Combined BPA and DIBP Exposure Induced Intestinal Mucosal Barrier Impairment Through the Notch Pathway and Gut Microbiota Dysbiosis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Experiments
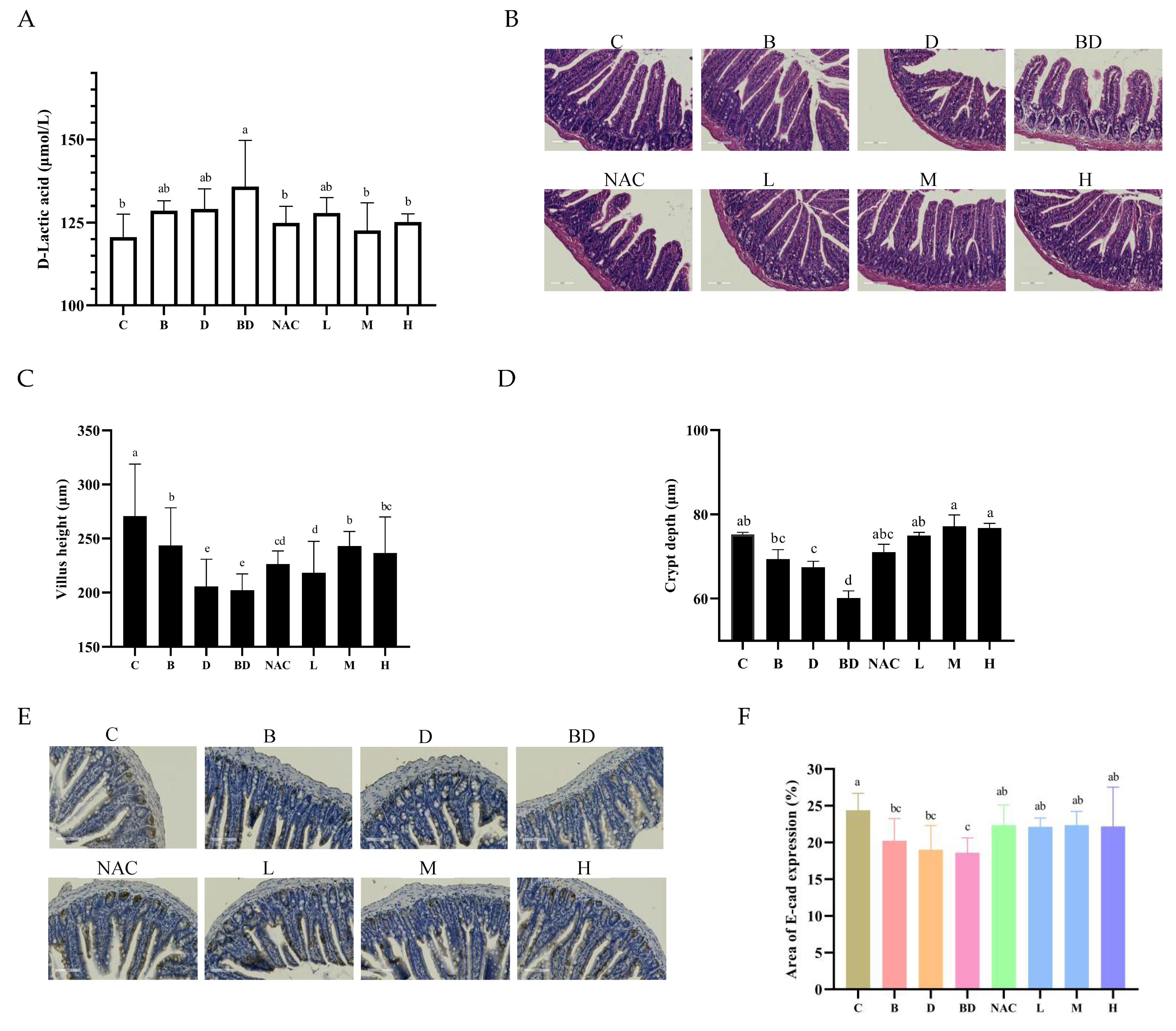
2.3. Determination of Intestinal Permeability
2.4. Histopathological Observation
2.5. Immunohistochemical Analysis
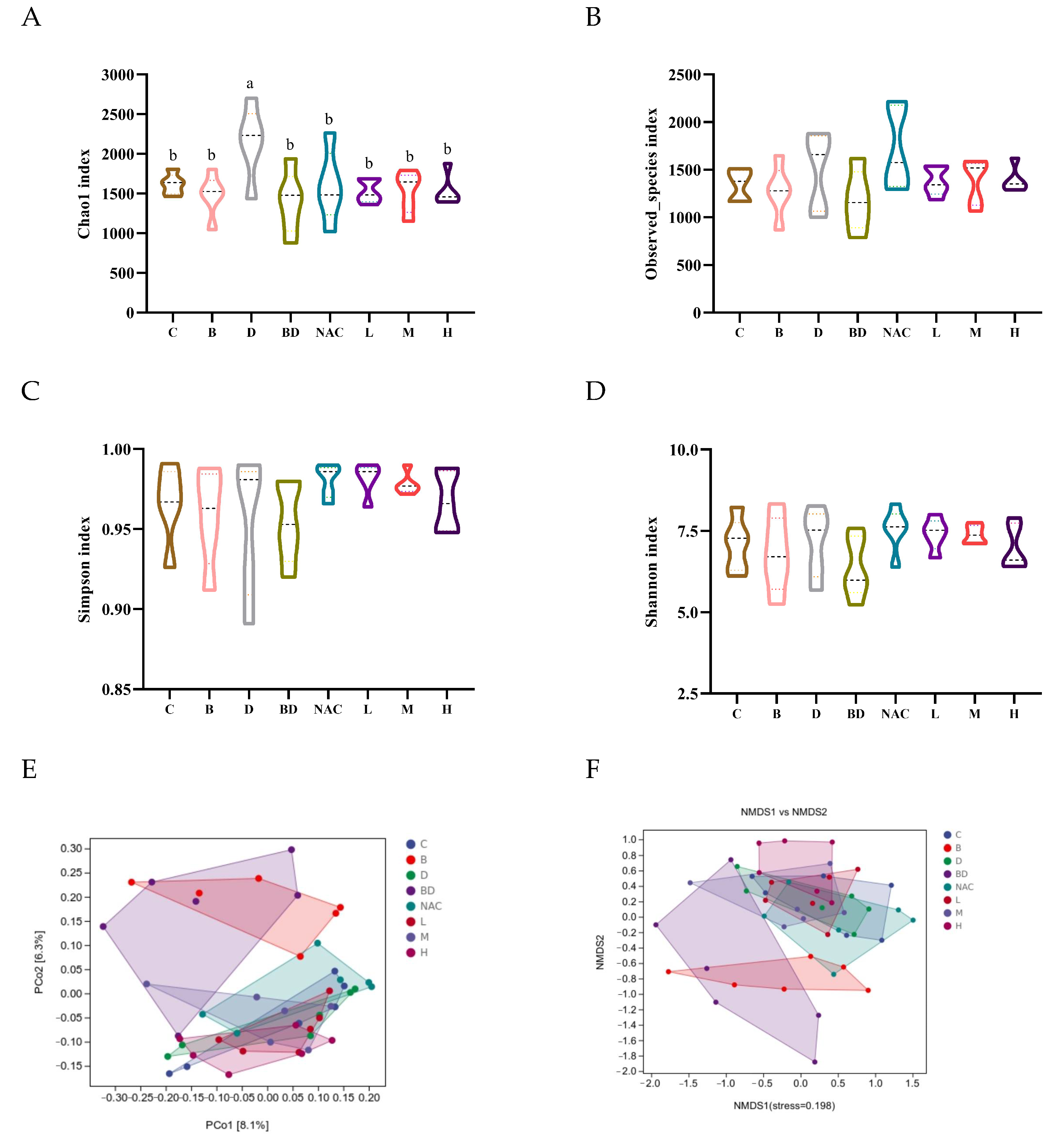
2.6. Gut Microbiota
2.7. Determination of Contents of Short-Chain Fatty Acids (SCFAs)
2.8. Cell Culture and Solution Preparation
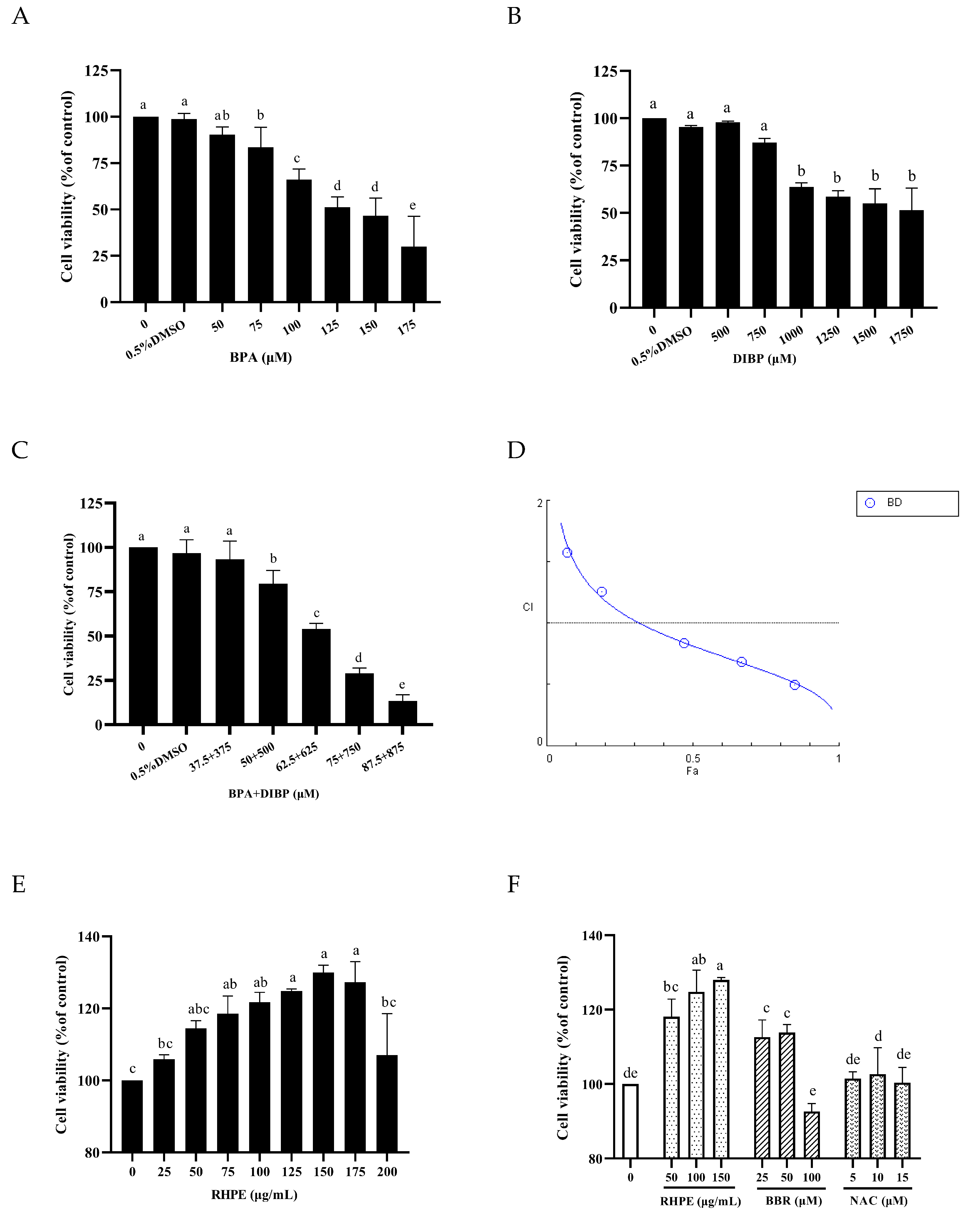
2.9. Cell Viability
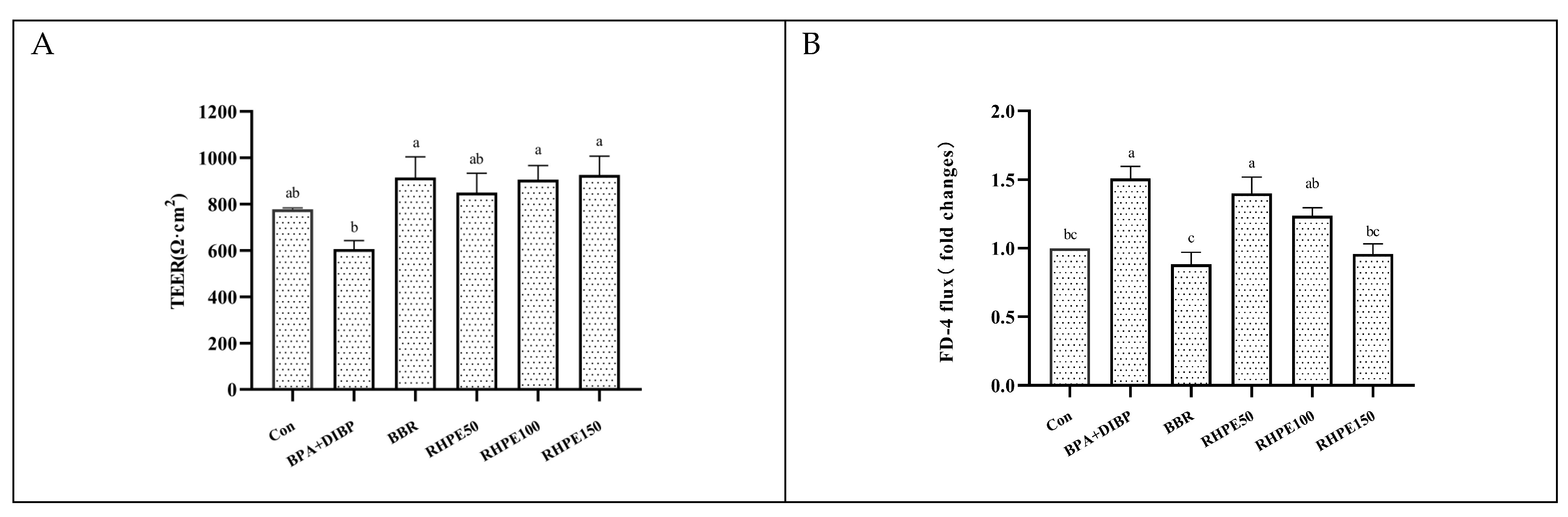
2.10. Monolayer Integrity
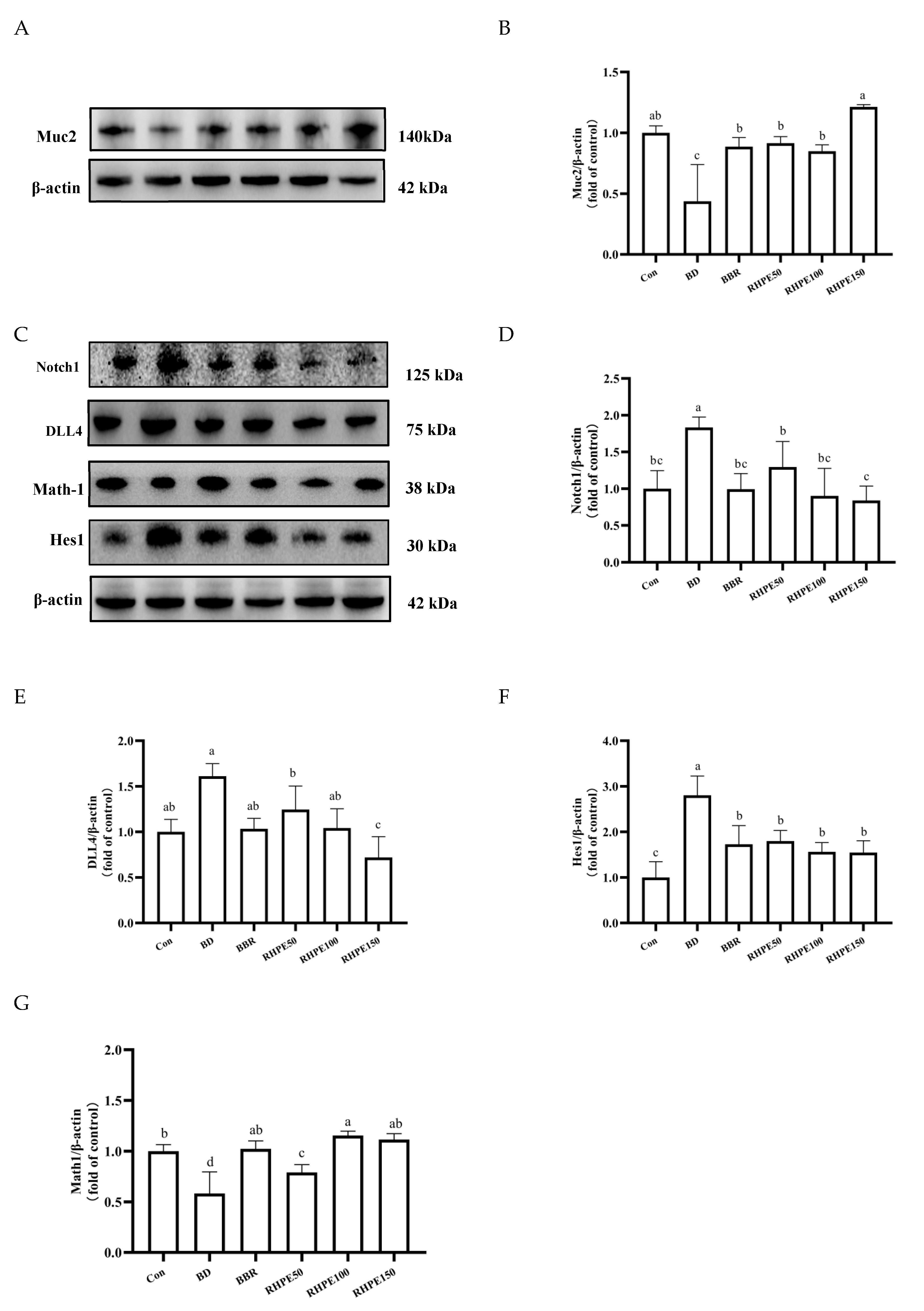
2.11. Western Blotting
2.12. Statistical Analysis
3. Results
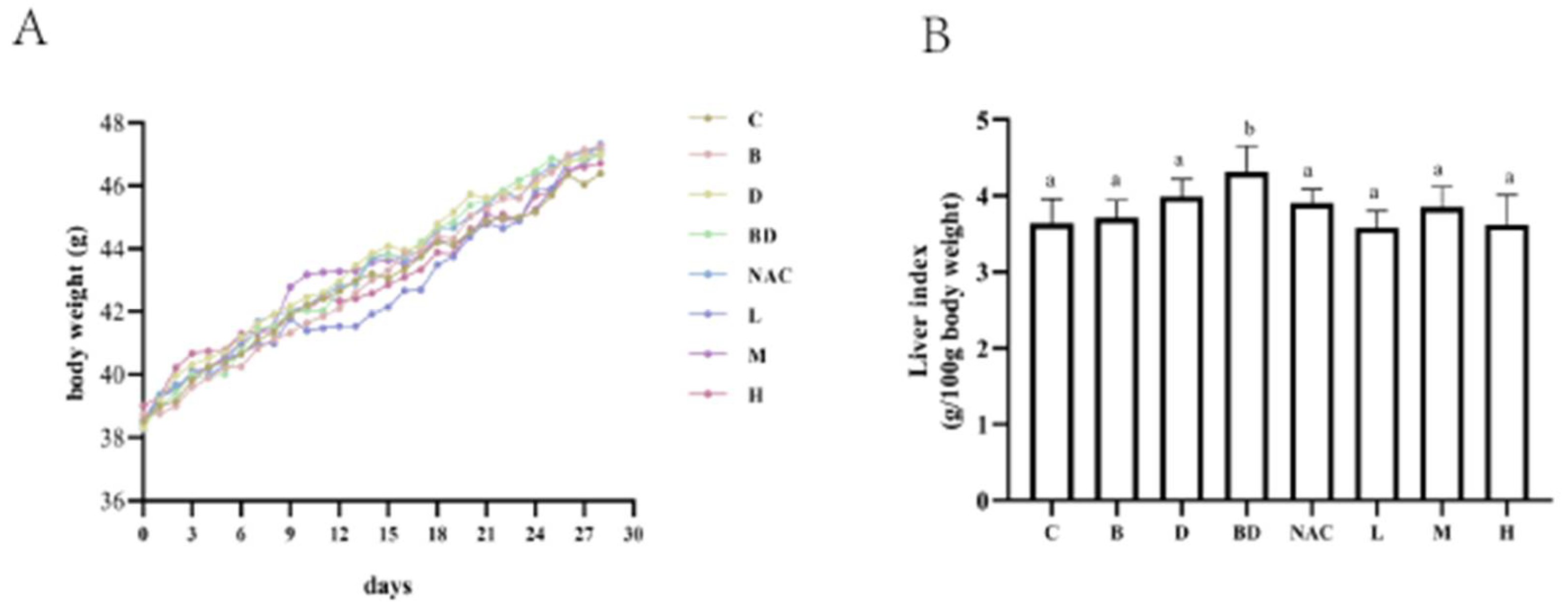
3.1. Effects of RHPE Interventions on Body Weight and Organ Index
3.2. Effects of RHPE Interventions on Intestinal Histopathology
3.3. RHPE Facilitated the Proliferation and Differentiation of ISCs
3.4. RHPE Protected Against BPA- and DIBP-Induced Intestinal Injury In Vitro
3.5. RHPE Improved Cell Barrier Integrity and Permeability of Caco-2/HT29-MTX
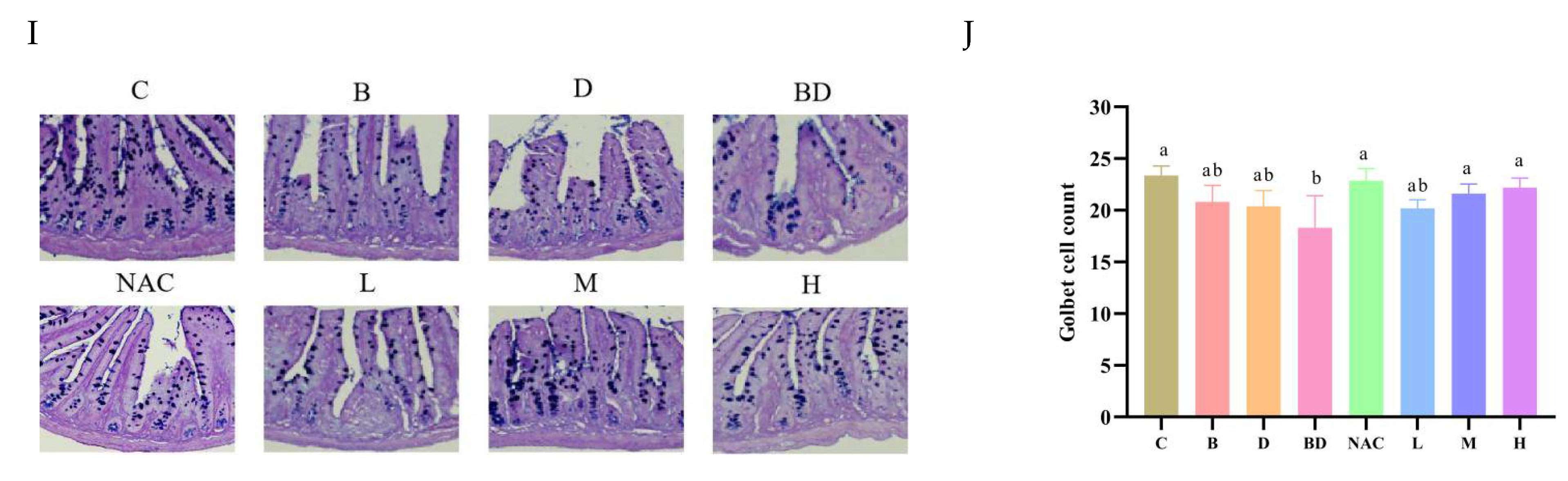
3.6. RHPE Alleviated Goblet Cell Damage Through Notch Pathway
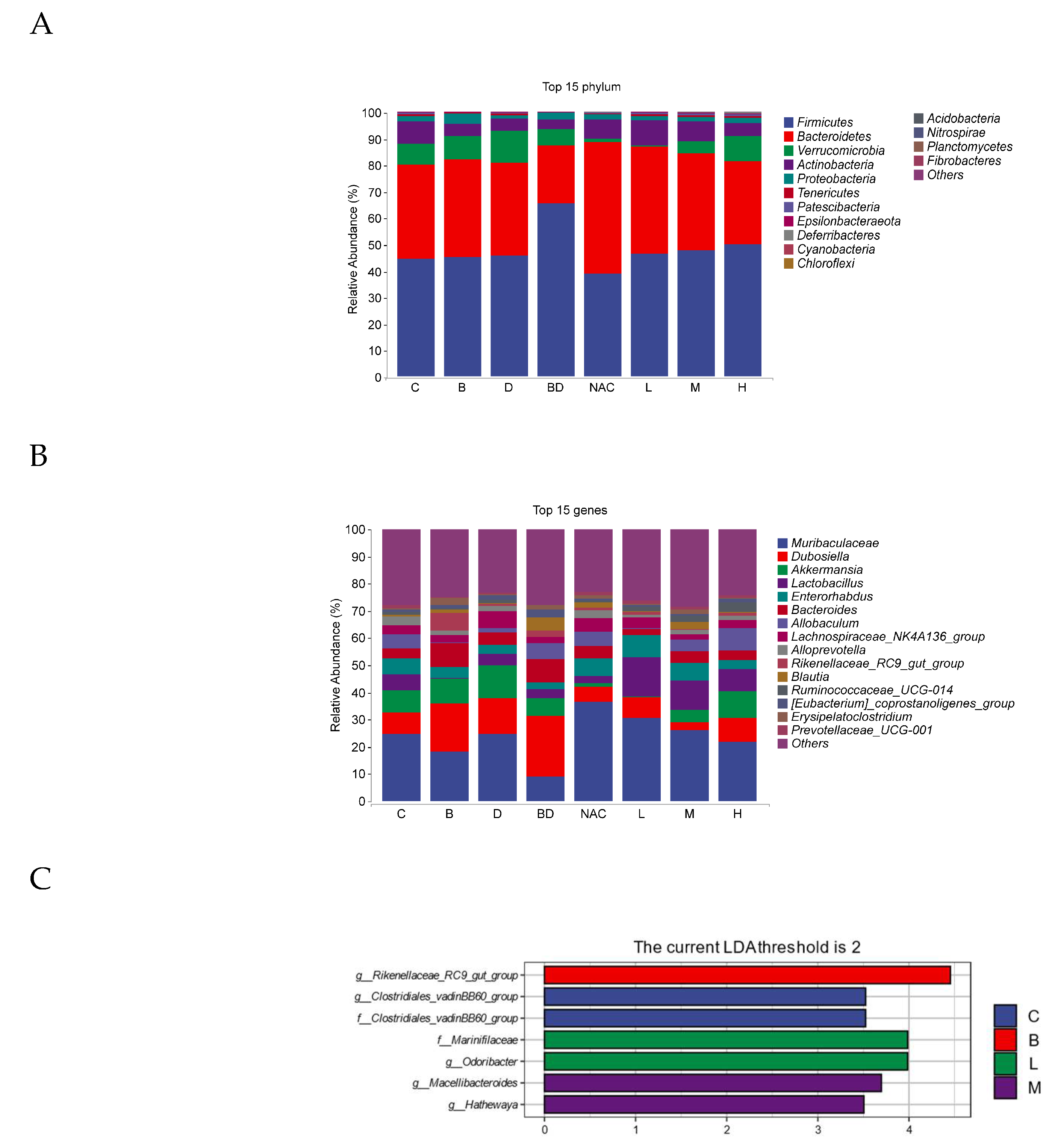
3.7. Effect of RHPE on Gut Microbiota
3.8. RHPE Improved SCFAs Abundance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Han, D.; Yao, Y.; Chen, L.; Miao, Z.; Xu, S. Apigenin ameliorates di(2-ethylhexyl) phthalate-induced ferroptosis: The activation of glutathione peroxidase 4 and suppression of iron intake. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2022, 164, 113089. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Alomirah, H.; Cho, H.S.; Minh, T.B.; Mohd, M.A.; Nakata, H.; Kannan, K. Occurrence of phthalate metabolites in human urine from several Asian countries. Environ. Sci. Technol. 2011, 45, 3138–3144. [Google Scholar] [CrossRef] [PubMed]
