Wimalawansa SJ. Reforming Food, Drug, and Nutraceutical Regulations to Improve Public Health and Reduce Healthcare Costs. Foods. 2025; 14(13):2328. https://doi.org/10.3390/foods14132328
Chicago/Turabian StyleWimalawansa, Sunil J. 2025. "Reforming Food, Drug, and Nutraceutical Regulations to Improve Public Health and Reduce Healthcare Costs" Foods 14, no. 13: 2328. https://doi.org/10.3390/foods14132328
APA StyleWimalawansa, S. J. (2025). Reforming Food, Drug, and Nutraceutical Regulations to Improve Public Health and Reduce Healthcare Costs. Foods, 14(13), 2328. https://doi.org/10.3390/foods14132328





