Nutritional, Phytochemical, and Antimicrobial Properties of Carica papaya Leaves: Implications for Health Benefits and Food Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Extraction
2.2.2. Nutritional Composition
Proximate Analysis
Mineral Analysis
2.2.3. Phytochemical Screening
Total Phenolic Content (TPC)
Total Flavonoid Content (TFC)
Tannin Content
Alkaloid Content
GC-MS Analysis
2.2.4. Assessment of Antioxidant Activity
DPPH Assay
FRAP Assay
2.2.5. FTIR Analysis
2.2.6. XRD Analysis
2.2.7. Thermal Properties
2.2.8. Antimicrobial Activity
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Nutritional, Phytochemical, and Antioxidant Activity
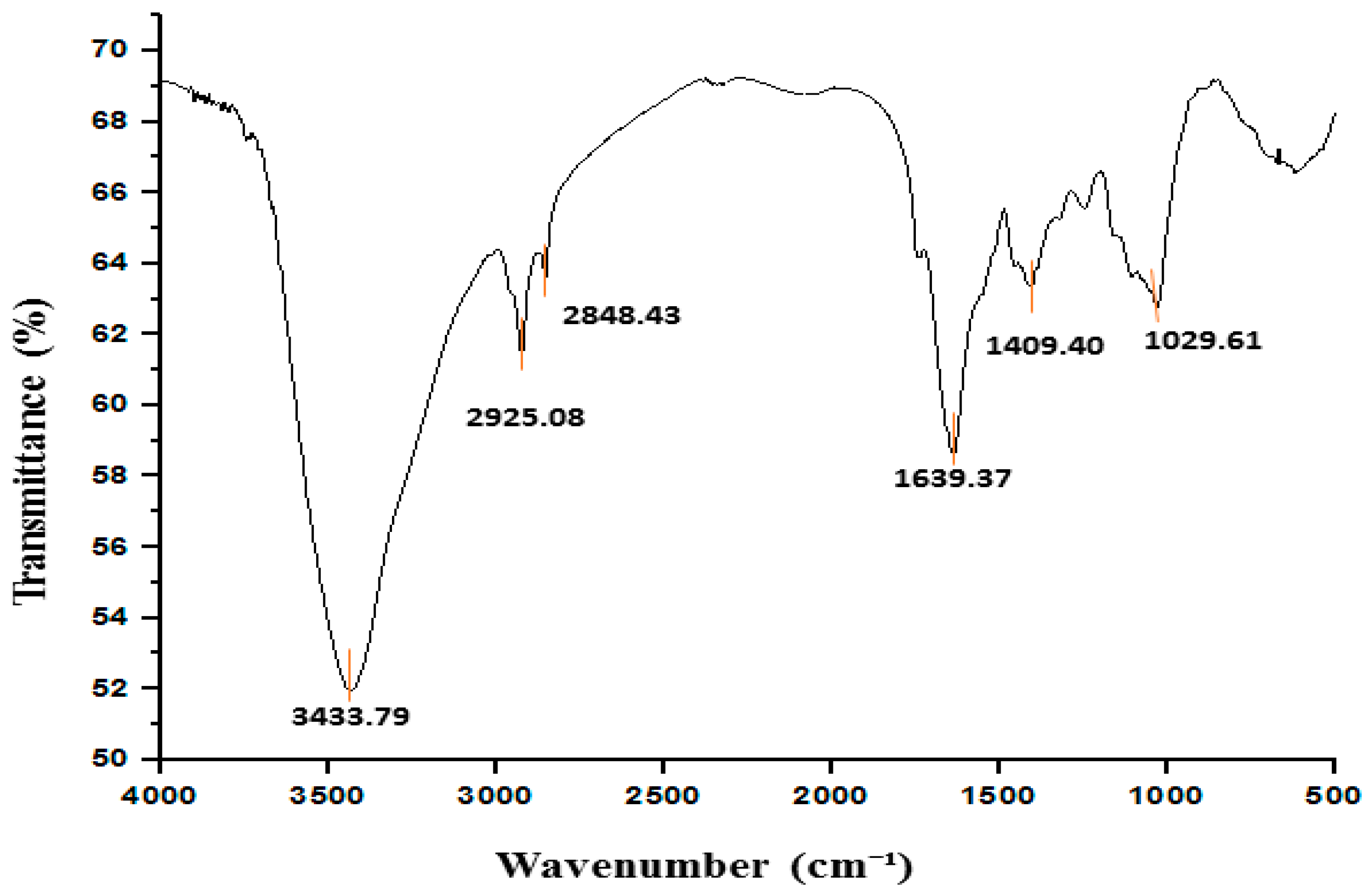
3.2. FTIR Findings
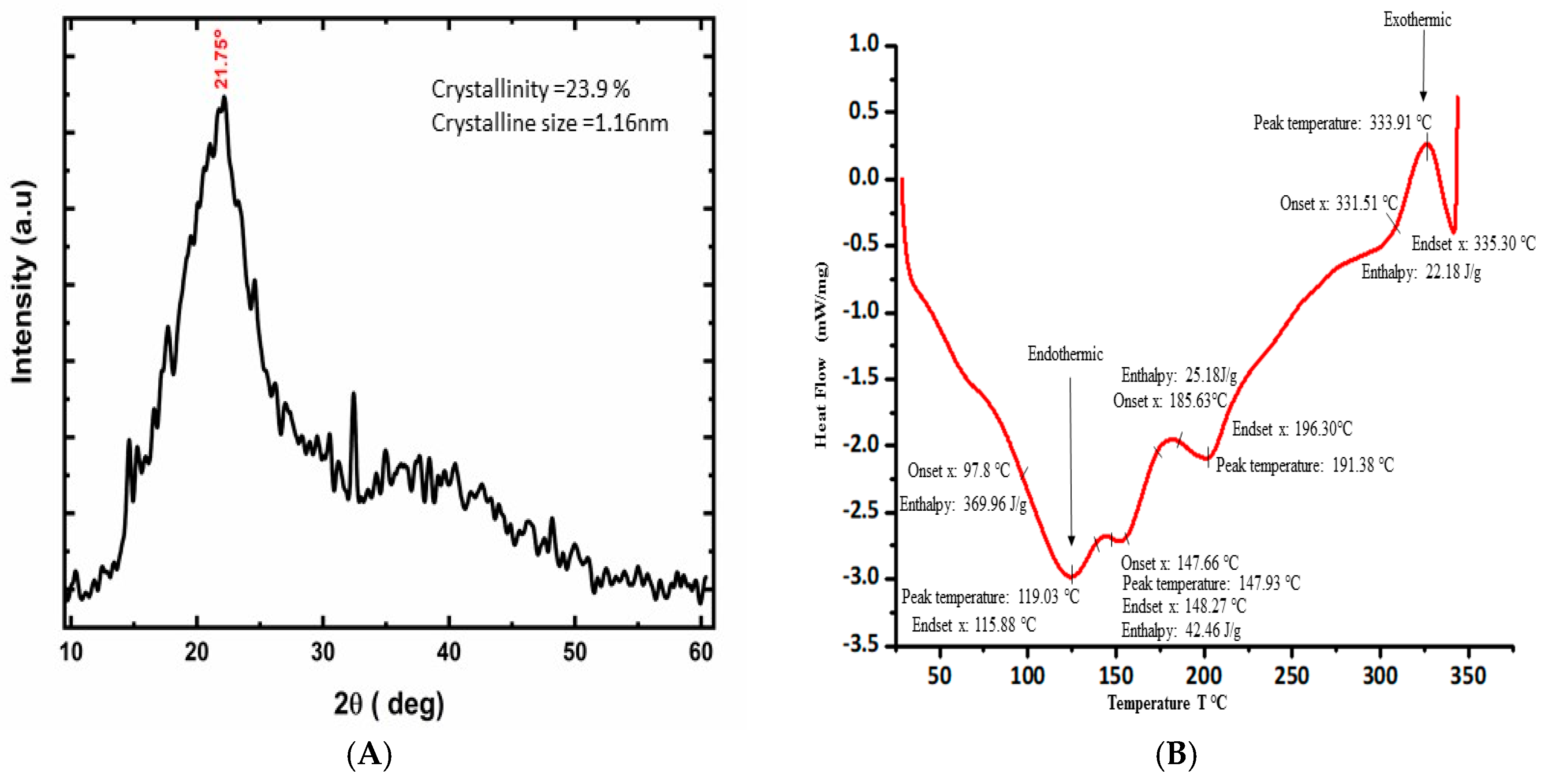
3.3. XRD Data and Observation
3.4. Thermal Analysis
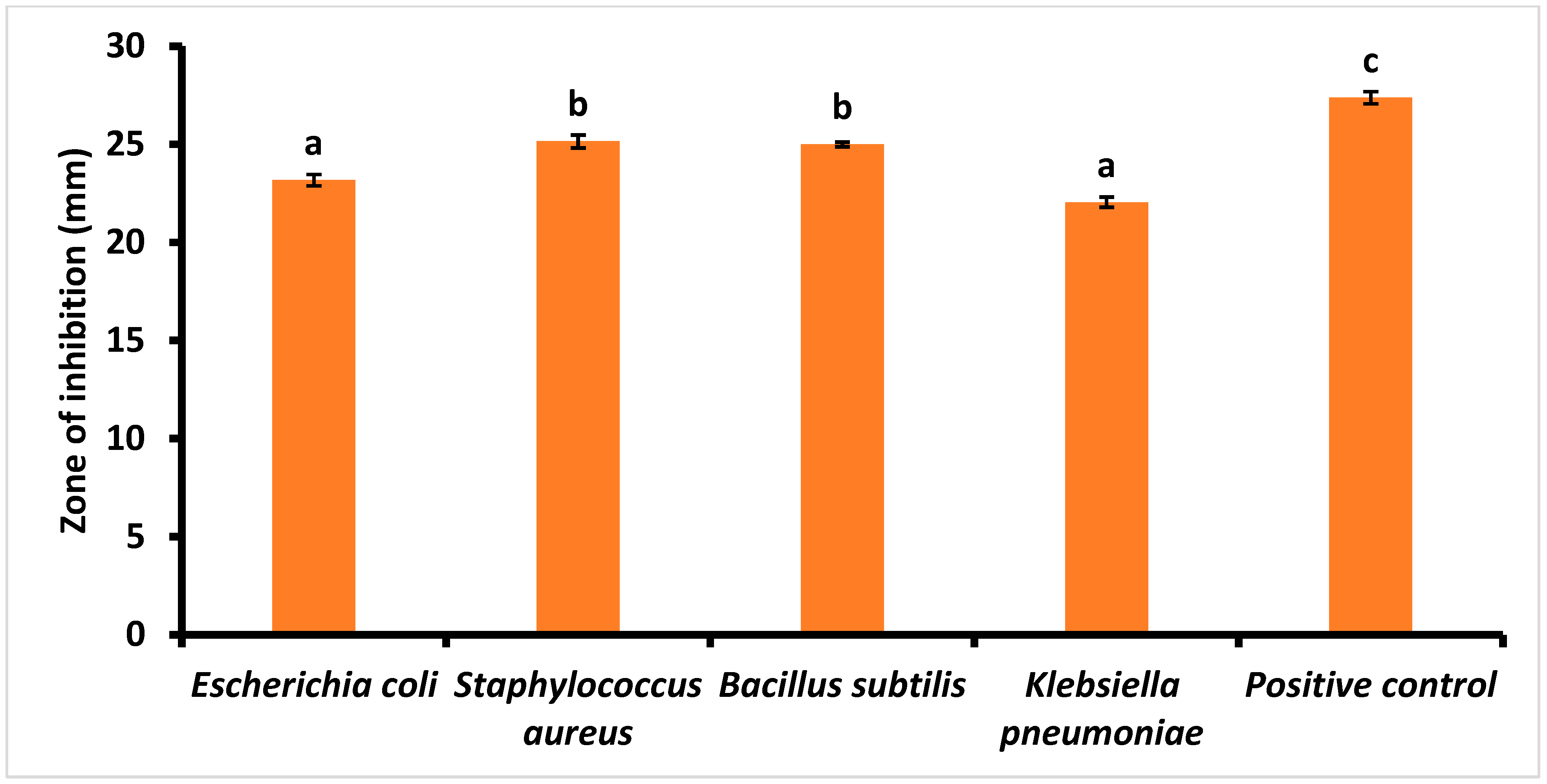
3.5. Antimicrobial Activity of PLs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Sharma, R.; Sharma, M.; Kumar, M.; Barbhai, M.D.; Lorenzo, J.M.; Sharma, S.; Samota, M.K.; Atanassova, M.; Caruso, G. Carica papaya L. leaves: Deciphering its antioxidant bioactives, biological activities, innovative products, and safety aspects. Oxidative Med. Cell. Longev. 2022, 2022, 2451733. [Google Scholar] [CrossRef] [PubMed]
- Munir, A.; Youssef, F.S.; Ishtiaq, S.; Kamran, S.H.; Sirwi, A.; Ahmed, S.A.; Ashour, M.L.; Elhady, S.S. Malva parviflora Leaves Mucilage: An Eco-Friendly and Sustainable Biopolymer with Antioxidant Properties. Polymers 2021, 13, 4251. [Google Scholar] [CrossRef]
- Jyothi, R.B.; Naganathan, L.; Chaitanya, B.; Sunitha, A.; Atyam VSSSG, S.J. Various Pharmacological Actions of Calendula officinalis, Tagetes erecta, Carica papaya, Hypericum perforatum and Salvia officinalis. Int. J. Pharm. Sci. Rev. Res. 2019, 59, 42–51. [Google Scholar]
