Directed Evolution of Microbial Communities in Fermented Foods: Strategies, Mechanisms, and Challenges
Abstract
:1. Introduction
2. Methods
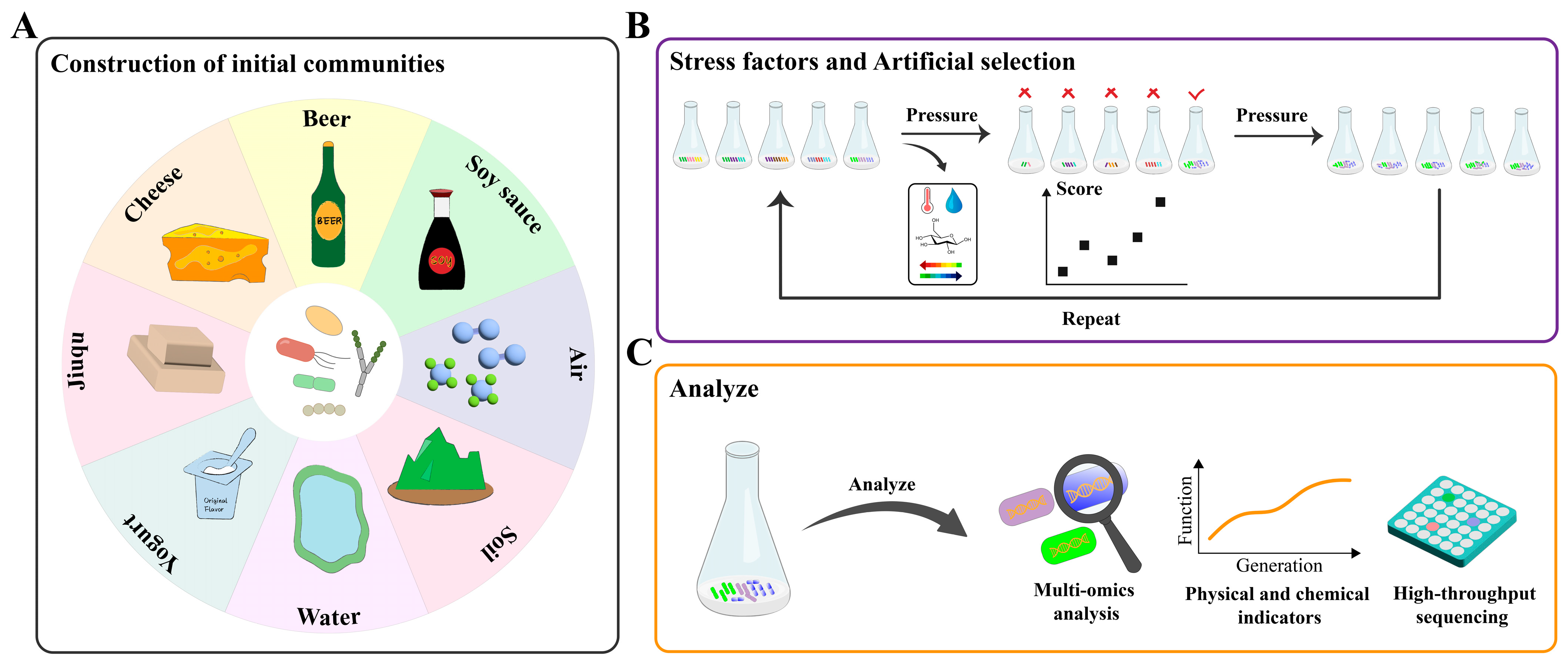
3. Strategies for DEMC
3.1. Construction of Initial Communities
3.2. Introduction of Stress Factors
3.3. Artificial Selection
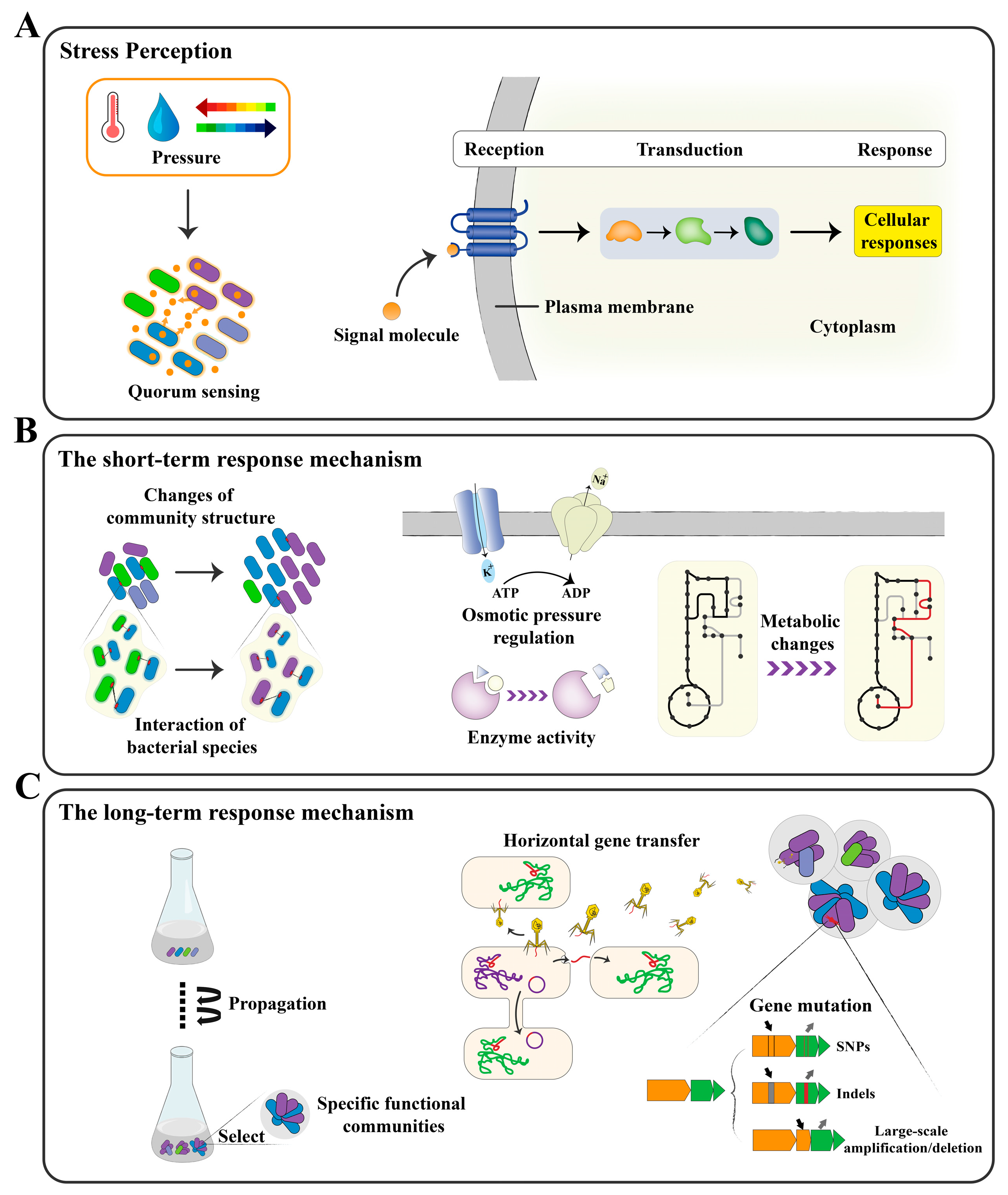
4. Mechanisms of DEMC
4.1. The Short-Term Mechanism
4.2. The Long-Term Mechanism
5. Challenges and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, Y.; Zhang, L.; Wen, R.; Chen, Q.; Kong, B. Role of lactic acid bacteria in flavor development in traditional chinese fermented foods: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2741–2755. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligne, B.; Ganzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Vrancken, G.; Ravyts, F.; Rimaux, T.; Weckx, S. Biodiversity, ecological determinants, and metabolic exploitation of sourdough microbiota. Food Microbiol. 2009, 26, 666–675. [Google Scholar] [CrossRef]
- Song, H.S.; Whon, T.W.; Kim, J.; Lee, S.H.; Kim, J.Y.; Kim, Y.B.; Choi, H.J.; Rhee, J.K.; Roh, S.W. Microbial niches in raw ingredients determine microbial community assembly during kimchi fermentation. Food Chem. 2020, 318, 126481. [Google Scholar] [CrossRef]
- Kong, H.; Kim, S.H.; Jeong, W.S.; Kim, S.Y.; Yeo, S.H. Microbiome analysis of traditional grain vinegar produced under different fermentation conditions in various regions in korea. Foods 2022, 11, 3573. [Google Scholar] [CrossRef]
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F.; Lanciotti, R. Role of yeasts on the sensory component of wines. Foods 2022, 11, 1921. [Google Scholar] [CrossRef]
- Gibson, B.; Geertman, J.A.; Hittinger, C.T.; Krogerus, K.; Libkind, D.; Louis, E.J.; Magalhaes, F.; Sampaio, J.P. New yeasts-new brews: Modern approaches to brewing yeast design and development. FEMS Yeast Res. 2017, 17, fox038. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding kombucha tea fermentation: A review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Zheng, X.-W.; Tabrizi, M.R.; Nout, M.J.R.; Han, B.-Z. Daqu—A traditional chinese liquor fermentation starter. J. Inst. Brew. 2011, 117, 82–90. [Google Scholar] [CrossRef]
- Dertli, E.; Çon, A.H. Microbial diversity of traditional kefir grains and their role on kefir aroma. LWT—Food Sci. Technol. 2017, 85, 151–157. [Google Scholar] [CrossRef]
- Lin, X.; Bakyrbay, S.; Liu, L.; Tang, X.; Liu, Y. Microbiota succession and chemical composition involved in lactic acid bacteria-fermented pickles. Fermentation 2023, 9, 330. [Google Scholar] [CrossRef]
- Hussain, B.; Chen, J.-S.; Hsu, B.-M.; Chu, I.-T.; Koner, S.; Chen, T.-H.; Rathod, J.; Chan, M.W.Y. Deciphering bacterial community structure, functional prediction and food safety assessment in fermented fruits using next-generation 16s rrna amplicon sequencing. Microorganisms 2021, 9, 1574. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Yin, F.H.; Amin, Z.; Mansa, R.F.; Ling, C.M.W.V. An overview of the role of lactic acid bacteria in fermented foods and their potential probiotic properties. Borneo Int. J. Biotechnol. 2023, 2, 65–83. [Google Scholar] [CrossRef]
- Tan, G.; Qi, S.; Wang, Y.; Li, X.; Li, X.; Li, M.; Li, L.; Zhao, L.; Hu, M. Uncovering differences in the composition and function of phage communities and phage-bacterium interactions in raw soy sauce. Front. Microbiol. 2023, 14, 1328158. [Google Scholar] [CrossRef]
- De Vrieze, J.; Christiaens, M.E.R.; Verstraete, W. The microbiome as engineering tool: Manufacturing and trading between microorganisms. New Biotechnol. 2017, 39, 206–214. [Google Scholar] [CrossRef]
- Rizo, J.; Guillen, D.; Farres, A.; Diaz-Ruiz, G.; Sanchez, S.; Wacher, C.; Rodriguez-Sanoja, R. Omics in traditional vegetable fermented foods and beverages. Crit. Rev. Food Sci. Nutr. 2020, 60, 791–809. [Google Scholar] [CrossRef]
- Mudoor Sooresh, M.; Willing, B.P.; Bourrie, B.C.T. Opportunities and challenges of understanding community assembly in spontaneous food fermentation. Foods 2023, 12, 673. [Google Scholar] [CrossRef]
- Parekh, S.; Vinci, V.A.; Strobel, R.J. Improvement of microbial strains and fermentation processes. Appl. Microbiol. Biotechnol. 2000, 54, 287–301. [Google Scholar] [CrossRef]
- Chen, G.-M.; Huang, Z.-R.; Wu, L.; Wu, Q.; Guo, W.-L.; Zhao, W.-H.; Liu, B.; Zhang, W.; Rao, P.-F.; Lv, X.-C.; et al. Microbial diversity and flavor of chinese rice wine (huangjiu): An overview of current research and future prospects. Curr. Opin. Food Sci. 2021, 42, 37–50. [Google Scholar] [CrossRef]
- Wang, S.; Xiong, W.; Wang, Y.; Nie, Y.; Wu, Q.; Xu, Y.; Geisen, S. Temperature-induced annual variation in microbial community changes and resulting metabolome shifts in a controlled fermentation system. mSystems 2020, 5, e00555-20. [Google Scholar] [CrossRef] [PubMed]
