Microbial Control in the Processing of Low-Temperature Meat Products: Non-Thermal Sterilization and Natural Antimicrobials
Abstract
1. Introduction
2. Non-Thermal Sterilization Techniques
| Physical Technology | Technical Parameters | Culture Condition | Target of an Action | Consequences of Action | Medium of Action | Bibliography | |
|---|---|---|---|---|---|---|---|
| Supercritical CO2 | 12 MPa, 50 °C, 15 min | 37 °C, 48 h | Listeria monocytogenes | Listeria monocytogenes inactivation 107 CFU/g. | - | Cured ham | [6] |
| 121 MPa, 50 °C, 5 min | - | mesophilic aerobic bacteria, psychrophile, Lactic Acid Bacteria, yeasts, mold, and Escherichia coli | Reductions of 3.0, 1.6, and 2.5 log CFU/g were observed for mesophilic aerobic bacteria, psychrophilic bacteria, and lactic acid bacteria, respectively. Meanwhile, yeasts, molds, and Escherichia coli were reduced to levels below the detection limit. | - | Hams | [16] | |
| Air conditioning | 30% CO2/70% N2 | −1 ± 0.1 °C | Pseudomonas spp., Lactic Acid Bacteria, and Enterobacteriaceae | Significantly inhibited the growth of Pseudomonas spp. | Extended storage period to 31 days. | Chicken meat | [17] |
| 25% CO2, 35% N2 | 4 °C | Pseudomonas spp., Lactobacillus, and Carn obacterium | - | Maximum shelf life of 9 days. | Frozen boneless beef | [18] | |
| 50% O2/40% CO2/10% N2 | 2 °C | Pseudomonas spp., Lactic Acid Bacteria, Enterobacteriaceae | - | Extends steak shelf life to 20 days. | Steak | [9] | |
| Ultra-high pressure | 600 MPa, 6 min, 31 °C | 4 °C | Lactic Acid Bacteria, enteric bacteria | After 60 days of pressurization, the counts of lactic acid bacteria were reduced by 6 logs compared to the counts in the blank samples. | Shelf life can be extended to at least 120 days. | Sliced corned beef and ham | [19] |
| 600 MPa, 20 °C, 180 s | 4 °C | Listeria monocytogenes, staphylococcus, Streptomyces thermophilus, coliform, yeasts, mold | Remains non-detectable for 95 days after pressure treatment. | The shelf life is significantly extended from approximately 45 to 50 days under refrigeration to at least 98 days. | Ready-to-eat (RTE) meat (low-fat pastrami, Strasbourg beef, export sausages, and Cajun beef) | [20] | |
| 310 MPa, 324 MPa, 345 MPa, 1 min, (25 ± 2) °C, | 4 °C | Aerobic bacteria (ATC) | Reduction in total ATC 1.37 log10 CFU g. | Shelf life up to 42 days. | Beef | [11] | |
| Irradiation | 10 kJ/m2 | 20 °C | Pseudomonas | The populations of total aerobic bacteria were significantly reduced by 1.76 log CFU/g. | Shelf life of 60 days. | Beef jerky | [21] |
| 0.5, 1, 2, 5, 10 kGy. | 4 °C | E. coli O157:H7, S. Typhimurium | For E. coli O157:H7 and S. Typhimurium, single or repeated irradiation at 0.5 kGy resulted in complete inactivation. | - | Fish products | [22] | |
| 3 kGy | 4 °C | Escherichia coli, Listeria monocytogenes | Effectively eliminates these bacteria over 4 log and 3 log units, respectively. | Safe storage for 12 days | Raw beef sausage | [23] | |
| 6. 0 kGy | 4 °C - | total bacterial count, coliforms, and Staphylococcus aureus | Total bacterial count, coliforms, and Staphylococcus aureus were reduced to national food safety standards. | 6. 0 kGy prolonged the shelf life to 10 days at least. | Spiced beef | [24] | |
| 2 kGy | 4 ± 0.5 °C | Salmonella, S. typhimurium | Salmonella, S. typhimurium, and total bacterial populations were reduced by 5, 3, and 3 log, respectively, at 2, kGy doses, 4 °C, and 45 days Salmonella levels were reduced to zero. | - | Beef sausage | [25] | |
| 7.5 kGy | 3 ± 1 °C | Staphylococcus aureus, Serratia marcescens, Enterobacter cloacae | Microbial community 7.23 log CFU/g to 1.56 log CFU/g. | Extended shelf life by two months. | Smoked pearl chicken | [26] | |
| microwave | 896 MHz, 3 °C, 7 kw, 2 min | 37 °C | the total plate count | On the seventh day, the total plate count (TPC) of intermediate moisture saury was 3.36 log CFU/g. | - | Intermediate moisture pacific saury | [27] |
| Super chilling | - | −2 ± 1 °C | Total aerobic bacteria | TAB < 7 log CFU/g for 14 day subcooled samples. | Subcooling can extend the shelf life of beef by at least two times compared to refrigeration. | Beef cuts | [28] |
| - | −3 °C | Total Viable Count | When stored for 6 days, the total viable count (TVC) values of cattle and buffalo tripe were 5.92 and 5.97 log values, respectively, which did not exceed the limit value. | Ultra-cold stored tripe has a shelf life of up to 6 days, double that of refrigerated. | Tripes | [29] | |
| - | –1 °C | total viable count (TVC), E. coli, V. parahaemolyticus | On day 28, TVC increased to <300 CFU/g, and levels of Vibrio parahaemolyticus (<3.0 MPN/g) and E. coli (<18 MPN/100 g) remained extremely low. | Subcooled storage can extend shelf life up to 21 days. | Crassostrea gigas | [30] | |
| Pulsed light (PL) | 11.9 J/cm2 | 4 °C | Listeria monocytogenes, S. typhimurium, Salmonella | 1.5 logcfu/cm2 to 1.8 logcfu/cm2. | - | Salami and pork tenderloin | [31] |
| Low concentration acidic electrolytic water (LCAEW) | Spray meat samples 120 s, 0.1% NaCl, 10 min | 4 °C | Yeasts, Mold, psychrophile | Decrease in total microorganisms by 3.25 logs, yeasts and molds by 2.68 logs, and total chilled bacteria by 3.10 logs. | - | Pork | [32] |
3. Biological Control Technology
3.1. Bacteriostatic Agents of Animal Origin
| Antibacterial Agent | Dosages | Culture Condition | Target of an Action | Consequences of Action | Medium of Action | Bibliography | |
|---|---|---|---|---|---|---|---|
| Chitosan | 0.5%, 1% | 4 °C | Lactic Acid Bacteria, Pseudomonas spp. | LAB counts were approximately 1 and 1.5 log CFU/g lower than control samples, respectively. | Acceptable level within 21 days | Pork sausage | [43] |
| 1.0% | 4 °C | Yeasts, mold, Lactic Acid Bacteria | The maximum colony size was 3 log CFU/g at 18 days. | Shelf life extended from 7 to 15 days | Pork | [44] | |
| 1.0% | 4 °C | Staphylococcus aureus, Pseudomonas, Proteus vulgaris, and Escherichia coli | 3.9 and 4.1 log CFU/g, respectively, until the end of the storage period. | Effectiveness for 20 days | Pork sausage | [45] | |