- Saillenfait, A.M.; Sabaté, J.P.; Gallissot, F. Developmental toxic effects of diisobutyl phthalate, the methyl-branched analogue of di-n-butyl phthalate, administered by gavage to rats. Toxicol. Lett. 2006, 165, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Du, X.; Chen, H.; Bai, C.; Lan, L. Systemic investigation of di-isobutyl phthalate (DIBP) exposure in the risk of cardiovascular via influencing the gut microbiota arachidonic acid metabolism in obese mice model. Regen. Ther. 2024, 27, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; et al. Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. Eur. Food Saf. Auth. 2023, 21, e06857. [Google Scholar] [CrossRef]
- Chen, W.Y.; Shen, Y.P.; Chen, S.C. Assessing bisphenol A (BPA) exposure risk from long-term dietary intakes in Taiwan. Sci. Total Environ. 2016, 543, 140–146. [Google Scholar] [CrossRef]
- Yadav, S.K.; Kumar, A.; Yadav, B.G.; Bijalwan, V.; Yadav, S.; Patil, G.P.; Sarkar, K.; Palkhade, R.; Das, S.; Singh, D.P. Sub-acute bisphenol A exposure induces proteomic alterations and impairs male reproductive health in mice. J. Biochem. Mol. Toxicol. 2024, 38, e23862. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Huang, Q.; Chi, Y.; Dong, S.; Fan, J. Bisphenol-A induces neurodegeneration through disturbance of intracellular calcium homeostasis in human embryonic stem cells-derived cortical neurons. Chemosphere 2019, 229, 618–630. [Google Scholar] [CrossRef]
- Zhu, M.; Zeng, R.; Wu, D.; Li, Y.; Chen, T.; Wang, A. Research progress of the effects of bisphenol analogues on the intestine and its underlying mechanisms: A review. Environ. Res. 2024, 243, 117891. [Google Scholar] [CrossRef]
- Yost, E.E.; Euling, S.Y.; Weaver, J.A.; Beverly, B.E.J.; Keshava, N.; Mudipalli, A.; Arzuaga, X.; Blessinger, T.; Dishaw, L.; Hotchkiss, A.J.E.I. Hazards of diisobutyl phthalate (DIBP) exposure: A systematic review of animal toxicology studies. Environ. Int. 2018, 125, 579–594. [Google Scholar] [CrossRef]
- Xiong, Z.; Zeng, Y.; Zhou, J.; Shu, R.; Xie, X.; Fu, Z. Exposure to dibutyl phthalate impairs lipid metabolism and causes inflammation via disturbing microbiota-related gut–liver axis. Acta Biochim. Biophys. Sin. 2020, 52, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Mustari, A.; Alam, M.; Miah, M.; Sujan, K.; Mahamud, A.; Chowdhury, E.H.C. Therapeutics, E. Restoration of hepatorenal dysfunction and injury by zinc and folic acid combination in bisphenol A-intoxicated mice. J. Adv. Biotechnol. Exp. Ther. 2023, 6, 541–551. [Google Scholar] [CrossRef]