- Raviraja, S.; Khanum, K.G.N.; Thangaraj, G.; Basalingappa, K. A review on significance of carica papaya linn: A promising medicinal plant. Int. J. Recent Sci. Res. 2020, 11, 37602–37607. [Google Scholar]
- Olumide, M.D.; Akintunde, A.O.; Shobo, B.A.; Akinboye, O.E. Nutrient evaluation and phytochemical analysis of fresh and dry leaves of Carica papaya. Indian J. Agric. Res. 2023, 57, 230–234. [Google Scholar] [CrossRef]
- Ghaffar, A.; Munir, B.; Jahangeer, M.; Ashiq, M.; Qamar, S.A.; Ahmad, B. Bioactivity prospection, antimicrobial, nutraceutical, and pharmacological potentialities of Carica papaya. In Antiviral and Antimicrobial Smart Coatings; Elsevier: Amsterdam, The Netherlands, 2023; pp. 587–606. [Google Scholar]
- Dwivedi, M.K.; Sonter, S.; Mishra, S.; Patel, D.K.; Singh, P.K. Antioxidant, antibacterial activity, and phytochemical characterization of Carica papaya flowers. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 23. [Google Scholar] [CrossRef]
- Leitão, M.; Ribeiro, T.; García, P.A.; Barreiros, L.; Correia, P. Benefits of Fermented Papaya in Human Health. Foods 2022, 11, 563. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Pudhuvai, B.; Sharma, C.; Kumar, A.; Sharma, V.; Yadav, D.; Jin, J.O. Carica papaya L.: A tropical fruit with benefits beyond the tropics. Diversity 2022, 14, 683. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists 2005. Official Methods of Analysis; AOAC: Rockville, MD, USA, 2005. [Google Scholar]