| Propolis | 0.5%, 1.0% 2.0% (w/v) | 4 °C | total mesophilic aerobic | The total mesophilic aerobic bacteria on day 49 for 0.5%, 1%, and 2% treatments were 5.41, 3.84, and 3.73 log CFU/g, respectively. | - | Tuscan sausage | [46] |
| 0.15 mg/mL | 4 °C | Clostridium | The addition of propolis reduced the number of non-toxic Clostridium difficile by 3 log CFU/g on day 5. | - | Fermented meat sausage | [47] | |
| α137-141 | 0.5% (w/w). | 4 °C | coliform | The coliform count was 5.07 ± 0.09 log CFU/g and had the slowest growth in viable colony counts. | Inhibits microbial growth for 14 days under refrigerated conditions | Beef | [48] |
3.2. Plant-Derived Bacteriostatic Agents
| Antibacterial Agent | Dosages | Culture Condition | Target of an Action | Consequences of Action | Medium of Action | Bibliography | |
|---|---|---|---|---|---|---|---|
| Peppermint Essential Oil | 0.5%, 1%, 1.5%, v/w | 4 °C | Pseudomonas spp. | 12 day bacterial population decreased by 1–4 log CFU/g. | - | Camel meat | [64] |
| Freeze-dried Allium sativum along with its spray-dried microencapsulated essential oil | 20% | 4–8 °C | Total aerobic mesophilic flora (GAMT), E. coli, Sulfite-reducing anaerobes (CSR) | On the sixth day of storage, the GAMT was 6.4 ± 0.4 log CFU/g. E. coli, and sulphite-reducing anaerobes (CSR) were not detected. | The shelf life of minced meat with satisfactory quality (x ≤ m) is extended by 4 days. | Pork | [65] |
| Eucommia ulmoides male flower extract (EUMFE) | 40, 80 mg/mL | 4 °C | Staphylococcus aureus | The number of Staphylococcus aureus strains in cooked beef was significantly reduced after treatment (p < 0.05). | - | Beef | [66] |
| Freeze-dried pomegranate peel nanoparticles (LPP-NP) | 1% | 4 ± 1 °C | psychrophile | A reduction of 2.91 log CFU/g in colony count was observed compared to the control group. | Storage for up to 15 days. | Beef meatballs | [67] |
| Extraction and encapsulation of Laurus nobilis leaf extract with nano-liposome | - | 4 ± 1 °C | Escherichia coli, Staphylococcus aureus | No Escherichia coli was observed in the 1500 ppm nanocapsule extract on day 8. | Storage for 16 days. | Spray ground beef | [68] |
| Addition of microencapsulated jabuticaba extract | 20 g/L (2%) | 1 ± 1 °C | Staphylococcus aureus, Escherichia coli | Microbiological reduction of more than 1 logCFU. | Storage for 15 days. | Pork sausage | [69] |
| Rosa Canina extract | 0.1% | 4 ± 1 °C | - | - | The shelf life was extended by one week. | Instant beef cocktail sausage | [70] |
| Black quinoa | 2.5% | 4 °C | MAB, LAB | During the 21 days of storage, MAB and LAB count remained below 4 log CFU/g and 3 log CFU/g, respectively. | - | Bologna-type sausages | [71] |
3.3. Bacteriostatic Agents of Microbial Origin
| Antibacterial Agent | Dosages | Culture Condition | Target of an Action | Consequences of Action | Medium of Action | Bibliography | |
|---|---|---|---|---|---|---|---|
| bacteriocinogenic Lactobacillus curvatus UFV-NPAC1 | 12.5, 6.25 mg/g | 7 °C | Listeria monocytogenes | Listeria monocytogenes reductions ranged from 1.0 to 3.0 log CFU/g. | Storage period 10 days | Sausages | [85] |
| Lactic acid bacteria | L. sakei subsp. carnosus/L. sakei + S. xylosus (1/2 ratio) | 4 ± 2 °C | B. thermosphacta | The 12 day colony count did not exceed the 3.1 log CFU/g level. | Shelf life extended to 12 days | Beef | [86] |
| Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus rhamnosus combination marinade (ML) | 4 °C | Escherichia coli, Listeria monocytogenes, and S. typhimurium | Escherichia coli O157:H7, Listeria monocytogenes, and S. typhimurium colony counts in the 0.7–2.7, 2.1–3.3, and 0.8–2.0 log CFU/g. | - | Beef | [87] | |
| Calcium propionate (CaP) and tea polyphenols (TPs) | 0.3% CaP + 0.03% TPs | 4 °C | TVC | TVC was consistently less than 4% during the 12 day storage period. | Shelf life is extended by at least 4 days. | Stewed beef | [88] |
4. Control of Microorganisms in Meat Products by Fencing Techniques
4.1. Composite Inhibitors and Composite Coatings
4.2. Physical Technology Linkage
4.3. Synergistic Treatment with Biotechnology and Physical Technology
| Physical Technology | Processing Parameter | Biotechnology | Dosages | Target of an Action | Consequences of Action | Medium of Action | Bibliography | |
|---|---|---|---|---|---|---|---|---|
| Radiographic irradiation | 3 kGy | Chitosan solution | 1.5% | - | The total colony count did not exceed 3.0 log CFU/g in 15 d. | - | Pork | [129] |
| cold nitrogen plasma (CNP) | (500 W, 120 s) | Lemongrass essential oil | 5 mg/mL, 30 min | Listeria monocytogenes | Listeria monocytogenes population decreased by 2.80 log CFU/g. | Shelf life extended by 4 days. | Pork | [130] |
| Radio-frequency heating (55 °C) | 27.12 MHz, 6 kW | Citric acid and potassium bicarbonate mixture | 0.5 per cent citric acid and 1.5 per cent potassium bicarbonate | Escherichia coli, Aerobic bacteria | Escherichia coli and aerobic bacteria plate counts were reduced by more than 4 log CFU/mL. | - | Ground beef (80/20; lean/fat) | [131] |
| Modified atmosphere packaging | 40% CO2:60% N2 | Ag/LDPE | Ag NPs (0.5% or 1%, w/w) | TVC, psychrotrophic bacteria, | The growth rate of psychrotrophic bacteria was slowed down by 16.67 percent. | Shelf life extended to 8 days. | Chicken breast fillets | [132] |
| Critical carbon dioxide combined with high power ultrasound (SC—CO2 + HPU) | 25 MPa, 46 °C and 10 min | Saline | 0.85% | Escherichia coli | Reduced colony count 3.62 ± 0.20 log CFU/g. | Storage 20 days. | Cured ham | [133] |
| LED | 405 nm, 19.2 J/cm2 | Riboflavin (vitamin B2) | 50 μM | Listeria monocytogenes | Reduced by 6.2 log CFU/mL. | - | Smoked salmon | [134] |
| High hydrostatic pressure (HHP) | 300 MPa/5 min after tumbling, 600 MPa/3 min for final product | KCl | 50 percent partial replacement of NaCl | - | - | Shelf life extended by 60 days. | Ready-to-eat (RTE) chicken breast | [135] |
| Oppressive | 600 MPa, 180 s | NaCl | 1.5% | Listeria monocytogenes | Decrease of 2.49 and 7.29 logarithms. | - | Chicken meat mince | [136] |
| EAP | 2.2 kHz and 8.4 kVpp, 15 min | Clove oil | 1.0% | Escherichia coli O157:H7 | Reduction of more than 7 log CFU/g. | - | Beef jerky | [137] |