- Javurek, A.B.; Spollen, W.G.; Johnson, S.A.; Bivens, N.J.; Bromert, K.H.; Givan, S.A.; Rosenfeld, C.S. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes 2016, 7, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhao, Z.; Ji, W. Bisphenol A induces apoptosis, oxidative stress and inflammatory response in colon and liver of mice in a mitochondria-dependent manner. Biomed. Pharmacother. 2019, 117, 109182. [Google Scholar] [CrossRef]
- Wang, X.; Sheng, N.; Cui, R.; Zhang, H.; Wang, J.; Dai, J. Gestational and lactational exposure to di-isobutyl phthalate via diet in maternal mice decreases testosterone levels in male offspring. Chemosphere 2017, 172, 260–267. [Google Scholar] [CrossRef]
- Ding, Q.; Guo, R.; Pei, L.; Lai, S.; Li, J.; Yin, Y.; Xu, T.; Yang, W.; Song, Q.; Han, Q.; et al. N-Acetylcysteine alleviates high fat diet-induced hepatic steatosis and liver injury via regulating the intestinal microecology in mice. Food Funct. 2022, 13, 3368–3380. [Google Scholar] [CrossRef]
- Lee, S.I.; Kang, K.S. N-acetylcysteine modulates lipopolysaccharide-induced intestinal dysfunction. Sci. Rep. 2019, 9, 1004. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Zhang, Q.; Qiu, C.; Jiang, W.; Feng, P.; Xue, X.; Bukhari, I.; Mi, Y.; Zheng, P. The impact of dioctyl phthalate exposure on multiple organ systems and gut microbiota in mice. Heliyon 2023, 9, e22677. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Sheng, J.Y.; Wang, S.Q.; Liu, K.H.; Zhu, B.; Zhang, Q.Y.; Qin, L.P.; Wu, J.J. Rubus chingii Hu: An overview of botany, traditional uses, phytochemistry, and pharmacology. Chin. J. Nat. Med. 2020, 18, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Jin, W.; Lin, J.; Wang, Z.; Ma, Y.; Zhang, W.; Zhu, Y.; Hu, Y.; Qu, Q.; Guo, S. Forsythia suspensa polyphenols regulate macrophage M1 polarization to alleviate intestinal inflammation in mice. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 125, 155336. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ru, M.; Zhai, Z.; Huang, J.; Wang, W.; Wang, R.; Zhang, Z.; Niu, K.M.; Wu, X. In vitro antibacterial effects of Broussonetia papyrifera leaf extract and its anti-colitis in DSS-treated mice. Front. Cell. Infect. Microbiol. 2023, 13, 1255127. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Wang, Y.; Li, C.; Yu, Q.; Xie, J.; Dong, R.; Xie, Y.; Li, B.; Tian, J.; Chen, Y. Natural variation on free, esterified, glycosylated and insoluble-bound phenolics of Rubus chingii Hu: Correlation between phenolic constituents and antioxidant activities. Food Res. Int. 2022, 162, 112043. [Google Scholar] [CrossRef]
- Wenzel, U.A.; Magnusson, M.K.; Rydström, A.; Jonstrand, C.; Hengst, J.; Johansson, M.E.; Velcich, A.; Öhman, L.; Strid, H.; Sjövall, H.; et al. Spontaneous colitis in Muc2-deficient mice reflects clinical and cellular features of active ulcerative colitis. PLoS ONE 2014, 9, e100217. [Google Scholar] [CrossRef]
- Béduneau, A.; Tempesta, C.; Fimbel, S.; Pellequer, Y.; Jannin, V.; Demarne, F.; Lamprecht, A. A tunable Caco-2/HT29-MTX co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure. Eur. J. Pharm. Biopharm. 2014, 87, 290–298. [Google Scholar] [CrossRef]
- Pan, F.; Han, L.; Zhang, Y.; Yu, Y.; Liu, J. Optimization of Caco-2 and HT29 co-culture in vitro cell models for permeability studies. Int. J. Food Sci. Nutr. 2015, 66, 680–685. [Google Scholar] [CrossRef]
- Ferraretto, A.; Bottani, M.; De Luca, P.; Cornaghi, L.; Arnaboldi, F.; Maggioni, M.; Fiorilli, A.; Donetti, E. Morphofunctional properties of a differentiated Caco2/HT-29 co-culture as an in vitro model of human intestinal epithelium. Biosci. Rep. 2018, 38, BSR20171497. [Google Scholar] [CrossRef]
- Burclaff, J.; Bliton, R.J.; Breau, K.A.; Ok, M.T.; Gomez-Martinez, I.; Ranek, J.S.; Bhatt, A.P.; Purvis, J.E.; Woosley, J.T.; Magness, S.T. A Proximal-to-Distal Survey of Healthy Adult Human Small Intestine and Colon Epithelium by Single-Cell Transcriptomics. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1554–1589. [Google Scholar] [CrossRef]
- Nikitas, G.; Deschamps, C.; Disson, O.; Niault, T.; Cossart, P.; Lecuit, M. Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. J. Exp. Med. 2011, 208, 2263–2277. [Google Scholar] [CrossRef]