- Kiani, A.; Arabameri, M.; Moazzen, M.; Shariatifar, N.; Aeenehvand, S.; Khaniki, G.J.; Abdel-Wahhab, M.; Shahsavari, S. Probabilistic health risk assessment of trace elements in baby food and milk powder using ICP-OES method. Biol. Trace Elem. Res. 2021, 200, 2486–2497. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Zin, N.B.M.; Azemin, A.; Rodi, M.M.M.; Mohd, K.S. Chemical composition and antioxidant activity of stingless bee propolis from different extraction methods. Int. J. Eng. Technol. 2018, 7, 90–95. [Google Scholar]
- Kumar, S.R.; Sadiq, M.B.; Anal, A.K. Comparative study of physicochemical and functional properties of soaked, germinated and pressure cooked Faba bean. J. Food Sci. Technol. 2022, 59, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.A.; Parihar, K.; Tabasum, U.; Kumari, E. Estimation of alkaloid, saponin and flavonoid, content in various extracts of Crocus sativa. J. Med. Plants Stud. 2016, 4, 171–174. [Google Scholar]
- Flieger, J.; Flieger, M.J.M. The [DPPH●/DPPH-H]-HPLC-DAD method on tracking the antioxidant activity of pure antioxidants and goutweed (Aegopodium podagraria L.) hydroalcoholic extracts. Molecules 2020, 25, 6005. [Google Scholar] [CrossRef] [PubMed]
- Nisa, F.Z.; Astuti, M.; Haryana, S.M.; Murdiati, A. Antioxidant activity and total flavonoid of Carica papaya L. leaves with different varieties, maturity and solvent. Agritech 2019, 39, 54–59. [Google Scholar] [CrossRef]
- Sutariya, S.; Ahmad Shah, A.; Bajpai, A.B.; Sharma, R.J.; Pandhurnekar, C.P.; Gupta, A. Fourier transform infrared spectroscopy (FTIR) analysis, antioxidant and anti-inflammatory activities of leaf and fruit extracts of Gymnosporia montana. Mater. Today Proc. 2023, 73, 134–141. [Google Scholar] [CrossRef]