| Air conditioning | MAP, 70% CO2, 30% N2 | Chitosan solution | 1 g/100 ml | Pseudomonas, lactic acid bacteria, and Enterobacteriaceae | Pseudomonas spp. control samples were 3.3 log CFU/g lower and Enterobacteriaceae populations remained unchanged between days 4 and 14 of storage (~1.0 log cfu/g). | Shelf life extended by 9 days. | Chicken breast | [138] |
| Modified atmosphere packaging (MAP) | 20% CO2, 80% N2 | Ziziphora clinopodioides essential oil and lysozyme | ZCEO (0.3%) lysozyme (400 μg/g) | Listeria monocytogenes | Storage 13 days Listeria monocytogenes 2.05 log CFU/g. | - | Balkan-style fresh sausage | [139] |
| High hydrostatic pressure | 400 MPa for 10 min | entertain LM-2 | 2560 AU/g | L. monocytogenes, S. enteritidis | Prevents the growth of Streptococcus enterocolitica, below the detection limit throughout the storage period at 4 °C. | Extending the shelf life to above 90 days. | Ready-to-eat sliced vacuum-packed cooked ham | [140] |
| Vacuum packaging | - | EO (CA, CI, and TH) | 2% | Pseudomonas aeruginosa | Reduces microbial populations by up to 4 to 6 log colony forming units (CFU)/g. | - | Chicken work | [141] |
| Low-voltage electric fields | 15 cm, 3000 V, 50 Hz, −1 ± 0.5 °C | Compound preservatives | 4% Lysozyme + 2% Nisin + 0.75% Eugenol | Bacillus subtilis, Pseudomonas | The absorbance values were reduced by 30.16% and 44.58%, respectively. | - | Mytilus edulis | [14] |
| High-pressure processing | 400 MPa, 10 min | Spice extracts | 0.05% clove + 0.05% cinnamon extracts | TVC, LAB, B. thermosphacta, and C. perfringens | TVC was significantly reduced to less than 3 log CFU/g in the 12 day treatment group, and no LAB was detected. | - | Low-salt Sausage | [142] |
| MAP | 80% N2, 20% CO2 | Aronia melanocarpa, Chaenomeles superba, and Cornus mas leaf extracts | 5% v/w | TVC, LAB, and Enterobacteriaceae | TVC was significantly reduced to less than 3 log CFU/g in the 12 day treatment group, and no LAB was detected. | - | Pork | [143] |
| Electron-beam Irradiation | 4 kGy | Leek extract on the quality | 0.5%, 1.0% | Escherichia coli, mold | No mold or Escherichia coli were detected. | - | Pork Jerky | [144] |
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thathsarani, A.; Alahakoon, A.U.; Liyanage, R. Current status and future trends of sous vide processing in meat industry; A review. Trends Food Sci. Technol. 2022, 129, 353–363. [Google Scholar] [CrossRef]
- Fei, N.; Li, F.; Dang, M.; Xia, X. Characteristics and application of non-thermal sterilization technology in meat products processing. J. Food Saf. Qual. 2015, 6, 540–544. [Google Scholar]
- Pan, Y.; Deng, Z.; Shahidi, F. Natural bioactive substances for the control of food-borne viruses and contaminants in food. Food Prod. Process. Nutr. 2020, 2, 27. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, Y.; Liu, J.; Wu, Z.; Zhang, Z. Research Progress on the Application of High-Pressure Carbon Dioxide in Meat Pasteurization and Preservation. Food Ind. Technol. 2022, 43, 471–479. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.-D.A. Current and future prospects for the use of pulsed electric field in the meat industry. Crit. Rev. Food Sci. Nutr. 2018, 59, 1660–1674. [Google Scholar] [CrossRef] [PubMed]
- Ferrentino, G.; Balzan, S.; Spilimbergo, S. Supercritical Carbon Dioxide Processing of Dry Cured Ham Spiked with Listeria monocytogenes: Inactivation Kinetics, Color, and Sensory Evaluations. Food Bioprocess Technol. 2012, 6, 1164–1174. [Google Scholar] [CrossRef]
- Kandeepan, G.; Tahseen, A. Modified Atmosphere Packaging (MAP) of Meat and Meat Products: A Review. J. Packag. Technol. Res. 2022, 6, 137–148. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Zhu, L.; Han, M.; Gao, S.; Luo, X. Effect of packaging atmospheres on storage quality characteristics of heavily marbled beef longissimus steaks. Meat Sci. 2016, 117, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Niu, L.; Zhu, L.; Liang, R.; Zhang, Y.; Luo, X. Shelf-Life Extension of Chill-Stored Beef Longissimus Steaks Packaged under Modified Atmospheres with 50% O2 and 40% CO2. J. Food Sci. 2016, 81, C1692–C1698. [Google Scholar] [CrossRef] [PubMed]
- Agregán, R.; Munekata, P.E.; Zhang, W.; Zhang, J.; Pérez-Santaescolástica, C.; Lorenzo, J.M. High-pressure processing in inactivation of Salmonella spp. in food products. Trends Food Sci. Technol. 2021, 107, 31–37. [Google Scholar] [CrossRef]
- Sánchez-Basurto, B.E.; Ramírez-Gilly, M.; Tecante, A.; Severiano-Pérez, P.; Wacher, C.; Valdivia-López, M.A. Effect of High Hydrostatic Pressure Treatment on the Preservation of Beef Meat. Ind. Eng. Chem. Res. 2011, 51, 5932–5938. [Google Scholar] [CrossRef]
- Reineke, K.; Mathys, A. Endospore Inactivation by Emerging Technologies: A Review of Target Structures and Inactivation Mechanisms. Annu. Rev. Food Sci. Technol. 2020, 11, 255–274. [Google Scholar] [CrossRef]
- Lung, H.-M.; Cheng, Y.-C.; Chang, Y.-H.; Huang, H.-W.; Yang, B.B.; Wang, C.-Y. Microbial decontamination of food by electron beam irradiation. Trends Food Sci. Technol. 2015, 44, 66–78. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Wu, Y.; Yi, C.; Lv, Y.; Luo, H.; Yang, T. The antibacterial mechanism of compound preservatives combined with low voltage electric fields on the preservation of steamed mussels (Mytilus edulis) stored at ice-temperature. Front. Nutr. 2023, 10, 1126456. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Yan, L.; Xiao, S.; Hou, B.; Zhou, X.; Wang, W.; Bai, T.; Zhu, K.; Cheng, J.; Zhang, J. Effects of Different Low-Temperature Storage Methods on the Quality and Processing Characteristics of Fresh Beef. Foods 2023, 12, 782. [Google Scholar] [CrossRef]
- Ferrentino, G.; Balzan, S.; Spilimbergo, S. Optimization of supercritical carbon dioxide treatment for the inactivation of the natural microbial flora in cubed cooked ham. Int. J. Food Microbiol. 2013, 161, 189–196. [Google Scholar] [CrossRef]
- Cha, H.; Liang, S.; Shi, K.; Xu, Z.; Ge, C.; Zhao, P.; Xiao, Z. Effect of modified atmosphere packaging on the quality characteristics and bacterial community succession of super-chilled chicken meat in biopreservation. LWT 2023, 189, 115547. [Google Scholar] [CrossRef]