- Ferrocino, I.; Bellio, A.; Giordano, M.; Macori, G.; Romano, A.; Rantsiou, K.; Decastelli, L.; Cocolin, L. Shotgun metagenomics and volatilome profile of the microbiota of fermented sausages. Appl. Environ. Microbiol. 2018, 84, e02120-17. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, Y.; Tian, Z.; Mao, Z.; Cheng, H.; Zhou, H.; Wang, W. Enhancing microbial community performance on acid resistance by modified adaptive laboratory evolution. Bioresour. Technol. 2019, 287, 121416. [Google Scholar] [CrossRef] [PubMed]
- Peter, H.; Beier, S.; Bertilsson, S.; Lindstrom, E.S.; Langenheder, S.; Tranvik, L.J. Function-specific response to depletion of microbial diversity. ISME J. 2011, 5, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhu, Y.; Wijffels, R.H.; Scott, W.T., Jr.; Xu, Y.; dos Santos, V.M. Regulating microbiome metabolic stability for stable indigenous liquor fermentation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Singh Nigam, P. An overview of microorganisms’; contribution and performance in alco- hol fermentation processing a variety of substrates. Curr. Biotechnol. 2017, 6, 9–16. [Google Scholar] [CrossRef]
- Grimalt-Alemany, A.; Lezyk, M.; Lange, L.; Skiadas, I.V.; Gavala, H.N. Enrichment of syngas-converting mixed microbial consortia for ethanol production and thermodynamics-based design of enrichment strategies. Biotechnol. Biofuels 2018, 11, 198. [Google Scholar] [CrossRef]
- Yu, S.R.; Zhang, Y.Y.; Zhang, Q.G. The effectiveness of artificial microbial community selection: A conceptual framework and a meta-analysis. Front. Microbiol. 2023, 14, 1257935. [Google Scholar] [CrossRef]
- Li, Y.C.; Rao, J.W.; Meng, F.B.; Wang, Z.W.; Liu, D.Y.; Yu, H. Combination of mutagenesis and adaptive evolution to engineer salt-tolerant and aroma-producing yeast for soy sauce fermentation. J. Sci. Food Agric. 2021, 101, 4288–4297. [Google Scholar] [CrossRef]
- Yu, J.; Tang, S.N.; Lee, P.K.H. Microbial communities in full-scale wastewater treatment systems exhibit deterministic assembly processes and functional dependency over time. Environ. Sci. Technol. 2021, 55, 5312–5323. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, Y.; Zhao, L.; Wang, Y.; Rao, L.; Liao, X. Pressure-resistant acclimation of lactic acid bacteria from a natural fermentation product using high pressure. Innov. Food Sci. Emerg. Technol. 2021, 69, 102660. [Google Scholar] [CrossRef]
- Rocca, J.D.; Simonin, M.; Blaszczak, J.R.; Ernakovich, J.G.; Gibbons, S.M.; Midani, F.S.; Washburne, A.D. The microbiome stress project: Toward a global meta-analysis of environmental stressors and their effects on microbial communities. Front. Microbiol. 2019, 9, 3272. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Vila, J.; Chang, C.-Y.; Diaz-Colunga, J.; Estrela, S.; Rebolleda-Gomez, M. Directed evolution of microbial communities. Annu. Rev. Biophys. 2020, 50, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Song, H.S.; Lee, S.H.; Ahn, S.W.; Kim, J.Y.; Rhee, J.K.; Roh, S.W. Effects of the main ingredients of the fermented food, kimchi, on bacterial composition and metabolite profile. Food Res. Int. 2021, 149, 110668. [Google Scholar] [CrossRef]
- Lee, S.H.; Whon, T.W.; Roh, S.W.; Jeon, C.O. Unraveling microbial fermentation features in kimchi: From classical to meta-omics approaches. Appl. Microbiol. Biotechnol. 2020, 104, 7731–7744. [Google Scholar] [CrossRef]
- Oshiro, M.; Momoda, R.; Tanaka, M.; Zendo, T.; Nakayama, J. Dense tracking of the dynamics of the microbial community and chemicals constituents in spontaneous wheat sourdough during two months of backslopping. J. Biosci. Bioeng. 2019, 128, 170–176. [Google Scholar] [CrossRef]
- Zang, J.; Xu, Y.; Xia, W.; Yu, D.; Gao, P.; Jiang, Q.; Yang, F. Dynamics and diversity of microbial community succession during fermentation of suan yu, a chinese traditional fermented fish, determined by high throughput sequencing. Food Res. Int. 2018, 111, 565–573. [Google Scholar] [CrossRef]
- Louw, N.L.; Lele, K.; Ye, R.; Edwards, C.B.; Wolfe, B.E. Microbiome assembly in fermented foods. Annu. Rev. Microbiol. 2023, 77, 381–402. [Google Scholar] [CrossRef]
- Li, Y.; Ge, Y.; Wang, J.; Shen, C.; Wang, J.; Liu, Y.J. Functional redundancy and specific taxa modulate the contribution of prokaryotic diversity and composition to multifunctionality. Mol. Ecol. 2021, 30, 2915–2930. [Google Scholar] [CrossRef]
- Hu, X.; Wang, K.; Chen, M.; Fan, J.; Han, S.; Hou, J.; Chi, L.; Liu, Y.; Wei, T. Profiling the composition and metabolic activities of microbial community in fermented grain for the chinese strong-flavor baijiu production by using the metatranscriptome, high-throughput 16s rrna and its gene sequencings. Food Res. Int. 2020, 138, 109765. [Google Scholar] [CrossRef]
- Chai, L.-J.; Qian, W.; Zhong, X.-Z.; Zhang, X.-J.; Lu, Z.-M.; Zhang, S.-Y.; Wang, S.-T.; Shen, C.-H.; Shi, J.-S.; Xu, Z.-H. Mining the factors driving the evolution of the pit mud microbiome under the impact of long-term production of strong-flavor baijiu. Appl. Environ. Microbiol. 2021, 87, e0088521. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tian, R.; Wang, K.; Cao, Z.; Yan, P.; Li, F.; Li, X.; Li, S.; He, P. The prokaryotic community, physicochemical properties and flavors dynamics and their correlations in fermented grains for chinese strong-flavor baijiu production. Food Res. Int. 2021, 148, 110626. [Google Scholar]