- Athmaselvi, K.A.; Kumar, C.; Balasubramanian, M.; Roy, I. Thermal, Structural, and Physical Properties of Freeze Dried Tropical Fruit Powder. J. Food Process. 2014, 2014, 524705. [Google Scholar] [CrossRef]
- Joshi, N.C.; Negi, T.; Gururani, P.J.I.; Chemistry, N.-M. Papaya (Carica papaya) leaves extract based synthesis, characterizations and antimicrobial activities of CeO2 nanoparticles (CeO2 NPs). Inorg. Nano-Met. Chem. 2023, 53, 795–802. [Google Scholar] [CrossRef]
- Nwofia, G.E.; Ojimelukwe, P.; Eji, C. Chemical composition of leaves, fruit pulp and seeds in some Carica papaya (L) morphotypes. Int. J. Med. Arom. Plants 2012, 2, 200–206. [Google Scholar]
- Ugo, N.J.; Ade, A.R.; Joy, A.T. Nutrient composition of Carica papaya leaves extracts. J. Food Sci. Nutr. Res. 2019, 2, 274–282. [Google Scholar]
- Joseph, B.; Sankarganesh, P.; Ichiyama, K.; Yamamoto, N. In vitro study on cytotoxic effect and anti-DENV2 activity of Carica papaya L. leaf. Front. Life Sci. 2015, 8, 18–22. [Google Scholar] [CrossRef]
- Dev, N.; Iqbal, A. Processing and quality evaluation of green papaya (Carica papaya L.) leaf tea. J. Agric. Crop Sci. 2015, 2, 1–6. [Google Scholar]
- Sobia, K.; Javaid, M.A.; Ahmad, M.S.; Rehmatullah, Q.; Hina, G.; Iram, B.; Pervaiz, A.; Farhana, B.; Nyla, J.; Gulfraz, M. Assessments of phytochemicals and hypoglycemic activity of leaves extracts of Carica papaya in diabetic mice. Int. J. Pharm. Sci. Res. 2016, 7, 3658. [Google Scholar]
- National Institutes of Health. Recommended Dietary Allowances and Adequate Intakes, Elements. Available online: https://ods.od.nih.gov/HealthInformation/nutrientrecommendations.aspx (accessed on 29 November 2024).