- Nyström, E.E.L.; Martinez-Abad, B.; Arike, L.; Birchenough, G.M.H.; Nonnecke, E.B.; Castillo, P.A.; Svensson, F.; Bevins, C.L.; Hansson, G.C.; Johansson, M.E.V. An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function. Sci. 2021, 372, 6539. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kang, W.; Mao, X.; Du, H.; Ge, L.; Hou, L.; Yuan, X.; Wang, M.; Chen, X.; Liu, Y.; et al. Low dose of arsenic exacerbates toxicity to mice and IPEC-J2 cells exposed with deoxynivalenol: Aryl hydrocarbon receptor and autophagy might be novel therapeutic targets. Sci. Total Environ. 2022, 832, 155027. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Y.; Wei, R.; Napier, D.L.; Sengoku, T.; Alstott, M.C.; Liu, J.; Wang, C.; Zaytseva, Y.Y.; Weiss, H.L.; et al. Glycolytic Regulation of Intestinal Stem Cell Self-Renewal and Differentiation. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 931–947. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Stanton, C.; Ross, R.P.; Zhao, J.; Chen, W.; Yang, B. Exopolysaccharides Produced by Bifidobacterium longum subsp. longum YS108R Ameliorates DSS-Induced Ulcerative Colitis in Mice by Improving the Gut Barrier and Regulating the Gut Microbiota. J. Agric. Food Chem. 2024, 72, 7055–7073. [Google Scholar] [CrossRef]
- Xie, L.; Chen, T.; Qi, X.; Li, H.; Xie, J.; Wang, L.; Xie, J.; Huang, Z. Exopolysaccharides from Genistein-Stimulated Monascus purpureus Ameliorate Cyclophosphamide-Induced Intestinal Injury via PI3K/AKT-MAPKs/NF-κB Pathways and Regulation of Gut Microbiota. J. Agric. Food Chem. 2023, 71, 12986–13002. [Google Scholar] [CrossRef]
- Lu, J.; Su, D.; Yang, Y.; Shu, M.; Wang, Y.; Zhou, X.; Yu, Q.; Li, C.; Xie, J.; Chen, Y. Disruption of intestinal epithelial permeability in the Co-culture system of Caco-2/HT29-MTX cells exposed individually or simultaneously to acrylamide and ochratoxin A. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2024, 186, 114582. [Google Scholar] [CrossRef]
- Yuan, J.; Che, S.; Ruan, Z.; Song, L.; Tang, R.; Zhang, L. Regulatory effects of flavonoids luteolin on BDE-209-induced intestinal epithelial barrier damage in Caco-2 cell monolayer model. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2021, 150, 112098. [Google Scholar] [CrossRef]