- Giraffa, G. Studying the dynamics of microbial populations during food fermentation. FEMS Microbiol. Rev. 2004, 28, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Li, H.; Zheng, C. Shifting product spectrum by ph adjustment during long-term continuous anaerobic fermentation of food waste. Bioresour. Technol. 2018, 270, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, J. Changes in microbial community structure during dark fermentative hydrogen production. Int. J. Hydrog. Energy 2019, 44, 25542–25550. [Google Scholar] [CrossRef]
- Temudo, M.F.; Muyzer, G.; Kleerebezem, R.; van Loosdrecht, M.C. Diversity of microbial communities in open mixed culture fermentations: Impact of the ph and carbon source. Appl. Microbiol. Biotechnol. 2008, 80, 1121–1130. [Google Scholar] [CrossRef]
- Junlin, W.; Jun, L.; Nie, Y.; Changwen, L.; Hai, D.; Yan, X. Amino acids drive the deterministic assembly process of fungal community and affect the flavor metabolites in baijiu fermentation. Microbiol. Spectr. 2023, 11, e0264022. [Google Scholar]
- Xiao, C.; Lu, Z.M.; Zhang, X.J.; Wang, S.T.; Ao, L.; Shen, C.H.; Shi, J.S.; Xu, Z.H. Bio-heat is a key environmental driver shaping the microbial community of medium-temperature daqu. Appl. Environ. Microbiol. 2017, 83, e01550-17. [Google Scholar] [CrossRef]
- Mugihito, O.; Masaru, T.; Zendo, T.; Nakayama, J. Impact of ph on succession of sourdough lactic acid bacteria communities and their fermentation properties. Biosci. Microbiota Food Health 2020, 39, 152–159. [Google Scholar]
- The-Thiri, M.; Bon-Yeob, G.; Mi Hwan, K.; Ryu, G. Fermentation of texturized vegetable proteins extruded at different moisture contents: Effect on physicochemical, structural, and microbial properties. Food Sci. Biotechnol. 2020, 29, 897–907. [Google Scholar]
- Liang, H.; He, Z.; Wang, X.; Song, G.; Chen, H.; Lin, X.; Ji, C.; Li, S. Effects of salt concentration on microbial diversity and volatile compounds during suancai fermentation. Food Microbiol. 2020, 91, 103537. [Google Scholar]
- Englezos, V.; Cravero, F.; Torchio, F.; Rantsiou, K.; Ortiz-Julien, A.; Lambri, M.; Gerbi, V.; Rolle, L.; Cocolin, L. Oxygen availability and strain combination modulate yeast growth dynamics in mixed culture fermentations of grape must with starmerella bacillaris and saccharomyces cerevisiae. Food Microbiol. 2018, 69, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, A.; Bruno, A.; Agostinetto, G.; Casiraghi, M.; Guzzetti, L.; Labra, M. Fermented food products in the era of globalization: Tradition meets biotechnology innovations. Curr. Opin. Biotechnol. 2021, 70, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Flores-Cosio, G.; Herrera-Lopez, E.J.; Arellano-Plaza, M.; Gschaedler-Mathis, A.; Kirchmayr, M.; Amaya-Delgado, L. Application of dielectric spectroscopy to unravel the physiological state of microorganisms: Current state, prospects and limits. Appl. Microbiol. Biotechnol. 2020, 104, 6101–6113. [Google Scholar] [CrossRef]
- Yap, M.; Ercolini, D.; Alvarez-Ordonez, A.; O’Toole, P.W.; O’Sullivan, O.; Cotter, P.D. Next-generation food research: Use of meta-omic approaches for characterizing microbial communities along the food chain. Annu. Rev. Food Sci. Technol. 2022, 13, 361–384. [Google Scholar] [CrossRef]
- Lee, J.; Shou, W.; Park, H.J. Artificial selection for microbial collective composition can succeed or fail depending on the initial and target values. bioRxiv 2024. [Google Scholar] [CrossRef]
- Raynaud, T.; Devers-Lamrani, M.; Spor, A.; Blouin, M. Community diversity determines the evolution of synthetic bacterial communities under artificial selection. Evolution 2022, 76, 1883–1895. [Google Scholar] [CrossRef]
- Chang, C.Y.; Osborne, M.L.; Bajic, D.; Sanchez, A. Artificially selecting bacterial communities using propagule strategies. Evolution 2020, 74, 2392–2403. [Google Scholar] [CrossRef]
- Vessman, B.; Guridi-Fernández, P.; Arias-Sánchez, F.I.; Mitri, S. Novel artificial selection method improves function of simulated microbial communities. bioRxiv 2023. [Google Scholar] [CrossRef]
- Arias-Sánchez, F.I.; Vessman, B.; Haym, A.; Alberti, G.; Mitri, S. Artificial selection optimizes pollutant-degrading bacterial communities. bioRxiv 2024. [Google Scholar] [CrossRef]
- Raynaud, T.; Devers, M.; Spor, A.; Blouin, M. Effect of the reproduction method in an artificial selection experiment at the community level. Front. Ecol. Evol. 2019, 7, 416. [Google Scholar] [CrossRef]
- Wright, R.J.; Gibson, M.I.; Christie-Oleza, J.A. Understanding microbial community dynamics to improve optimal microbiome selection. Microbiome 2019, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Barrangou, R. Combining omics technologies with crispr-based genome editing to study food microbes. Curr. Opin. Biotechnol. 2020, 61, 198–208. [Google Scholar] [CrossRef]
- Yang, L.; Fan, W.; Xu, Y. Metaproteomics insights into traditional fermented foods and beverages. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2506–2529. [Google Scholar] [CrossRef]