- Sharma, D.K.; Tiwari, B.; Singh, R.K.; Sahu, S.; Mathur, S.C.; Singh, R.M.; Singh, G.N. Estimation of minerals in Carica papaya L. Leaf found in Northern India by using ICP-OES technique. Int. J. Sci. Eng. Res. 2013, 4, 2012–2019. [Google Scholar]
- Houston, M.C. The importance of potassium in managing hypertension. Curr. Hypertens. Rep. 2011, 13, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Jayachitra, J.; Janani, B.; Bharathi, V.; Manikandan, R. Phytochemical analysis and mineral composition of Methanolic extract of Boerhavia diffusa L. Res. J. Pharm. Technol. 2020, 13, 4856–4860. [Google Scholar] [CrossRef]
- Ayoola, P.B.; Adeyeye, A. Phytochemical and nutrient evaluation of Carica papaya (pawpaw) leaves. Ijrras 2010, 5, 325–328. [Google Scholar]
- Palanisamy, P.; Basalingappa, K. Phytochemical analysis and antioxidant properties of leaf extracts of Carica papaya. Phytochem. Anal. 2020, 13, 58–62. [Google Scholar]
- Shams, R.; Bakht, J. Phytochemical analysis, antioxidant activity and antibacterial activity of ilex dipyrena. Pak. J. Bot. 2024, 56, 2011–2018. [Google Scholar] [CrossRef]
- Chetehouna, S.; Derouiche, S.; Reggami, Y.; Boulaares, I.; Frahtia, A. Gas Chromatography Analysis, Mineral Contents and Anti-inflammatory Activity of Sonchus maritimus. Trop. J. Nat. Prod. Res. 2024, 8, 6787–6798. [Google Scholar]
- Younis, A.; Saleh, H. Phytochemical Screening and Assessment of Antioxidant and Antimicrobial Potentialities of Two Egyptian Medicinal Plants. Egypt. J. Pure Appl. Sci. 2021, 59, 49–57. [Google Scholar] [CrossRef]
- Ganie, S.Y.; Javaid, D.; Singh, A.; Jawaid, F.; Anjum, S.; Kumari, M.; Singh, S.K.; Bhagat, M.; Reshi, M.S. Chemoprofiling and in vitro evaluation of anticancer, antioxidant and antibacterial activities of Asparagus racemosus (Willd). Pharmacol. Res.-Nat. Prod. 2024, 2, 100015. [Google Scholar] [CrossRef]
- Gideon, M.; Ladan, Z.; Yakubu, Y. Acid Induced Phytoconstituents of Calotropis procera Extract Combined with Ampicillin in Combating Clinical Resistant Isolates of Staphylococcus aureus and Salmonella spp. Fine Chem. Eng. 2024, 5, 111–122. [Google Scholar] [CrossRef]
- Okorie, U.; Sunday-Adeoye, I.; Obuna, J.; Daniyan, A.; Ekwedigwe, K. Metal and organic characterization of bladder stones removed surgically from VesicoVaginal Fistula patients at the National Obstetric Fistula Centre, Abakaliki, Ebonyi State. Arch. Urol. Res. 2022, 6, 1–13. [Google Scholar] [CrossRef]
- Altameme, M. Biochemical analysis of leaves for the species Fagonia burguieri DC., Zygophyllum coccineum L. Zygophyllum fabago L. (Zygophyllaceae) in Bahr al-Najaf depression in Iraq based on GC-Mass technology. Turk. J. Physiother. Rehabil. 2021, 32, 3. [Google Scholar]
- Musthafa, K.S.; Sahu, S.K.; Ravi, A.V.; Kathiresan, K. Anti-quorum sensing potential of the mangrove Rhizophora annamalayana. World J. Microbiol. Biotechnol. 2013, 29, 1851–1858. [Google Scholar] [CrossRef]
- Hamza, L.F.; Kadhim, S.A.H.; Hameed, I.H. Metabolites Produced by Burkholderia cepacia Using Gas Chromatography-Mass Spectrometry Technique and Determination of Its Anti-Fungal and Anti-Bacterial Activity. Indian J. Public Health Res. Dev. 2018, 9, 1173. [Google Scholar] [CrossRef]
- Selim, S.; Abdel Aziz, M.; Mashait, M.; Warrad, M. Antibacterial activities, chemical constitutes and acute toxicity of Egyptian Origanum majorana L., Peganum harmala L. and Salvia officinalis L. essential oils. Afr. J. Pharm. Pharmacol. 2013, 7, 725–735. [Google Scholar]