- Hou, X.; Zhao, H.; Yan, L.; Li, S.; Chen, X.; Fan, J. Effect of CO2 on the preservation effectiveness of chilled fresh boneless beef knuckle in modified atmosphere packaging and microbial diversity analysis. LWT 2023, 187, 115262. [Google Scholar] [CrossRef]
- Garriga, M.; Grèbol, N.; Aymerich, M.; Monfort, J.; Hugas, M. Microbial inactivation after high-pressure processing at 600 MPa in commercial meat products over its shelf life. Innov. Food Sci. Emerg. Technol. 2004, 5, 451–457. [Google Scholar] [CrossRef]
- Gilstrap, O.; Liu, C.; Nindo, C.; Parveen, S. Pilot Scale Assessment of High-Pressure Processing (HPP) to Enhance Microbiological Quality and Shelf Life of Fresh Ready-to-Eat (RTE) Blue Crab Meat. Microorganisms 2023, 11, 2909. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Chun, H.-H.; Song, H.-J.; Song, K.-B. Effects of electron beam irradiation on the microbial growth and quality of beef jerky during storage. Radiat. Phys. Chem. 2010, 79, 1165–1168. [Google Scholar] [CrossRef]
- Je, G.-S.; Chung, D.-H.; Shim, W.-B. Reducing Effect of Microorganism on Meat and Fish Products by Repeated γ-Irradiation at Low Dose. J. Food Hyg. Saf. 2015, 30, 92–97. [Google Scholar] [CrossRef]
- Badr, H.M. Elimination of Escherichia coli O157:H7 and Listeria monocytogenes from raw beef sausage by γ-irradiation. Mol. Nutr. Food Res. 2005, 49, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Effect of Electron Beam Irradiation on Quality of Spiced Beef. J. Anhui Agric. Sci. 2014, 42, 4441–4443. [Google Scholar]
- Bouzarjomehri, F.; Dad, V.; Hajimohammadi, B.; Shirmardi, S.; Salimi, A.Y.-G. The effect of electron-beam irradiation on microbiological properties and sensory characteristics of sausages. Radiat. Phys. Chem. 2020, 168, 108524. [Google Scholar] [CrossRef]
- Otoo, E.A.; Ocloo, F.C.; Appiah, V. Effect of gamma irradiation on shelf life of smoked guinea fowl (Numida meleagris) meat stored at refrigeration temperature. Radiat. Phys. Chem. 2022, 194, 110041. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Wang, Y.; Zhu, Q.; Wang, X.; Luan, D. Effects of Microwave Pasteurization on the Quality and Shelf-Life of Low-Sodium and Intermediate-Moisture Pacific Saury (Cololabis saira). Foods 2023, 12, 2000. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kwon, J.A.; Kim, M.; Lee, Y.E.; Ryu, M.; Jo, C. Effect of supercooling on storage ability of different beef cuts in comparison to traditional storage methods. Meat Sci. 2023, 199, 109137. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; He, Z.F.; Zhang, X.; Li, X.M.; Liao, L.; Wang, Q.; Cheng, C.P.; Lee, H.J. Effects of micro-freezing and cold storage on the quality of two kinds of tripes. Food Ferment. Ind. 2022, 48, 257–266. [Google Scholar]
- Dong, S.; Niu, Y.; Wei, H.; Lin, Y.; Lu, X.; Yamashita, T.; Yu, K.; Takaki, K.; Yuan, C. Effect of super-chilling storage on maintenance of quality and freshness of the Pacific oyster (Crassostrea gigas). Food Qual. Saf. 2023, 7, fyad008. [Google Scholar] [CrossRef]
- Ganan, M.; Hierro, E.; Hospital, X.F.; Barroso, E.; Fernández, M. Use of pulsed light to increase the safety of ready-to-eat cured meat products. Food Control 2013, 32, 512–517. [Google Scholar] [CrossRef]
- Brychcy, E.; Malik, M.; Drożdżewski, P.; Ulbin-Figlewicz, N.; Jarmoluk, A. Low-concentrated acidic electrolysed water treatment of pork: Inactivation of surface microbiota and changes in product quality. Int. J. Food Sci. Technol. 2015, 50, 2340–2350. [Google Scholar] [CrossRef]
- Chen, X.; Lan, W.; Xie, J. Natural phenolic compounds: Antimicrobial properties, antimicrobial mechanisms, and potential utilization in the preservation of aquatic products. Food Chem. 2024, 440, 138198. [Google Scholar] [CrossRef]
- Vaz, J.M.; Pezzoli, D.; Chevallier, P.; Campelo, C.S.; Candiani, G.; Mantovani, D. Antibacterial Coatings Based on Chitosan for Pharmaceutical and Biomedical Applications. Curr. Pharm. Des. 2018, 24, 866–885. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Haghighi, H.; Leugoue, S.K.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Fava, P.; Pulvirenti, A. Development of antimicrobial films based on chitosan-polyvinyl alcohol blend enriched with ethyl lauroyl arginate (LAE) for food packaging applications. Food Hydrocoll. 2020, 100, 105419. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Król, W.; Bankova, V.; Sforcin, J.M.; Szliszka, E.; Czuba, Z.; Kuropatnicki, A.K. Propolis: Properties, Application, and Its Potential. Evid.-Based Complement. Altern. Med. 2013, 2013, 807578. [Google Scholar] [CrossRef]
- Frozza, C.O.d.S.; Garcia, C.S.C.; Gambato, G.; de Souza, M.D.O.; Salvador, M.; Moura, S.; Padilha, F.F.; Seixas, F.K.; Collares, T.; Borsuk, S.; et al. Chemical characterization, antioxidant and cytotoxic activities of Brazilian red propolis. Food Chem. Toxicol. 2013, 52, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Medina, L.; Guzmán, F.; Álvarez, C.; Delgado, J.P.; Carbonell-M, B. Ramosin: The First Antibacterial Peptide Identified on Bolitoglossa ramosi Colombian Salamander. Pharmaceutics 2022, 14, 2579. [Google Scholar] [CrossRef]
- Drayton, M.; Deisinger, J.P.; Ludwig, K.C.; Raheem, N.; Müller, A.; Schneider, T.; Straus, S.K. Host Defense Peptides: Dual Antimicrobial and Immunomodulatory Action. Int. J. Mol. Sci. 2021, 22, 11172. [Google Scholar] [CrossRef] [PubMed]
- Ookubo, M.; Tashiro, Y.; Asano, K.; Kamei, Y.; Tanaka, Y.; Honda, T.; Yokoyama, T.; Honda, M. “Rich arginine and strong positive charge” antimicrobial protein protamine: From its action on cell membranes to inhibition of bacterial vital functions. Biochim. Biophys. Acta (BBA)—Biomembr. 2024, 1866, 184323. [Google Scholar] [CrossRef] [PubMed]
- Soultos, N.; Tzikas, Z.; Abrahim, A.; Georgantelis, D.; Ambrosiadis, I. Chitosan effects on quality properties of Greek style fresh pork sausages. Meat Sci. 2008, 80, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, S.; Board, R.; Roller, S. Chitosan inhibits growth of spoilage micro-organisms in chilled pork products. Food Microbiol. 2002, 19, 175–182. [Google Scholar] [CrossRef]