- Takagi, H. Molecular mechanisms and highly functional development for stress tolerance of the yeast saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 2021, 85, 1017–1037. [Google Scholar] [CrossRef]
- Koska, M.; Kordes, A.; Erdmann, J.; Willger, S.D.; Thoming, J.G.; Bahre, H.; Haussler, S. Distinct long- and short-term adaptive mechanisms in pseudomonas aeruginosa. Microbiol. Spectr. 2022, 10, e0304322. [Google Scholar] [CrossRef]
- Vasilakou, E.; van Loosdrecht, M.C.M.; Wahl, S.A. Escherichia coli metabolism under short-term repetitive substrate dynamics: Adaptation and trade-offs. Microb. Cell Fact. 2020, 19, 116. [Google Scholar] [CrossRef]
- Gan, Y.; Qi, X.; Lin, Y.; Guo, Y.; Zhang, Y.; Wang, Q. A hierarchical transcriptional regulatory network required for long-term thermal stress tolerance in an industrial saccharomyces cerevisiae strain. Front. Bioeng. Biotechnol. 2021, 9, 826238. [Google Scholar] [CrossRef]
- Rose, C.J.; Hammerschmidt, K.; Pichugin, Y.; Rainey, P.B. Meta-population structure and the evolutionary transition to multicellularity. Ecol. Lett. 2020, 23, 1380–1390. [Google Scholar] [CrossRef]
- Zong, E.; Bo, T.; Dang, L.; Zhang, J.; Li, H.; Lv, N.; He, Y.; Bai, B.; Zhang, J.; Fan, S. Different functions can be provided by low temperature daqu with different appearance features due to variations in the microbial community structure during fermentation. LWT 2024, 193, 115763. [Google Scholar] [CrossRef]
- Li, D.; Jia, F.; Wang, L.; Chang, F. The initial composition and structure of microbial community determined the yield and quality of baijiu during the spontaneous fermentation. Int. Microbiol. 2024, 27, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Pattanakittivorakul, S.; Tsuzuno, T.; Kosaka, T.; Murata, M.; Kanesaki, Y.; Yoshikawa, H.; Limtong, S.; Yamada, M. Evolutionary adaptation by repetitive long-term cultivation with gradual increase in temperature for acquiring multi-stress tolerance and high ethanol productivity in kluyveromyces marxianus dmku 3-1042. Microorganisms 2022, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.K.; Akhtar, N.; Sher, F.; Navarrete, A.A.; Americo-Pinheiro, J.H.P. Microbial adaptation to different environmental conditions: Molecular perspective of evolved genetic and cellular systems. Arch. Microbiol. 2022, 204, 144. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Griffiths, B.S.; Langenheder, S. Microbial community resilience across ecosystems and multiple disturbances. Microbiol. Mol. Biol. Rev. 2021, 85, e00026-20. [Google Scholar] [CrossRef]
- Dhar, R.; Sägesser, R.; Weikert, C.; Yuan, J.; Wagner, A. Adaptation of to saline stress through laboratory evolution. J. Evol. Biol. 2011, 24, 1135–1153. [Google Scholar] [CrossRef]
- Oleinikova, Y.A.; Kuznetsova, T.V.; Saubenova, M.G.; Aitzhanova, A.A.; Shormanova, M.M.; Raimbekova, L.T. Selective approach to efficient ethanol production using adaptation of producer saccharomyces cerevisiae to hyperosmotic medium. Int. J. Eng. Res. Appl. 2017, 7, 01–05. [Google Scholar] [CrossRef]
- Ekberg, J.; Rautio, J.; Mattinen, L.; Vidgren, V.; Londesborough, J.; Gibson, B.R. Adaptive evolution of the lager brewing yeast saccharomyces pastorianus for improved growth under hyperosmotic conditions and its influence on fermentation performance. FEMS Yeast Res. 2013, 13, 335–349. [Google Scholar] [CrossRef]
- Tirloni, L.; Heidrich, D.; Souza, C.F.V.d. Adaptive laboratory evolution to obtain lactic acid bacteria strains of industrial interest-a review. Braz. J. Food Technol. 2023, 26, e2023053. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; González-Fernández, C.; Tomás-Pejó, E. Evolutionary engineering of lactobacillus pentosus improves lactic acid productivity from xylose-rich media at low ph. Bioresour. Technol. 2019, 288, 121540. [Google Scholar] [CrossRef]
- Mladenovic, D.; Pejin, J.; Kocic-Tanackov, S.; Djukic-Vukovic, A.; Mojovic, L. Enhanced lactic acid production by adaptive evolution of lactobacillus paracasei on agro-industrial substrate. Appl. Biochem. Biotechnol. 2019, 187, 753–769. [Google Scholar] [CrossRef]
- Ming, H.; Xu, D.; Guo, Z.; Liu, Y. Adaptive evolution of lactobacillus casei under acidic conditions enhances multiple-stress tolerance. Food Sci. Technol. Res. 2016, 22, 331–336. [Google Scholar] [CrossRef]
- Chen, J.; Shen, J.; Ingvar Hellgren, L.; Ruhdal Jensen, P.; Solem, C. Adaptation of lactococcus lactis to high growth temperature leads to a dramatic increase in acidification rate. Sci. Rep. 2015, 5, 14199. [Google Scholar] [CrossRef] [PubMed]
- Randez-Gil, F.; Prieto, J.A.; Rodriguez-Puchades, A.; Casas, J.; Sentandreu, V.; Estruch, F. Myriocin-induced adaptive laboratory evolution of an industrial strain of saccharomyces cerevisiae reveals its potential to remodel lipid composition and heat tolerance. Microb. Biotechnol. 2020, 13, 1066–1081. [Google Scholar] [CrossRef] [PubMed]