- Pohanka, M. D-Lactic Acid as a Metabolite: Toxicology, Diagnosis, and Detection. BioMed Res. Int. 2020, 2020, 3419034. [Google Scholar] [CrossRef]
- Perry, J.K.; Lins, R.J.; Lobie, P.E.; Mitchell, M.D. Regulation of invasive growth: Similar epigenetic mechanisms underpin tumour progression and implantation in human pregnancy. Clin. Sci. 2009, 118, 451–457. [Google Scholar] [CrossRef] [PubMed]
- van der Post, S.; Jabbar, K.S.; Birchenough, G.; Arike, L.; Akhtar, N.; Sjovall, H.; Johansson, M.E.V.; Hansson, G.C. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 2019, 68, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Qureshi, M.Z.; Khalid, S.; Attar, R.; Martinelli, C.; Sabitaliyevich, U.Y.; Nurmurzayevich, S.B.; Taverna, S.; Poltronieri, P.; Xu, B. Regulation of Cell Signaling Pathways by Berberine in Different Cancers: Searching for Missing Pieces of an Incomplete Jig-Saw Puzzle for an Effective Cancer Therapy. Cancers 2019, 11, 478. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Ahmadi, Z.; Tavakol, S.; Ashrafizadeh, M. Berberine as a potential autophagy modulator. J. Cell. Physiol. 2019, 234, 14914–14926. [Google Scholar] [CrossRef]
- Dong, Y.; Fan, H.; Zhang, Z.; Jiang, F.; Li, M.; Zhou, H.; Guo, W.; Zhang, Z.; Kang, Z.; Gui, Y.; et al. Berberine ameliorates DSS-induced intestinal mucosal barrier dysfunction through microbiota-dependence and Wnt/β-catenin pathway. Int. J. Biol. Sci. 2022, 18, 1381–1397. [Google Scholar] [CrossRef]
- Yang, Y.N.; Han, B.; Zhang, M.Q.; Chai, N.N.; Yu, F.L.; Qi, W.H.; Tian, M.Y.; Sun, D.Z.; Huang, Y.; Song, Q.X.; et al. Therapeutic effects and mechanisms of isoxanthohumol on DSS-induced colitis: Regulating T cell development, restoring gut microbiota, and improving metabolic disorders. Inflammopharmacology 2024, 32, 1983–1998. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, Z.; Shen, R.; Wang, M.; Huang, Y.; Ren, H.; Zhang, Y.; Lemos, B. Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environ. Int. 2020, 143, 105916. [Google Scholar] [CrossRef]