- Georgantelis, D.; Ambrosiadis, I.; Katikou, P.; Blekas, G.; Georgakis, S.A. Effect of rosemary extract, chitosan and α-tocopherol on microbiological parameters and lipid oxidation of fresh pork sausages stored at 4 °C. Meat Sci. 2007, 76, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Viera, V.B.; Piovesan, N.; Moro, K.I.B.; Rodrigues, A.S.; Scapin, G.; da Rosa, C.S.; Kubota, E.H. Preparation and microbiological analysis of Tuscan sausage with added propolis extract. Food Sci. Technol. 2016, 36 (Suppl. S1), 37–41. [Google Scholar] [CrossRef]
- Castro, S.M.; Teixeira, P.; Yildiz, F.; Casquete, R.; Jácome, S. Antimicrobial activity of ethanolic extract of propolis in “Alheira”, a fermented meat sausage. Cogent Food Agric. 2016, 2, 1125773. [Google Scholar] [CrossRef]
- Przybylski, R.; Firdaous, L.; Châtaigné, G.; Dhulster, P.; Nedjar, N. Production of an antimicrobial peptide derived from slaughterhouse by-product and its potential application on meat as preservative. Food Chem. 2016, 211, 306–313. [Google Scholar] [CrossRef]
- He, K. Research Progress of Plant Active Ingredient Antibacterial Abilities and Mechanism. Bot. Res. 2018, 7, 29–36. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Huang, J.; Huang, K.; Liu, S.; Luo, Q.; Xu, M. Adsorption properties of tea polyphenols onto three polymeric adsorbents with amide group. J. Colloid Interface Sci. 2007, 315, 407–414. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Zhang, J.; Xu, D.; Li, Y.; Liu, Y.; Zhang, X.; Zhang, R.; Wu, Z.; Weng, P. Antimicrobial Effect of Tea Polyphenols against Foodborne Pathogens: A Review. J. Food Prot. 2021, 84, 1801–1808. [Google Scholar] [CrossRef]
- Chan, E.W.; Tie, P.P.; Soh, E.Y.; Law, Y.P. Antioxidant and antibacterial properties of green, black, and herbal teas of Camellia sinensis. Pharmacogn. Res. 2011, 3, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Landry, K.S.; Chang, Y.; McClements, D.J.; McLandsborough, L. Effectiveness of a novel spontaneous carvacrol nanoemulsion against Salmonella enterica Enteritidis and Escherichia coli O157:H7 on contaminated mung bean and alfalfa seeds. Int. J. Food Microbiol. 2014, 187, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of Four Monoterpenes Contained in Essential Oils with Model Membranes: Implications for Their Antibacterial Activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef]
- Liu, X.; Liao, W.; Xia, W. Recent advances in chitosan based bioactive materials for food preservation. Food Hydrocoll. 2023, 140, 108612. [Google Scholar] [CrossRef]
- Michalczyk, M.; Satora, P.; Banaś, J.; Fiutak, G. Effect of Adding Essential Oils of Caraway and Rosemary on Volatile Aroma Compounds Derived from Stored Vacuum Packaged Minced Turkey Meat. Ann. Anim. Sci. 2021, 21, 1133–1147. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2020, 61, 149–178. [Google Scholar] [CrossRef]
- Mashau, M.E.; Kgatla, T.E.; Makhado, M.V.; Mikasi, M.S.; Ramashia, S.E. Nutritional composition, polyphenolic compounds and biological activities of marula fruit (Sclerocarya birrea) with its potential food applications: A review. Int. J. Food Prop. 2022, 25, 1549–1575. [Google Scholar] [CrossRef]
- Skrinjar, M.; Nemet, N. Antimicrobial effects of spices and herbs essential oils. Acta Period. Technol. 2009, 40, 195–209. [Google Scholar] [CrossRef]
- Ateş, D.A.; Turgay Erdoğrul, Ö. Antimicrobial Activities of Various Medicinal and Commercial Plant Extracts. Turk. J. Biol. 2003, 27, 157–162. [Google Scholar]
- Kim, H.-O.; Park, S.-W.; Park, H.-D. Inactivation of Escherichia coli O157:H7 by cinnamic aldehyde purified from Cinnamomum cassia shoot. Food Microbiol. 2004, 21, 105–110. [Google Scholar] [CrossRef]
- Park, I.-K.; Lee, H.-S.; Lee, S.-G.; Park, J.-D.; Ahn, Y.-J. Insecticidal and Fumigant Activities of Cinnamomum cassia Bark-Derived Materials against Mechoris ursulus (Coleoptera: Attelabidae). J. Agric. Food Chem. 2000, 48, 2528–2531. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, Y.; Karami, N.; Shavisi, N. Effect of Mentha spicata essential oil on chemical, microbial, and sensory properties of minced camel meat during refrigerated storage. J. Food Saf. 2017, 38, e12375. [Google Scholar] [CrossRef]
- Najjaa, H.; Chekki, R.; Elfalleh, W.; Tlili, H.; Jaballah, S.; Bouzouita, N. Freeze-dried, oven-dried, and microencapsulation of essential oil from Allium sativum as potential preservative agents of minced meat. Food Sci. Nutr. 2020, 8, 1995–2003. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Liu, S.; Yu, Y.; Guo, L.; Wang, Y.; Feng, Y.; Fei, P.; Kang, H.; Ali, A. Antibacterial Mode of Eucommia ulmoides Male Flower Extract Against Staphylococcus aureus and Its Application as a Natural Preservative in Cooked Beef. Front. Microbiol. 2022, 13, 846622. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.K.; Mekawi, E.; Elsabagh, R. Impact of pomegranate peel nanoparticles on quality attributes of meatballs during refrigerated storage. LWT 2018, 89, 489–495. [Google Scholar] [CrossRef]
- Tometri, S.S.; Ahmady, M.; Ariaii, P.; Soltani, M.S. Extraction and encapsulation of Laurus nobilis leaf extract with nano-liposome and its effect on oxidative, microbial, bacterial and sensory properties of minced beef. J. Food Meas. Charact. 2020, 14, 3333–3344. [Google Scholar] [CrossRef]
- Baldin, J.C.; Michelin, E.C.; Polizer, Y.J.; Rodrigues, I.; de Godoy, S.H.S.; Fregonesi, R.P.; Pires, M.A.; Carvalho, L.T.; Fávaro-Trindade, C.S.; de Lima, C.G.; et al. Microencapsulated jabuticaba (Myrciaria cauliflora) extract added to fresh sausage as natural dye with antioxidant and antimicrobial activity. Meat Sci. 2016, 118, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Pamuk, A.; Gedikoğlu, A.; Sökmen, M. Use of a natural antioxidant, Cistus criticus extract, on lipid oxidation and shelf life of ready-to-eat beef cocktail sausages. J. Food Process. Preserv. 2022, 46, e16913. [Google Scholar] [CrossRef]
- Fernández-López, J.; Lucas-González, R.; Roldán-Verdú, A.; Viuda-Martos, M.; Sayas-Barberá, E.; Ballester-Sánchez, J.; Haros, C.M.; Pérez-Álvarez, J.A. Effects of Black Quinoa Wet-Milling Coproducts on the Quality Properties of Bologna-Type Sausages During Cold Storage. Foods 2020, 9, 274. [Google Scholar] [CrossRef]
- Tavares, T.D.; Ribeiro, A.R.; Silva, C.; Antunes, J.C.; Felgueiras, H.P. Combinatory effect of nisin antimicrobial peptide with bioactive molecules: A review. J. Drug Deliv. Sci. Technol. 2024, 91, 105246. [Google Scholar] [CrossRef]