- Bagheri, G.; Ayatollahi, S.A.; Ramírez-Alarcón, K.; Fernández, M.; Salehi, B.; Forman, K.; Martorell, M.; Moghadam, M.H.; Sharifi-Rad, J. Phytochemical screening of Alstonia scholaris leaf and bark extracts and their antimicrobial activities. Cell. Mol. Biol. 2020, 66, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Pasdaran, A.; Pasdaran, A.; Mamedov, N. Antibacterial and antioxidant activities of the volatile composition of the flower and fruit of Solanum sisymbriifolium (Litchi Tomato). Pharm. Sci. 2017, 23, 66–71. [Google Scholar] [CrossRef]
- Meena, H.; Mishra, R.; Ranganathan, S.; Sarma, V.V.; Ampasala, D.R.; Kalia, V.C.; Lee, J.-K.; Siddhardha, B. Phomopsis tersa as Inhibitor of Quorum Sensing System and Biofilm Forming Ability of Pseudomonas aeruginosa. Indian J. Microbiol. 2020, 60, 70–77. [Google Scholar] [CrossRef]
- Youssef, A.M.M.; Maaty, D.A.M.; Al-Saraireh, Y.M. Phytochemistry and Anticancer Effects of Mangrove (Rhizophora mucronata Lam.) Leaves and Stems Extract against Different Cancer Cell Lines. Pharmaceuticals 2022, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Wigati, D.; Setyowati, E.P.; Pratiwi, S.U.T.; Nugraha, A.S. Promising sponge derived marine fungi as antibacterial and biofilm inhibitors. J. Appl. Pharm. Sci. 2024, 14, 014–034. [Google Scholar] [CrossRef]
- Fadare, O.; Durosinmi, O.; Fadare, R.; Izevbekhai, O.; Awonyemi, I.O.; Obafemi, C.A. ATR-FTIR and HPLC spectroscopic studies and evaluation of mineral content of Carica papaya leaves and flowers. J. Phytomed. 2015, 1, 1–7. [Google Scholar]
- Alam, M.W.; Al Qahtani, H.S.; Aamir, M.; Abuzir, A.; Khan, M.S.; Albuhulayqah, M.; Mushtaq, S.; Zaidi, N.; Ramya, A. Phyto synthesis of manganese-doped zinc nanoparticles using Carica papaya leaves: Structural properties and its evaluation for catalytic, antibacterial and antioxidant activities. Polymers 2022, 14, 1827. [Google Scholar] [CrossRef]
- Das, P.; Ghosh, S.; Baskey, M. Heterogeneous catalytic reduction of 4-nitroaniline by RGO-Ni nanocomposite for water resource management. J. Mater. Sci. Mater. Electron. 2019, 30, 19731–19737. [Google Scholar] [CrossRef]
- Tafu, N.N.; Jideani, V.A. Characterization of Novel Solid Dispersions of Moringa oleifera Leaf Powder Using Thermo-Analytical Techniques. Processes 2021, 9, 2230. [Google Scholar] [CrossRef]
- Bello, O.S.; Adegoke, K.A.; Akinyunni, O.O. Preparation and characterization of a novel adsorbent from Moringa oleifera leaf. Appl. Water Sci. 2017, 7, 1295–1305. [Google Scholar] [CrossRef]
- Correia, L.P.; Procópio, J.V.V.; de Santana, C.P.; Santos, A.F.O.; de Medeiros Cavalcante, H.M.; Macêdo, R.O. Characterization of herbal medicine with different particle sizes using pyrolysis GC/MS, SEM, and thermal techniques. J. Therm. Anal. Calorim. 2013, 111, 1691–1698. [Google Scholar] [CrossRef]
- Alencar Fernandes, F.; Santana, C.; Santos, R.; Correia, L.; Conceição, M.; Macêdo, R.; Medeiros, A.C. Thermal characterization of dried extract of medicinal plant by DSC and analytical techniques. J. Therm. Anal. Calorim. 2013, 113, 443–447. [Google Scholar] [CrossRef]
- Devi, D.R.; Battu, G.R. Qualitative phytochemical screening and ftir spectroscopic analysis of grewia tilifolia (vahl) leaf extracts. Int. J. Curr. Pharm. Res. 2019, 11, 100–107. [Google Scholar] [CrossRef]