- Ingelman, H.; Heffernan, J.K.; Harris, A.; Brown, S.D.; Shaikh, K.M.; Saqib, A.Y.; Pinheiro, M.J.; de Lima, L.A.; Martinez, K.R.; Gonzalez-Garcia, R.A.; et al. Autotrophic adaptive laboratory evolution of the acetogen clostridium autoethanogenum delivers the gas-fermenting strain labrini with superior growth, products, and robustness. New Biotechnol. 2024, 83, 1–15. [Google Scholar] [CrossRef]
- Lairon-Peris, M.; Castiglioni, G.L.; Routledge, S.J.; Alonso-Del-Real, J.; Linney, J.A.; Pitt, A.R.; Melcr, J.; Goddard, A.D.; Barrio, E.; Querol, A. Adaptive response to wine selective pressures shapes the genome of a saccharomyces interspecies hybrid. Microb. Genom. 2021, 7, 000628. [Google Scholar] [CrossRef]
- Kayacan, Y.; Van Mieghem, T.; Delvaux, F.; Delvaux, F.R.; Willaert, R. Adaptive evolution of industrial brewer’s yeast strains towards a snowflake phenotype. Fermentation 2020, 6, 20. [Google Scholar] [CrossRef]
- da Silveira, F.A.; de Oliveira Soares, D.L.; Bang, K.W.; Balbino, T.R.; de Moura Ferreira, M.A.; Diniz, R.H.S.; de Lima, L.A.; Brandao, M.M.; Villas-Boas, S.G.; da Silveira, W.B. Assessment of ethanol tolerance of kluyveromyces marxianus cct 7735 selected by adaptive laboratory evolution. Appl. Microbiol. Biotechnol. 2020, 104, 7483–7494. [Google Scholar] [CrossRef]
- Stock, A.M.; Robinson, V.L.; Goudreau, P.N. Two-component signal transduction. Annu. Rev. Biochem. 2000, 69, 183–215. [Google Scholar] [CrossRef]
- Brown, A.J.; Budge, S.; Kaloriti, D.; Tillmann, A.; Jacobsen, M.D.; Yin, Z.; Ene, I.V.; Bohovych, I.; Sandai, D.; Kastora, S.; et al. Stress adaptation in a pathogenic fungus. J. Exp. Biol. 2014, 217, 144–155. [Google Scholar] [CrossRef]
- Hohmann, S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 2002, 66, 300–372. [Google Scholar] [CrossRef]
- Williams, P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 2007, 153, 3923–3938. [Google Scholar] [CrossRef] [PubMed]
- Giannakara, M.; Koumandou, V.L. Evolution of two-component quorum sensing systems. Access Microbiol. 2022, 4, 000303. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.G.G.; Pinto, U.M.; Matos, C.B.; Frazilio, D.A.; Braga, V.F.; von Zeska-Kress, M.R.; De Martinis, E.C.P. Does quorum sensing play a role in microbial shifts along spontaneous fermentation of cocoa beans? An in silico perspective. Food Res. Int. 2020, 131, 109034. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.T.; Russo, M.; Fernandes, A.A.R.; Broach, J.R.; Fernandes, P.M.B. Transcriptional response of multi-stress-tolerant saccharomyces cerevisiae to sequential stresses. Fermentation 2023, 9, 195. [Google Scholar] [CrossRef]
- Imhoff, J.F. Osmotic adaptation in halophilic and halotolerant microorganisms. In The Biology of Halophilic Bacteria; CRC Press: Boca Raton, FL, USA, 2020; pp. 211–253. [Google Scholar]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef]
- Cirat, R.; Capozzi, V.; Benmechernene, Z.; Spano, G.; Grieco, F.; Fragasso, M. Lab antagonistic activities and their significance in food biotechnology: Molecular mechanisms, food targets, and other related traits of interest. Fermentation 2024, 10, 222. [Google Scholar] [CrossRef]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef]
- Tan, Y.-S.; Zhang, R.-K.; Liu, Z.-H.; Li, B.-Z.; Yuan, Y.-J. Microbial adaptation to enhance stress tolerance. Front. Microbiol. 2022, 13, 888746. [Google Scholar] [CrossRef]
- Hernando, M.P.; Schloss, I.R.; De La Rosa, F.; De Troch, M. Fatty acids in microalgae and cyanobacteria in a changing world: Contrasting temperate and cold environments. Biocell 2022, 46, 607–621. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Vila, J.C.C.; Bender, M.; Li, R.; Mankowski, M.C.; Bassette, M.; Borden, J.; Golfier, S.; Sanchez, P.G.L.; Waymack, R.; et al. Engineering complex communities by directed evolution. Nat. Ecol. Evol. 2021, 5, 1011–1023. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Sarengaowa; Ji, Y.; Guan, Y.; Feng, K. Microbial dynamics and volatilome profiles during the fermentation of chinese northeast sauerkraut by leuconostoc mesenteroides orc 2 and lactobacillus plantarum hbuas 51041 under different salt concentrations. Food Res. Int. 2020, 130, 108926. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Hodgson, D.J.; Buckling, A. The interplay between microevolution and community structure in microbial populations. Curr. Opin. Biotechnol. 2013, 24, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Zelezniak, A.; Andrejev, S.; Ponomarova, O.; Mende, D.R.; Bork, P.; Patil, K.R. Metabolic dependencies drive species co-occurrence in diverse microbial communities. Proc. Natl. Acad. Sci. USA 2015, 112, 6449–6454. [Google Scholar] [CrossRef] [PubMed]
- Senne de Oliveira Lino, F.; Bajic, D.; Vila, J.C.C.; Sanchez, A.; Sommer, M.O.A. Complex yeast-bacteria interactions affect the yield of industrial ethanol fermentation. Nat. Commun. 2021, 12, 1498. [Google Scholar] [CrossRef]
- An, F.; Wu, J.; Feng, Y.; Pan, G.; Ma, Y.; Jiang, J.; Yang, X.; Xue, R.; Wu, R.; Zhao, M. A systematic review on the flavor of soy-based fermented foods: Core fermentation microbiome, multisensory flavor substances, key enzymes, and metabolic pathways. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2773–2801. [Google Scholar] [CrossRef]