- Bakhshi, A. Use of Nisin as a Natural Preservative in Food Industry. J. Nutr. Disord. Ther. 2020, 10, 5. [Google Scholar]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a Food Preservative: Part 1: Physicochemical Properties, Antimicrobial Activity, and Main Uses. Crit. Rev. Food Sci. Nutr. 2015, 56, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- de Arauz, L.J.; Jozala, A.F.; Mazzola, P.G.; Penna, T.C.V. Nisin biotechnological production and application: A review. Trends Food Sci. Technol. 2009, 20, 146–154. [Google Scholar] [CrossRef]
- Smaoui, S.; Ben Hlima, H.; Ben Braïek, O.; Ennouri, K.; Mellouli, L.; Khaneghah, A.M. Recent advancements in encapsulation of bioactive compounds as a promising technique for meat preservation. Meat Sci. 2021, 181, 108585. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, M.; Bhandari, B.; Xu, J.; Yang, C. Effects of nanoemulsion-based active coatings with composite mixture of star anise essential oil, polylysine, and nisin on the quality and shelf life of ready-to-eat Yao meat products. Food Control 2020, 107, 106771. [Google Scholar] [CrossRef]
- Geornaras, I.; Yoon, Y.; Belk, K.; Smith, G.; Sofos, J. Antimicrobial Activity of ε-Polylysine against Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes in Various Food Extracts. J. Food Sci. 2007, 72, M330–M334. [Google Scholar] [CrossRef]
- Ning, H.-Q.; Li, Y.-Q.; Lin, H.; Wang, J.-X. Apoptosis-induction effect of ε-poly-lysine against Staphylococcus aureus and its application on pasteurized milk. LWT 2021, 137, 110493. [Google Scholar] [CrossRef]
- Jia, Z.; Li, C.; Fang, T.; Chen, J. Predictive Modeling of the Effect of ε-Polylysine Hydrochloride on Growth and Thermal Inactivation of Listeria monocytogenes in Fish Balls. J. Food Sci. 2018, 84, 127–132. [Google Scholar] [CrossRef]
- Zhao, B.; Yuan, M.; Wang, L.; Liu, Z.; Fu, X.; Mukhtar, H.; Zhu, C.; Sun, H.; Yao, M.; Mou, H. Antibacterial activity of bifunctional bacterial cellulose composite grafted with glucose oxidase and l-arginine. Cellulose 2023, 30, 8973–8984. [Google Scholar] [CrossRef]
- Yousefi, M.; Nematollahi, A.; Shadnoush, M.; Mortazavian, A.M.; Khorshidian, N. Antimicrobial Activity of Films and Coatings Containing Lactoperoxidase System: A Review. Front. Nutr. 2022, 9, 828065. [Google Scholar] [CrossRef]
- Kaveh, S.; Hashemi, S.M.B.; Abedi, E.; Amiri, M.J.; Conte, F.L. Bio-Preservation of Meat and Fermented Meat Products by Lactic Acid Bacteria Strains and Their Antibacterial Metabolites. Sustainability 2023, 15, 10154. [Google Scholar] [CrossRef]
- Danielski, G.M.; Imazaki, P.H.; Cavalari, C.M.d.A.; Daube, G.; Clinquart, A.; de Macedo, R.E.F. Carnobacterium maltaromaticum as bioprotective culture in vitro and in cooked ham. Meat Sci. 2020, 162, 108035. [Google Scholar] [CrossRef] [PubMed]
- de Castilho, N.P.A.; Todorov, S.D.; Oliveira, L.L.; Bersot, L.d.S.; Nero, L.A. Inhibition of Listeria monocytogenes in fresh sausage by bacteriocinogenic Lactobacillus curvatus UFV-NPAC1 and its semi-purified bacteriocin. LWT 2020, 118, 108757. [Google Scholar] [CrossRef]
- Comi, G.; Tirloni, E.; Andyanto, D.; Manzano, M.; Iacumin, L. Use of bio-protective cultures to improve the shelf-life and the sensorial characteristics of commercial hamburgers. LWT 2015, 62, 1198–1202. [Google Scholar] [CrossRef]
- Gargi, A.; Sengun, I.Y. Marination liquids enriched with probiotics and their inactivation effects against food-borne pathogens inoculated on meat. Meat Sci. 2021, 182, 108624. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Lei, Y.; Wei, M.; Liu, X.; Yu, H.; Xie, P.; Sun, B. Preservation of stewed beef chunks by using calcium propionate and tea polyphenols. LWT 2023, 176, 114491. [Google Scholar] [CrossRef]
- Zuo, H.; Wang, B.; Zhang, J.; Zhong, Z.; Tang, Z. Research Progress on Bacteria-Reducing Pretreatment Technology of Meat. Foods 2024, 13, 2361. [Google Scholar] [CrossRef]
- Yin, W.; Qiu, C.; Ji, H.; Li, X.; Sang, S.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z. Recent advances in biomolecule-based films and coatings for active and smart food packaging applications. Food Biosci. 2023, 52, 102378. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Kaveh, S.; Abedi, E.; Phimolsiripol, Y. Polysaccharide-Based Edible Films/Coatings for the Preservation of Meat and Fish Products: Emphasis on Incorporation of Lipid-Based Nanosystems Loaded with Bioactive Compounds. Foods 2023, 12, 3268. [Google Scholar] [CrossRef]
- Qin, Y.-Y.; Yang, J.-Y.; Lu, H.-B.; Wang, S.-S.; Yang, J.; Yang, X.-C.; Chai, M.; Li, L.; Cao, J.-X. Effect of chitosan film incorporated with tea polyphenol on quality and shelf life of pork meat patties. Int. J. Biol. Macromol. 2013, 61, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Deng, S.; Tian, H.; Kou, X.; Yu, H.; Huang, J.; Lou, X.; Yuan, H. Novel bioactive sponge mats composed of oxidized bacterial cellulose and chitosan-gum Arabic microcapsules loaded with cinnamon essential oil for enhancing meat preservation. Food Hydrocoll. 2024, 148, 109496. [Google Scholar] [CrossRef]
- Chen, L.; Niu, X.; Fan, X.; Liu, Y.; Yang, J.; Xu, X.; Zhou, G.; Zhu, B.; Ullah, N.; Feng, X. Highly absorbent antibacterial chitosan-based aerogels for shelf-life extension of fresh pork. Food Control 2022, 136, 108644. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Kang, H.; Peng, X. Effect of tannic acid-grafted chitosan coating on the quality of fresh pork slices during cold storage. Meat Sci. 2022, 188, 108779. [Google Scholar] [CrossRef] [PubMed]
- Montaño-Sánchez, E.; Torres-Martínez, B.d.M.; Vargas-Sánchez, R.D.; Huerta-Leidenz, N.; Sánchez-Escalante, A.; Beriain, M.J.; Torrescano-Urrutia, G.R. Effects of Chitosan Coating with Green Tea Aqueous Extract on Lipid Oxidation and Microbial Growth in Pork Chops during Chilled Storage. Foods 2020, 9, 766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, Q.; Lu, F.; Bie, X.; Zhao, H.; Lu, Z.; Lu, Y. A Novel Class IIb Bacteriocin-Plantaricin EmF Effectively Inhibits Listeria monocytogenes and Extends the Shelf Life of Beef in Combination with Chitosan. J. Agric. Food Chem. 2022, 70, 2187–2196. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, X.; Zhong, H.; Li, C.; Shi, C.; Cui, H.; Lin, L. Ethyl cellulose/gelatin-carboxymethyl chitosan bilayer films doped with Euryale ferox seed shell polyphenol for cooked meat preservation. Int. J. Biol. Macromol. 2024, 256, 128286. [Google Scholar] [CrossRef] [PubMed]