- Du, H.; Wang, Q.; Li, T.; Ren, D.; Yang, X. Grape seed proanthocyanidins reduced the overweight of C57BL/6J mice through modulating adipose thermogenesis and gut microbiota. Food Funct. 2021, 12, 8467–8477. [Google Scholar] [CrossRef]
- Zhong, X.; Zhao, Y.; Huang, L.; Liu, J.; Wang, K.; Gao, X.; Zhao, X.; Wang, X. Remodeling of the gut microbiome by Lactobacillus johnsonii alleviates the development of acute myocardial infarction. Front. Microbiol. 2023, 14, 1140498. [Google Scholar] [CrossRef]
- Zhang, T.; Ji, X.; Lu, G.; Zhang, F. The potential of Akkermansia muciniphila in inflammatory bowel disease. Appl. Microbiol. Biotechnol. 2021, 105, 5785–5794. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Wu, C.C.; Huang, C.L.; Chang, M.Y.; Cheng, S.H.; Lin, C.T.; Tsai, Y.C. Lactobacillus plantarum PS128 Promotes Intestinal Motility, Mucin Production, and Serotonin Signaling in Mice. Probiotics Antimicrob. Proteins 2022, 14, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Dolan, B.; Ermund, A.; Martinez-Abad, B.; Johansson, M.E.V.; Hansson, G.C. Clearance of small intestinal crypts involves goblet cell mucus secretion by intracellular granule rupture and enterocyte ion transport. Sci. Signal. 2022, 15, eabl5848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, X.; Liu, L.; Guo, M.; Huang, T.; Zhou, W.; Geng, F.; Cui, S.W.; Nie, S. Glucomannan from Aloe vera Gel Promotes Intestinal Stem Cell-Mediated Epithelial Regeneration via the Wnt/β-Catenin Pathway. J. Agric. Food Chem. 2021, 69, 10581–10591. [Google Scholar] [CrossRef]
- Jiang, H.; Shi, G.F.; Fang, Y.X.; Liu, Y.Q.; Wang, Q.; Zheng, X.; Zhang, D.J.; Zhang, J.; Yin, Z.Q. Aloin A prevents ulcerative colitis in mice by enhancing the intestinal barrier function via suppressing the Notch signaling pathway. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 106, 154403. [Google Scholar] [CrossRef]
- Johansson, M.E.; Gustafsson, J.K.; Holmén-Larsson, J.; Jabbar, K.S.; Xia, L.; Xu, H.; Ghishan, F.K.; Carvalho, F.A.; Gewirtz, A.T.; Sjövall, H.; et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014, 63, 281–291. [Google Scholar] [CrossRef]