- Zhao, G.; Ding, L.-L.; Yao, Y.; Cao, Y.; Pan, Z.-H.; Kong, D.-H. Extracellular proteome analysis and flavor formation during soy sauce fermentation. Front. Microbiol. 2018, 9, 1872. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, P.; Cao, M.; Yu, T.; Lane, S.T.; Zhao, H. Directed evolution: Methodologies and applications. Chem. Rev. 2021, 121, 12384–12444. [Google Scholar] [CrossRef]
- Arnold, B.J.; Huang, I.T.; Hanage, W.P. Horizontal gene transfer and adaptive evolution in bacteria. Nat. Rev. Microbiol. 2022, 20, 206–218. [Google Scholar] [CrossRef]
- Woods, L.C.; Gorrell, R.J.; Taylor, F.; Connallon, T.; Kwok, T.; McDonald, M.J. Horizontal gene transfer potentiates adaptation by reducing selective constraints on the spread of genetic variation. Proc. Natl. Acad. Sci. USA 2020, 117, 26868–26875. [Google Scholar] [CrossRef]
- Zheng, Y.; Hong, K.; Wang, B.; Liu, D.; Chen, T.; Wang, Z. Genetic diversity for accelerating microbial adaptive laboratory evolution. ACS Synth. Biol. 2021, 10, 1574–1586. [Google Scholar] [CrossRef]
- Blakely, G.W. Mechanisms of horizontal gene transfer and DNA recombination. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 309–324. [Google Scholar]
- Wang, R.; Wu, J.; Jiang, N.; Lin, H.; An, F.; Wu, C.; Yue, X.; Shi, H.; Wu, R. Recent developments in horizontal gene transfer with the adaptive innovation of fermented foods. Crit. Rev. Food Sci. Nutr. 2023, 63, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Bonham, K.S.; Wolfe, B.E.; Dutton, R.J. Extensive horizontal gene transfer in cheese-associated bacteria. bioRxiv 2017. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Rodríguez, C.; Marsit, S.; Galeote, V. Diversity of oligopeptide transport in yeast and its impact on adaptation to winemaking conditions. Front. Genet. 2020, 11, 602. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Wang, J.; Zhou, A.; Ma, C.; Wu, X.; Moore, J.; Millar, B.; Xu, J. Characterization and transfer of antibiotic resistance in lactic acid bacteria from fermented food products. Curr. Microbiol. 2011, 62, 1081–1089. [Google Scholar] [CrossRef]
- Xie, M.; An, F.; Wu, J.; Liu, Y.; Shi, H.; Wu, R. Meta-omics reveal microbial assortments and key enzymes in bean sauce mash, a traditional fermented soybean product. J. Sci. Food Agric. 2019, 99, 6522–6534. [Google Scholar] [CrossRef]
- Baquero, F.; Martinez, J.L.; Lanza, V.F.; Rodriguez-Beltran, J.; Galan, J.C.; San Millan, A.; Canton, R.; Coque, T.M. Evolutionary pathways and trajectories in antibiotic resistance. Clin. Microbiol. Rev. 2021, 34, e0005019. [Google Scholar] [CrossRef]
- Gao, L.; Wu, X.; Zhu, C.; Jin, Z.; Wang, W.; Xia, X. Metabolic engineering to improve the biomanufacturing efficiency of acetic acid bacteria: Advances and prospects. Crit. Rev. Biotechnol. 2020, 40, 522–538. [Google Scholar] [CrossRef]
- Niehus, R.; Mitri, S.; Fletcher, A.G.; Foster, K.R. Migration and horizontal gene transfer divide microbial genomes into multiple niches. Nat. Commun. 2015, 6, 8924. [Google Scholar] [CrossRef]
- Alekseeva, A.Y.; Groenenboom, A.E.; Smid, E.J.; Schoustra, S.E. Eco-evolutionary dynamics in microbial communities from spontaneous fermented foods. Int. J. Environ. Res. Public Health 2021, 18, 10093. [Google Scholar] [CrossRef]
- Chen, L.; Wang, G.; Teng, M.; Wang, L.; Yang, F.; Jin, G.; Du, H.; Xu, Y. Non-gene-editing microbiome engineering of spontaneous food fermentation microbiota-limitation control, design control, and integration. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1902–1932. [Google Scholar] [CrossRef]
- Xie, L.; Yuan, A.E.; Shou, W. Simulations reveal challenges to artificial community selection and possible strategies for success. PLoS Biol. 2019, 17, e3000295. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, B.; Swings, T.; Fauvart, M.; Michiels, J. Experimental design, population dynamics, and diversity in microbial experimental evolution. Microbiol. Mol. Biol. Rev. 2018, 82, e00008-18. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yuan, A.E.; Shou, W. A quantitative genetics framework for understanding the selection response of microbial communities. bioRxiv 2023. [Google Scholar] [CrossRef]
- Thomas, J.L.; Rowland-Chandler, J.; Shou, W. Artificial selection of microbial communities: What have we learnt and how can we improve? Curr. Opin. Microbiol. 2024, 77, 102400. [Google Scholar] [CrossRef]
- Herron, M.D.; Zamani-Dahaj, S.A.; Ratcliff, W.C. Trait heritability in major transitions. BMC Biol. 2018, 16, 145. [Google Scholar] [CrossRef]
- Schoustra, S.E.; Kasase, C.; Toarta, C.; Kassen, R.; Poulain, A.J. Microbial community structure of three traditional zambian fermented products: Mabisi, chibwantu and munkoyo. PLoS ONE 2013, 8, e63948. [Google Scholar] [CrossRef]
| Fermented Foods | Microbial Communities | Main Functions | References |
|---|---|---|---|
| Yogurt | Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus | Produces lactic acid, texture improvement | [3] |
| Sourdough Bread | Lactic acid bacteria (LAB), wild yeasts | Provides unique sour flavor, improves texture | [4] |