- Khouchaf, L.; Boulahya, K.; Das, P.P.; Nicolopoulos, S.; Kis, V.K.; Lábár, J.L. Study of the microstructure of amorphous silica nanostructures using high-resolution electron microscopy, electron energy loss spectroscopy, X-ray powder diffraction, and electron pair distribution function. Materials 2020, 13, 4393. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liang, K.-h.; Liu, Q.; Qiu, J.; Wang, J.; Zhu, H. Superfine grinding affects physicochemical, thermal and structural properties of Moringa Oleifera leaf powders. Ind. Crop. Prod. 2020, 151, 112472. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X.; Wang, Y.; Jing, R.; Yue, F. Comparing physicochemical properties of hawthorn superfine and fine powders. J. Food Process. Preserv. 2017, 41, e12834. [Google Scholar] [CrossRef]
- Sakr, H.; Ammar, A.; Zaki, H.; Salama, M.A.; Ali, M. Impact of ball milling on physicochemical, structural, and functional properties of Moringa oleifera L. leaf powders. J. Food Meas. Charact. 2023, 18, 320–330. [Google Scholar] [CrossRef]
- Wan Nadirah, W.O.; Jawaid, M.; Al Masri, A.A.; Abdul Khalil, H.P.S.; Suhaily, S.S.; Mohamed, A.R. Cell wall morphology, chemical and thermal analysis of cultivated pineapple leaf fibres for industrial applications. J. Polym. Environ. 2012, 20, 404–411. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Wadhwa, S.S. Industry-relevant approaches for minimising the bitterness of bioactive compounds in functional foods: A review. Food Bioprocess Technol. 2013, 6, 607–627. [Google Scholar] [CrossRef]
- Patil, N.D.; Bains, A.; Sridhar, K.; Rashid, S.; Kaur, S.; Ali, N.; Chawla, P.; Sharma, M. Effect of Sustainable Pretreatments on the Nutritional and Functionality of Chickpea Protein: Implication for Innovative Food Product Development. J. Food Biochem. 2024, 2024, 5173736. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Sansone, F.; Mencherini, T.; Picerno, P.; Esposito, T.; Del Gaudio, P.; Russo, P.; Pepe, G.; Lauro, M.R.; Aquino, R.P. Microencapsulation by spray drying of Lannea microcarpa extract: Technological characteristics and antioxidant activity. J. Pharm. Pharmacogn. Res. 2014, 2, 100–109. [Google Scholar] [CrossRef]
- Veeramachineni, A.K.; Sathasivam, T.; Muniyandy, S.; Janarthanan, P.; Langford, S.J.; Yan, L.Y. Optimizing extraction of cellulose and synthesizing pharmaceutical grade carboxymethyl sago cellulose from malaysian sago pulp. Appl. Sci. 2016, 6, 170. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, L.; Li, M.-C.; Wu, Q.; Zhou, D. Cellulose nanocrystals (CNCs) from corn stalk: Activation energy analysis. Materials 2017, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Callixte, C.; Baptiste, N.J.; Arwati, H. Phytochemical screening and antimicrobial activities of methanolic and aqueous leaf extracts of Carica papaya grown in Rwanda. Mol. Cell. Biomed. Sci. 2020, 4, 39–44. [Google Scholar] [CrossRef]
- Khadam, S.; Afzal, U.; Gul, H.; Hira, S.; Satti, M.; Yaqub, A.; Ajab, H.; Gulfraz, M. Phytochemical screening and bioactivity assessment of leaves and fruits extract of Carica papaya. Pak. J. Pharm. Sci. 2019, 32, 1941–1948. [Google Scholar]
- Medawati, A.; Andriani, I.; Rahmawati, A.D.; Hidayati, N. The Activity of Active Compounds of Papaya Leaf (Carica Papaya L.) in Inhibiting the Growth of Fungus Candida Albicans in the Oral Cavity. Formosa J. Sustain. Res. 2023, 2, 1717–1728. [Google Scholar] [CrossRef]

| S. No. | Parameter | Concentration |
|---|---|---|
| 1 | Moisture (g/100 g) | 15.16 ± 1.15 |
| 2 | Ash (g/100 g) | 3.73 ± 0.21 |
| 3 | Fat (g/100 g) | 4.50 ± 0.86 |
| 4 | Crude fiber (g/100 g) | 9.06 ± 0.92 |
| 5 | Crude protein (g/100 g) | 25.75 ± 0.10 |
| 6 | Total soluble protein (mg/g) | 52.37 ± 0.28 |
| 7 | Carbohydrates (g/100 g) | 41.49 ± 0.10 |
| 8 | Ca (mg/kg) | 1079 ± 0.75 |