- Sallé, J.; Gervais, L.; Boumard, B.; Stefanutti, M.; Siudeja, K.; Bardin, A.J. Intrinsic regulation of enteroendocrine fate by Numb. EMBO J. 2017, 36, 1928–1945. [Google Scholar] [CrossRef]
- Yin, X.; Farin, H.F.; van Es, J.H.; Clevers, H.; Langer, R.; Karp, J.M. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat. Methods 2014, 11, 106–112. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, H.; Matjeke, R.S.; Zhao, J.; Yu, Q. Bacillus coagulans protect against Salmonella enteritidis-induced intestinal mucosal damage in young chickens by inducing the differentiation of goblet cells. Poult. Sci. 2022, 101, 101639. [Google Scholar] [CrossRef]
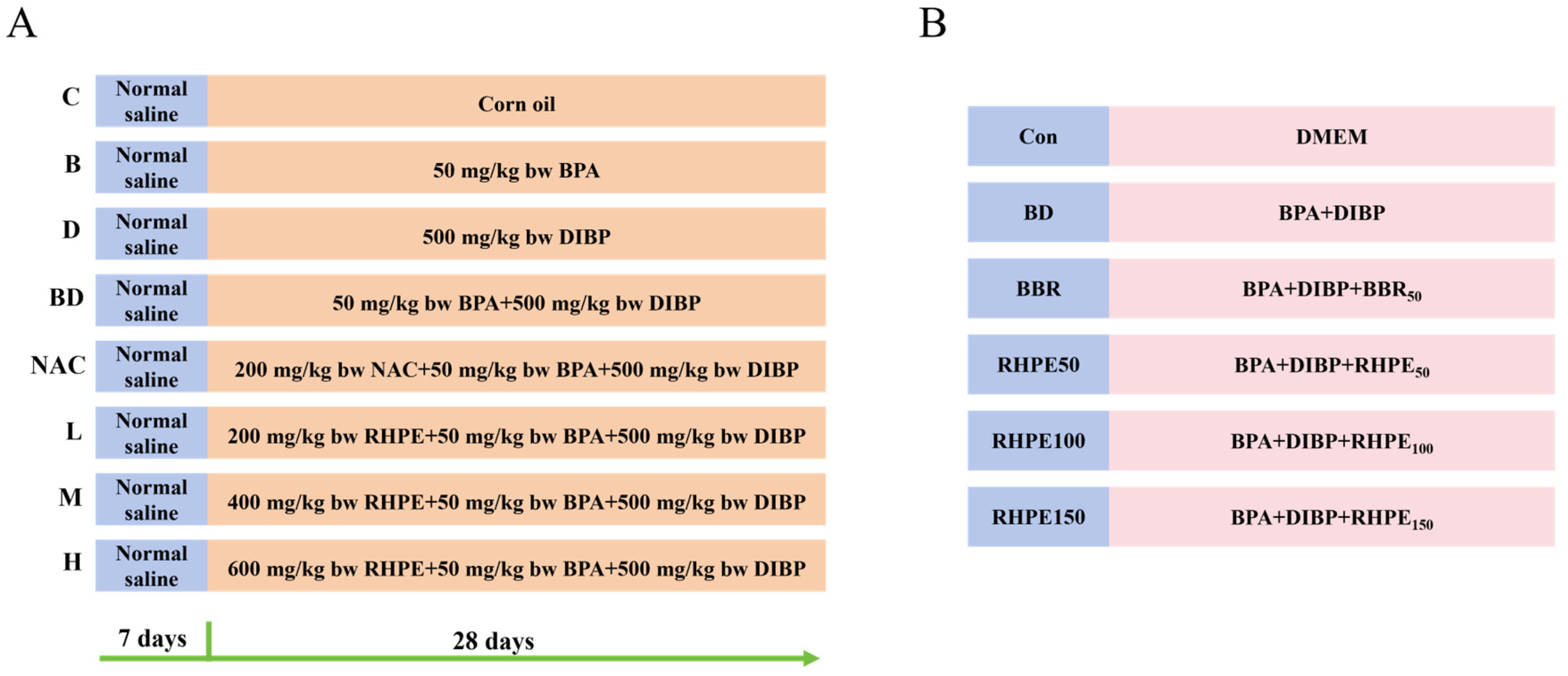


| Group | Days 1–7 | Days 8–28 | |
|---|---|---|---|
| 8:30 a.m. | 10:30 a.m. | ||
| C | Normal saline | Corn oil | Corn oil |
| B | Corn oil | BPA | |
| D | Corn oil | DIBP | |
| BD | Corn oil | BPA + DIBP | |
| NAC | NAC | BPA + DIBP | |
| L | RHPE200 | BPA + DIBP | |
| M | RHPE400 | BPA + DIBP | |
| H | RHPE600 | BPA + DIBP | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, M.; Wang, Y.; Chen, S.; Lu, J.; Dong, R.; Yu, Q.; Xie, J.; Chen, Y. Combined BPA and DIBP Exposure Induced Intestinal Mucosal Barrier Impairment Through the Notch Pathway and Gut Microbiota Dysbiosis in Mice. Foods 2025, 14, 214. https://doi.org/10.3390/foods14020214
Duan M, Wang Y, Chen S, Lu J, Dong R, Yu Q, Xie J, Chen Y. Combined BPA and DIBP Exposure Induced Intestinal Mucosal Barrier Impairment Through the Notch Pathway and Gut Microbiota Dysbiosis in Mice. Foods. 2025; 14(2):214. https://doi.org/10.3390/foods14020214
Chicago/Turabian StyleDuan, Mengge, Yuting Wang, Shiyu Chen, Jiawen Lu, Ruihong Dong, Qiang Yu, Jianhua Xie, and Yi Chen. 2025. "Combined BPA and DIBP Exposure Induced Intestinal Mucosal Barrier Impairment Through the Notch Pathway and Gut Microbiota Dysbiosis in Mice" Foods 14, no. 2: 214. https://doi.org/10.3390/foods14020214
APA StyleDuan, M., Wang, Y., Chen, S., Lu, J., Dong, R., Yu, Q., Xie, J., & Chen, Y. (2025). Combined BPA and DIBP Exposure Induced Intestinal Mucosal Barrier Impairment Through the Notch Pathway and Gut Microbiota Dysbiosis in Mice. Foods, 14(2), 214. https://doi.org/10.3390/foods14020214