- Karam, L.; Chehab, R.; Osaili, T.M.; Savvaidis, I.N. Antimicrobial effect of thymol and carvacrol added to a vinegar-based marinade for controlling spoilage of marinated beef (Shawarma) stored in air or vacuum packaging. Int. J. Food Microbiol. 2020, 332, 108769. [Google Scholar] [CrossRef] [PubMed]
- Tajik, H.; Aminzare, M.; Raad, T.; Hashemi, M.; Hassanzadazar, H.; Raeisi, M.; Naghili, H. Effect of Zataria Multiflora Boiss Essential Oil and Grape Seed Extract on the Shelf Life of Raw Buffalo Patty and Fate of Inoculated Listeria Monocytogenes. Soc. Sci. Res. Netw. 2015, 39, 3005–3013. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, Y.; Li, R. Effects of chitosan-based coatings incorporated with ε-polylysine and ascorbic acid on the shelf-life of pork. Food Chem. 2022, 390, 133206. [Google Scholar] [CrossRef]
- Guo, Z.; Han, L.; Yu, Q.-L.; Lin, L. Effect of a sea buckthorn pomace extract-esterified potato starch film on the quality and spoilage bacteria of beef jerky sold in supermarket. Food Chem. 2020, 326, 127001. [Google Scholar] [CrossRef]
- Albertos, I.; Avena-Bustillos, R.J.; Martín-Diana, A.B.; Du, W.-X.; Rico, D.; McHugh, T.H. Antimicrobial Olive Leaf Gelatin films for enhancing the quality of cold-smoked Salmon. Food Packag. Shelf Life 2017, 13, 49–55. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Xiao, Z.; Bi, W. Effect of chitosan nanoparticles loaded with cinnamon essential oil on the quality of chilled pork. LWT-Food Sci. Technol. 2015, 63, 519–526. [Google Scholar] [CrossRef]
- Kim, G.H.; Chin, K.B. Effect of sea tangle extract on the quality characteristics of reduced-salt, low-fat sausages using pre-rigor muscle during refrigerated storage. Anim. Biosci. 2023, 36, 1738–1746. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Tang, H.; Zhang, J.; Zhang, L.; Yang, X.; Wang, F.; Chen, L. Effect of lysozyme and Chinese liquor on Staphylococcus aureus growth, microbiome, flavor profile, and the quality of dry fermented sausage. LWT 2021, 150, 112059. [Google Scholar] [CrossRef]
- Woo, S.-H.; Sung, J.-M.; Park, H.J.; Kim, J.; Kim, Y.-J.; Kim, T.-K.; Lee, H.; Choi, Y.-S. Inhibitory effect of natural extract mixtures on microbial growth and lipid oxidation of sausages during storage. J. Anim. Sci. Technol. 2023, 65, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Rehmani, N.; Alsubaie, L.; Kim, C.; Sismour, E.; Scales, A. Tapioca starch active nanocomposite films and their antimicrobial effectiveness on ready-to-eat chicken meat. Food Packag. Shelf Life 2018, 16, 86–91. [Google Scholar] [CrossRef]
- Moradi, E.; Moosavi, M.H.; Hosseini, S.M.; Mirmoghtadaie, L.; Moslehishad, M.; Khani, M.R.; Jannatyha, N.; Shojaee-Aliabadi, S. Prolonging shelf life of chicken breast fillets by using plasma-improved chitosan/low density polyethylene bilayer film containing summer savory essential oil. Int. J. Biol. Macromol. 2020, 156, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ye, K.; Liu, P.; Yuan, A.; Chen, S.; He, Y. Effect of a Konjac glucomannan/chitosan antibacterial composite membrane microencapsulated with oregano essential oil on the quality of chilled pork. Appl. Food Res. 2023, 3, 100249. [Google Scholar] [CrossRef]
- Esmaeili, H.; Cheraghi, N.; Khanjari, A.; Rezaeigolestani, M.; Basti, A.A.; Kamkar, A.; Aghaee, E.M. Incorporation of nanoencapsulated garlic essential oil into edible films: A novel approach for extending shelf life of vacuum-packed sausages. Meat Sci. 2020, 166, 108135. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Gidley, M.; Dykes, G. Potential of a nisin-containing bacterial cellulose film to inhibit Listeria monocytogenes on processed meats. Food Microbiol. 2008, 25, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Churklam, W.; Chaturongakul, S.; Ngamwongsatit, B.; Aunpad, R. The mechanisms of action of carvacrol and its synergism with nisin against Listeria monocytogenes on sliced bologna sausage. Food Control 2020, 108, 106864. [Google Scholar] [CrossRef]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life 2020, 23, 100439. [Google Scholar] [CrossRef]
- Zhong, C.; Hou, P.-F.; Li, Y.-X.; Yang, W.-Y.; Shu, M.; Wu, G.-P. Characterization, antioxidant and antibacterial activities of gelatin film incorporated with protocatechuic acid and its application on beef preservation. LWT 2021, 151, 112154. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Noipha, S. Active film from chitosan incorporating green tea extract for shelf life extension of pork sausages. Food Hydrocoll. 2012, 27, 102–108. [Google Scholar] [CrossRef]
- Surendhiran, D.; Cui, H.; Lin, L. Encapsulation of Phlorotannin in Alginate/PEO blended nanofibers to preserve chicken meat from Salmonella contaminations. Food Packag. Shelf Life 2019, 21, 100346. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Kang, H. Chitosan coatings incorporated with free or nano-encapsulated Paulownia Tomentosa essential oil to improve shelf-life of ready-to-cook pork chops. LWT 2019, 116, 108580. [Google Scholar] [CrossRef]
- Abdou, E.S.; Galhoum, G.F.; Mohamed, E.N. Curcumin loaded nanoemulsions/pectin coatings for refrigerated chicken fillets. Food Hydrocoll. 2018, 83, 445–453. [Google Scholar] [CrossRef]
- Moon, H.; Kim, N.H.; Kim, S.H.; Kim, Y.; Ryu, J.H.; Rhee, M.S. Teriyaki sauce with carvacrol or thymol effectively controls Escherichia coli O157:H7, Listeria monocytogenes, Salmonella typhimurium, and indigenous flora in marinated beef and marinade. Meat Sci. 2017, 129, 147–152. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Lotfy, T.M.R.; Shawir, S.M.S. Preparation and antibacterial activity of chitosan-silver nanoparticles for application in preservation of minced meat. Bull. Natl. Res. Cent. 2019, 43, 1–14. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Shi, H.; Zhang, X.; Dong, P.; Luo, X.; Qin, H.; Zhang, Y.; Mao, Y.; Holman, B.W. Influence of low-energy electron beam irradiation on the quality and shelf-life of vacuum-packaged pork stored under chilled and superchilled conditions. Meat Sci. 2023, 195, 109019. [Google Scholar] [CrossRef]