| 9 | Cu (mg/kg) | 13.24 ± 0.01 |
| 10 | Fe (mg/kg) | 228.2 ± 0.08 |
| 11 | K (mg/kg) | 4071 ± 0.41 |
| 12 | Mg (mg/kg) | 789.2 ± 0.32 |
| 13 | Mn (mg/kg) | 22.15 ± 0.01 |
| 14 | Zn (mg/kg) | 70.92 ± 0.04 |
| 15 | Na (mg/kg) | 361.2 ± 1.74 |
| 16 | Total phenolic content (mg GAE/g) | 8.85 ± 0.01 |
| 17 | Total flavonoid content (mg QE/g) | 21.00 ± 0.18 |
| 18 | Tannin content (mg TAE/g) | 430 ± 0.08 |
| 19 | Alkaloid content (g/100 g) | 11.4 ± 0.10 |
| 20 | DPPH (%) | 77.55 ± 2.21 |
| 21 | FRAP (mmol/mg) | 25.34 ± 2.45 |
| S. No | Name | Formula | RT | Area (%) | Biological Effects | References |
|---|---|---|---|---|---|---|
| 1 | Hydroperoxide, hexyl | C6H14O2 | 4.01 | 15.69 | Antioxidant | [32] |
| 2 | cyclopentasiloxane, decamethyl | C5H10O | 8.28 | 3.32 | Antiviral | [33] |
| 3 | Methane, isothiocyanato- | C2H3NS | 12.00 | 1.41 | Antimicrobial | [34] |
| 4 | cyclohexasiloxane, decamethyl | C12H36O6Si6 | 12.87 | 5.51 | Antimicrobial | [35] |
| 5 | 1,1,1,3,5,5,7,7,7-Nonamethyl-3(trimethyl siloxy) tetrasiloxane | C12H36O4Si5 | 15.90 | 2.90 | Antimicrobial | [36] |
| 6 | 4-Aminosalicylic acid, 3TMS derivative | C13H33NO2Si3 | 18.41 | 2.69 | Antitubercular | [37] |
| 7 | 1,1,1,5,7,7,7-Heptamethyl-3,3-bis (trimethylsiloxy) tetrasiloxane | C13H39O5Si6 | 20.53 | 2.05 | Antiquorum | [38,39] |
| 8 | Piperazine, 1-nitroso | C4H9N3O | 22.75 | 1.21 | Antibacterial, Antifungal | [40] |
| 9 | Heptane, 4-azido- | C7H15N3 | 24.81 | 3.48 | Antibacterial | [41] |
| 10 | 1,1,1,3,5,7,7,7-Octamethyl-3,5-bis (trimethyl siloxy) tetrasiloxane | C14H42O5Si6 | 25.99 | 1.09 | Antimicrobial | [42] |
| 11 | 9,12,15-Octadecatrienal | C18H30O | 28.97 | 11.25 | Antioxidant | [43] |
| 12 | Ethanethioic acid, S-(2-methyl butyl) ester | C7H14OS | 29.38 | 2.99 | Antimicrobial | [44] |
| 13 | 1,1,1,3,5,5,7,7,7-Nonamethyl-3- (trimethyl siloxy) tetrasiloxane | C12H36O4Si5 | 31.13 | 1.29 | Antimicrobial | [36] |
| 14 | 1,1,1,3,5,7,7,7-Octamethyl-3,5-bis (trimethyl siloxy) tetrasiloxane | C14H42O5Si6 | 32.99 | 1.21 | Antimicrobial | [42] |
| 15 | Hexasiloxane, tetradecamethyl | C14H42O5Si6 | 35.22 | 1.11 | Anticancer | [45] |
| 16 | 1,1,1,3,5,5,7,7,7-Nonamethyl-3- (trimethyl siloxy) tetra siloxane | C12H36O4Si5 | 38.16 | 0.89 | Antimicrobial | [36] |
| 17 | 1H-1,2,4-Triazole-3-carboxaldehyde, 5-methyl | C4H5N3O | 39.94 | 1.07 | Antifungal | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhary, R.; Kaushik, R.; Akhtar, A.; Manna, S.; Sharma, J.; Bains, A. Nutritional, Phytochemical, and Antimicrobial Properties of Carica papaya Leaves: Implications for Health Benefits and Food Applications. Foods 2025, 14, 154. https://doi.org/10.3390/foods14020154
Choudhary R, Kaushik R, Akhtar A, Manna S, Sharma J, Bains A. Nutritional, Phytochemical, and Antimicrobial Properties of Carica papaya Leaves: Implications for Health Benefits and Food Applications. Foods. 2025; 14(2):154. https://doi.org/10.3390/foods14020154
Chicago/Turabian StyleChoudhary, Rajni, Ravinder Kaushik, Ansab Akhtar, Suvendu Manna, Jyoti Sharma, and Aarti Bains. 2025. "Nutritional, Phytochemical, and Antimicrobial Properties of Carica papaya Leaves: Implications for Health Benefits and Food Applications" Foods 14, no. 2: 154. https://doi.org/10.3390/foods14020154
APA StyleChoudhary, R., Kaushik, R., Akhtar, A., Manna, S., Sharma, J., & Bains, A. (2025). Nutritional, Phytochemical, and Antimicrobial Properties of Carica papaya Leaves: Implications for Health Benefits and Food Applications. Foods, 14(2), 154. https://doi.org/10.3390/foods14020154