| Kimchi | Lactic acid bacteria (Leuconostoc spp., Lactobacillus spp., Weissella spp.) | Enhances preservation, adds flavor, health benefits | [5] |
| Natto | Bacillus subtilis natto | Produces nattokinase, enhances soybean digestibility | [4] |
| Vinegar | Acetobacter aceti, Acetobacter pasteurianus | Produces acetic acid, imparts sour flavor | [6] |
| Wine | Saccharomyces cerevisiae, Oenococcus oeni | Alcoholic fermentation, enhances flavor and aroma | [7] |
| Beer | Saccharomyces cerevisiae, Lactic acid bacteria | Alcohol production, contributes to flavor formation | [8] |
| Kombucha | SCOBY (Symbiotic Culture Of Bacteria and Yeast), including Gluconacetobacter xylinus | Produces organic acids, alcohol, and gasses, health benefits | [9] |
| Baijiu | Saccharomycopsis, Aspergillus spp., Lactic acid bacteria | Alcohol production, develops complex flavor profiles | [10] |
| Kefir | Lactobacillus kefiranofaciens, Saccharomyces cerevisiae, Dipodascaceae family yeasts | Contributes to aroma formation and unique flavor profile | [11] |
| Pickles | Lactic acid bacteria (Lactobacillus plantarum, Lactobacillus pentosus), Weissella, Enterobacteriaceae | Enhances acid production (lactic and malic acids), enriches flavor through esters | [12] |
| Fermented Fruits | Diverse bacterial genera (Sphingomonas spp., Acinetobacter spp.) | Ensures food safety through microbial diversity, inhibits pathogens | [13] |
| Fermented Dairy Products | Lactic acid bacteria, Streptococcus thermophilus, Lactococcus lactis | Improves gut microbiome, provides antioxidant benefits | [14] |
| Soy Sauce | Tetragenococcus halophilus, Zygosaccharomyces rouxii, Candida versatilis, Weissella, Bacillus | Microbial succession enhances complex flavor profiles, acidifies the mash, and improves aroma formation | [15] |
| Methods | Advantages | Limitations | Examples | References |
|---|---|---|---|---|
| Spontaneous Fermentation | High microbial diversity; contributes to complex flavors and resilience | Variability in outcomes; potential safety issues | Kimchi, sauerkraut, sourdough | [18,34,35] |
| Backslopping | Continuity in microbial composition and flavor; suitable for maintaining batch consistency | Reduced diversity over time; limits resilience and flavor complexity | Sourdough, cheese | [36] |
| Defined Starters | Reliable and standardized results; ensures product safety and uniformity | May lack complex and nuanced flavor profiles due to limited microbial interactions | Beer, wine, yogurt | [37] |
| Stress Factors | Impact and Role | Cases | References |
|---|---|---|---|
| Nutrition | Specific nutrients (e.g., sugars, proteins, and fats) activate distinct metabolic pathways in microbes, influencing the flavor and shelf-life of fermented foods. | Serine promotes the growth of Zygosaccharomyces and ethanol production, impacting the flavor profile of Baijiu | [47] |
| Temperature | Temperature is a critical factor influencing microbial metabolism and growth rates in fermented foods. It affects microbial community dynamics, metabolic pathways, and the production of flavor compounds, ultimately impacting the quality and safety of the final product. | Temperature dynamics significantly correlate with the quick succession of MT-Daqu microbiota in the first 12 days of fermentation, and sustained bio-heat inhibits most microbes’ growth. | [48] |
| pH | pH significantly influences the structure and function of microbial communities by modifying enzyme activity and metabolite production. | Lower pH is favorable for the succession of sourdough lactic acid bacteria communities, leading to Lactobacillus dominance in the final stages of fermentation. | [49] |
| Moisture | Moisture content plays a crucial role in the fermentation process of various foods, affecting microbial growth, metabolic activity, and the overall quality of the fermented product. | Fermenting texturized vegetable proteins (TVPs) at 50% moisture content maintains higher chewiness, hardness, integrity index, and layered structure compared to 40% moisture content. | [50] |
| Salinity | Salt content affects microbial community structure by inhibiting salt-sensitive microbes and selecting for salt-tolerant species. | 6% salt addition in suancai fermentation leads to a higher Lactobacillus abundance and better taste quality. | [51] |
| Oxygen | Oxygen availability influences microbial metabolic pathways and fermentation products. | Oxygen availability affects yeast growth dynamics in mixed culture fermentations, increasing survival time of Starmerella bacillaris and decreasing growth rate of Saccharomyces cerevisiae strains. | [52] |
| Microbial Communities | Stress Conditions | Experimental Outcomes | Mechanisms | Foods/Fermentation Processes | References |
|---|---|---|---|---|---|
| Saccharomyces cerevisiae | Salt stress (0.7M NaCl) | The yeast showed improved growth rates and survival under high salt conditions | Genome rearrangement and upregulation of specific stress-responsive genes facilitated osmotic balance and ion homeostasis under saline stress | Bread, soy sauce, wine | [75] |
| Saccharomyces cerevisiae | Ethanol stress (8% v/v ethanol) | Yeast demonstrated increased ethanol tolerance, leading to improved fermentation efficiency | Reconfiguration of metabolic pathways and upregulation of genes involved in ethanol detoxification and membrane stabilization were critical for adaptation | Beer, wine, ethanol production | [76] |
| Saccharomyces pastorianus | Low-temperature stress (<10 °C) | Enhanced fermentation capacity and metabolic efficiency were observed in yeast at low temperatures | Genetic adaptations, including mutations in glycolytic and respiratory pathways, improved energy production efficiency under cold stress | Lager beer, cold brew | [77] |
| Lactobacillus spp. | Salt stress (6% NaCl) | Lactic acid bacteria exhibited increased salt tolerance and lactic acid production | Genomic reorganization and the activation of salt-specific regulatory networks improved ionic balance and osmotic pressure resistance | Pickled vegetables, kimchi, suancai | [78] |
| Lactobacillus pentosus | Acid stress (pH 3.5) | Lactobacillus pentosus showed enhanced acid tolerance and metabolic stability | Upregulation of acid stress response genes and modifications of membrane lipid composition enhanced cellular integrity under acidic conditions | Pickled foods, sourdough | [79] |
| Saccharomyces cerevisiae | High-temperature stress (>40 °C) | Yeast showed increased thermotolerance, with improved cell viability at elevated temperatures | Accumulation of heat shock proteins and chaperones, along with alterations in the protein folding and degradation pathways, facilitated thermotolerance | Baijiu, bread, wine | [80] |
| Lactobacillus casei | Lactose stress (high lactose concentration) | Lactobacillus casei adapted to high lactose conditions, showing improved growth and lactose metabolism | Genetic mutations in the lactose operon and enhanced expression of β-galactosidase contributed to efficient lactose utilization and adaptation | Yogurt, cheese | [81] |
| Lactococcus lactis | High-temperature stress (>38 °C) | Mutant TM29 grows 33% faster and has a 12% higher lactate production rate at 38 °C than the wild type. | Mutations enhanced thermal tolerance through improved protein expression, membrane synthesis, and gene deletion. | Cheese, sour cream | [82] |
| Saccharomyces cerevisiae | Temperature stress (increased temperature) | Yeast showed improved adaptation to elevated temperatures, enhancing fermentation performance | Genome-wide mutations led to optimized protein quality control and heat shock response, stabilizing cellular functions under thermal stress | Baijiu, beer, wine | [83] |
| Clostridium autoethanogenum | CO2 stress (high CO2 concentration) | Clostridium autoethanogenum showed increased robustness and productivity under high CO2 conditions | Genetic adaptations, including modifications in CO2 fixation pathways and energy conservation mechanisms, enhanced autotrophic growth and product yield | Biofuel fermentation, ethanol production | [84] |
| Saccharomyces cerevisiae | Alcohol selective pressure (high ethanol concentration) | Yeast strains demonstrated enhanced ethanol tolerance and survival in high-ethanol environments | Mutations in genes related to membrane lipid biosynthesis and stress-responsive pathways contributed to increased membrane integrity and stress resistance | Beer, wine, ethanol production | [85] |
| Kluyveromyces lactis | Ethanol tolerance stress (high ethanol concentration) | Kluyveromyces lactis showed enhanced ethanol tolerance under high ethanol conditions | The regulation of ethanol-responsive genes and stabilization of cellular membranes via lipid modification played key roles in adaptation | Wine, ethanol production | [86] |
| Lactobacillus casei | Freezing stress (−20 °C) | Lactobacillus casei exhibited improved survival and metabolic stability under prolonged freezing conditions | Upregulation of cold-shock proteins and restructuring of membrane lipids improved cellular resilience to freezing-induced damage | Frozen yogurt, frozen fermented foods | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Z.; Xie, T.; Deng, H.; Xiao, S.; Yang, T. Directed Evolution of Microbial Communities in Fermented Foods: Strategies, Mechanisms, and Challenges. Foods 2025, 14, 216. https://doi.org/10.3390/foods14020216
Yao Z, Xie T, Deng H, Xiao S, Yang T. Directed Evolution of Microbial Communities in Fermented Foods: Strategies, Mechanisms, and Challenges. Foods. 2025; 14(2):216. https://doi.org/10.3390/foods14020216
Chicago/Turabian StyleYao, Zihan, Ting Xie, Hongjie Deng, Shuzhi Xiao, and Tao Yang. 2025. "Directed Evolution of Microbial Communities in Fermented Foods: Strategies, Mechanisms, and Challenges" Foods 14, no. 2: 216. https://doi.org/10.3390/foods14020216
APA StyleYao, Z., Xie, T., Deng, H., Xiao, S., & Yang, T. (2025). Directed Evolution of Microbial Communities in Fermented Foods: Strategies, Mechanisms, and Challenges. Foods, 14(2), 216. https://doi.org/10.3390/foods14020216