- Gertzou, I.N.; Karabagias, I.K.; Drosos, P.E.; Riganakos, K.A. Effect of combination of ozonation and vacuum packaging on shelf life extension of fresh chicken legs during storage under refrigeration. J. Food Eng. 2017, 213, 18–26. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.; He, J.; Wu, Z.; Sun, Y.; Pan, D.; Tian, H.; Xia, Q. Combining e-beam irradiation and modified atmosphere packaging as a preservation strategy to improve physicochemical and microbiological properties of sauced duck product. Food Control 2022, 136, 108889. [Google Scholar] [CrossRef]
- Botsaris, G.; Taki, A. Effect of High-Pressure Processing on the Microbial Quality throughout the Shelf Life of Vacuum-Packed Sliced Ham and Frankfurters. J. Food Process. Preserv. 2014, 39, 840–845. [Google Scholar] [CrossRef]
- Liu, N.; Liang, M.; Tan, Y.; Xu, X.; Deng, L.; He, L.; Zheng, X.; Liang, C.; Zhang, R.; Zhou, J.; et al. Optimization of Combined Pulsed Light and UV Treatment for Extending the Shelf Life of Sliced Cured Pork. Meat Res. 2017, 31, 16–21. [Google Scholar]
- Chen, K.; Zhang, M.; Deng, D. The synergistic antimicrobial effects and mechanism of cinnamon essential oil and high voltage electrostatic field and their application in minced pork. Food Control 2024, 163, 110475. [Google Scholar] [CrossRef]
- Smaoui, S.; Ben Hlima, H.; Tavares, L.; Ben Braïek, O.; Ennouri, K.; Abdelkafi, S.; Mellouli, L.; Khaneghah, A.M. Application of eco-friendly active films and coatings based on natural antioxidant in meat products: A review. Prog. Org. Coat. 2022, 166, 106780. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Yang, T.; Liu, X.; Ding, X. Combined effect of chitosan and gamma ray on quality of chilled fresh pork. J. Zhejiang Univ. (Agric. Life Sci.) 2016, 42, 220–227. [Google Scholar]
- Cui, H.; Wu, J.; Li, C.; Lin, L. Promoting anti-listeria activity of lemongrass oil on pork loin by cold nitrogen plasma assist. J. Food Saf. 2016, 37, e12316. [Google Scholar] [CrossRef]
- Nagaraj, G.; Purohit, A.; Harrison, M.; Singh, R.; Hung, Y.-C.; Mohan, A. Radiofrequency pasteurization of inoculated ground beef homogenate. Food Control 2016, 59, 59–67. [Google Scholar] [CrossRef]
- Azlin-Hasim, S.; Cruz-Romero, M.C.; Morris, M.A.; Cummins, E.; Kerry, J.P. Effects of a combination of antimicrobial silver low density polyethylene nanocomposite films and modified atmosphere packaging on the shelf life of chicken breast fillets. Food Packag. Shelf Life 2015, 4, 26–35. [Google Scholar] [CrossRef]
- Castillo-Zamudio, R.I.; Paniagua-Martínez, I.; Ortuño-Cases, C.; García-Alvarado, M.; Larrea, V.; Benedito, J. Use of high-power ultrasound combined with supercritical fluids for microbial inactivation in dry-cured ham. Innov. Food Sci. Emerg. Technol. 2021, 67, 102557. [Google Scholar] [CrossRef]
- Kim, M.-J.; Da Jeong, M.; Zheng, Q.; Yuk, H.-G. Antimicrobial activity of 405 nm light-emitting diode (LED) in the presence of riboflavin against Listeria monocytogenes on the surface of smoked salmon. Food Sci. Biotechnol. 2021, 30, 609–618. [Google Scholar] [CrossRef]
- Orel, R.; Tabilo-Munizaga, G.; Cepero-Betancourt, Y.; Reyes-Parra, J.E.; Badillo-Ortiz, A.; Pérez-Won, M. Effects of high hydrostatic pressure processing and sodium reduction on physicochemical properties, sensory quality, and microbiological shelf life of ready-to-eat chicken breasts. LWT 2020, 127, 109352. [Google Scholar] [CrossRef]
- Balamurugan, S.; Ahmed, R.; Chibeu, A.; Gao, A.; Koutchma, T.; Strange, P. Effect of salt types and concentrations on the high-pressure inactivation of Listeria monocytogenes in ground chicken. Int. J. Food Microbiol. 2016, 218, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Baek, K.H.; Heo, Y.S.; Yong, H.I.; Jo, C. Synergistic bactericidal effect of clove oil and encapsulated atmospheric pressure plasma against Escherichia coli O157:H7 and Staphylococcus aureus and its mechanism of action. Food Microbiol. 2021, 93, 103611. [Google Scholar] [CrossRef] [PubMed]
- Latou, E.; Mexis, S.; Badeka, A.; Kontakos, S.; Kontominas, M. Combined effect of chitosan and modified atmosphere packaging for shelf life extension of chicken breast fillets. LWT 2014, 55, 263–268. [Google Scholar] [CrossRef]
- Ajourloo, M.; Khanjari, A.; Misaghi, A.; Basti, A.A.; Kamkar, A.; Yadegar, F.; Gholami, F.; Khansavar, F.; Fallah, F. Combined effects of Ziziphora clinopodioides essential oil and lysozyme to extend shelf life and control Listeria monocytogenes in Balkan-style fresh sausage. Food Sci. Nutr. 2021, 9, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, Y.; Gui, M.; Zheng, H.; Dai, R.; Li, P. Combined effect of high hydrostatic pressure and enterocin LM-2 on the refrigerated shelf life of ready-to-eat sliced vacuum-packed cooked ham. Food Control 2012, 24, 64–71. [Google Scholar] [CrossRef]
- Osaili, T.M.; Al-Nabulsi, A.A.; Hasan, F.; Dhanasekaran, D.K.; Ismail, L.C.; Hashim, M.; Hasan, H.; Ayyash, M.; Olaimat, A.; Hussain, A.Z.S.; et al. Inhibition of spoilage bacteria on marinated chicken by essential oils under aerobic and vacuum packaging. J. Food Sci. 2022, 88, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Xu, M.; Xu, B.; Chen, C.; Deng, J.; Li, P. Combined Effect of High-Pressure Processing with Spice Extracts on Quality of Low-Salt Sausage during Refrigerated Storage. Foods 2021, 10, 2610. [Google Scholar] [CrossRef] [PubMed]
- Efenberger-Szmechtyk, M.; Gałązka-Czarnecka, I.; Otlewska, A.; Czyżowska, A.; Nowak, A. Aronia melanocarpa (Michx.) Elliot, Chaenomeles superba Lindl. and Cornus mas L. Leaf Extracts as Natural Preservatives for Pork Meat Products. Molecules 2021, 26, 3009. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kang, M.; Yong, H.I.; Bae, Y.S.; Jung, S.; Jo, C. Synergistic Effects of Electron-beam Irradiation and Leek Extract on the Quality of Pork Jerky during Ambient Storage. Asian-Australas. J. Anim. Sci. 2013, 26, 596–602. [Google Scholar] [CrossRef] [PubMed]
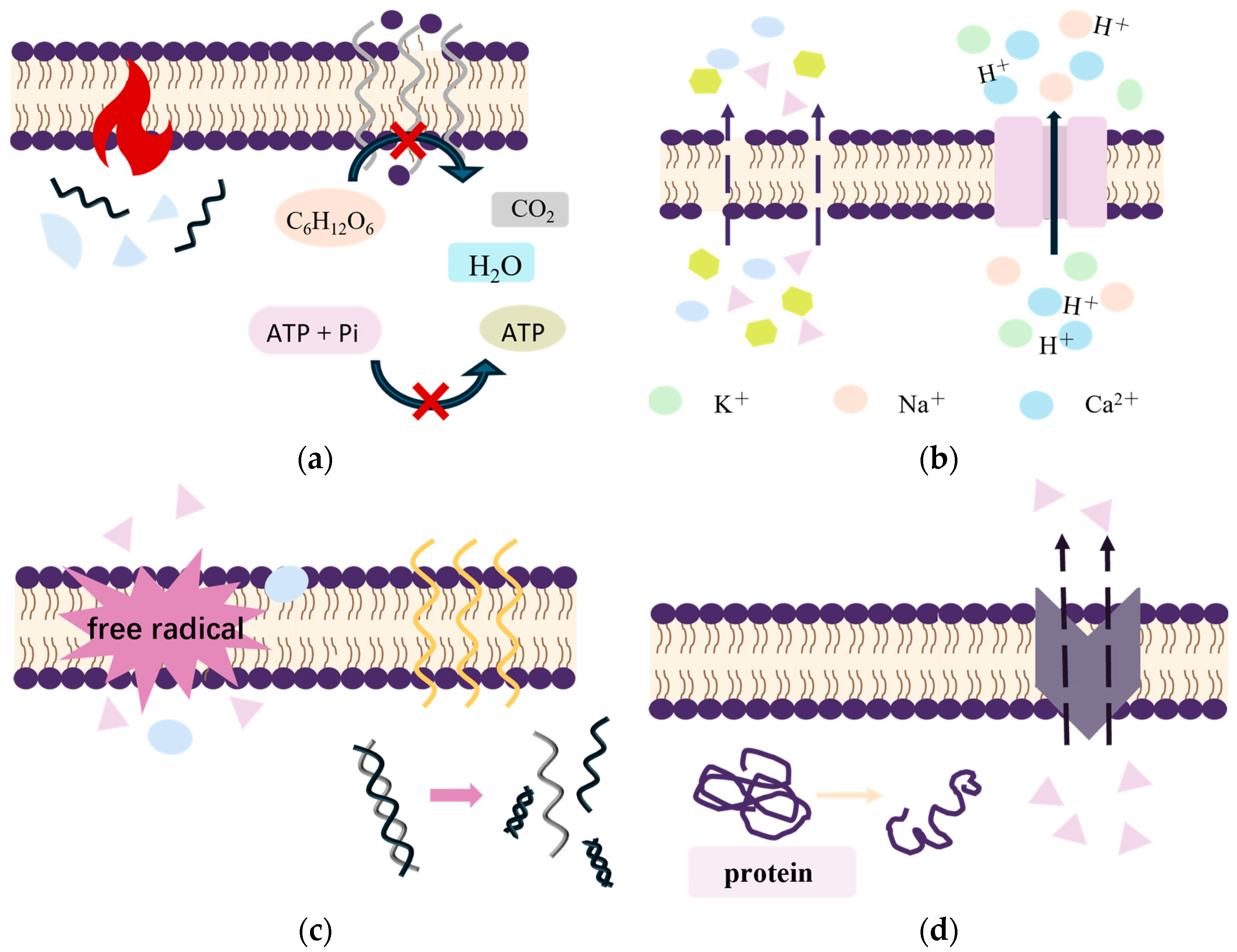
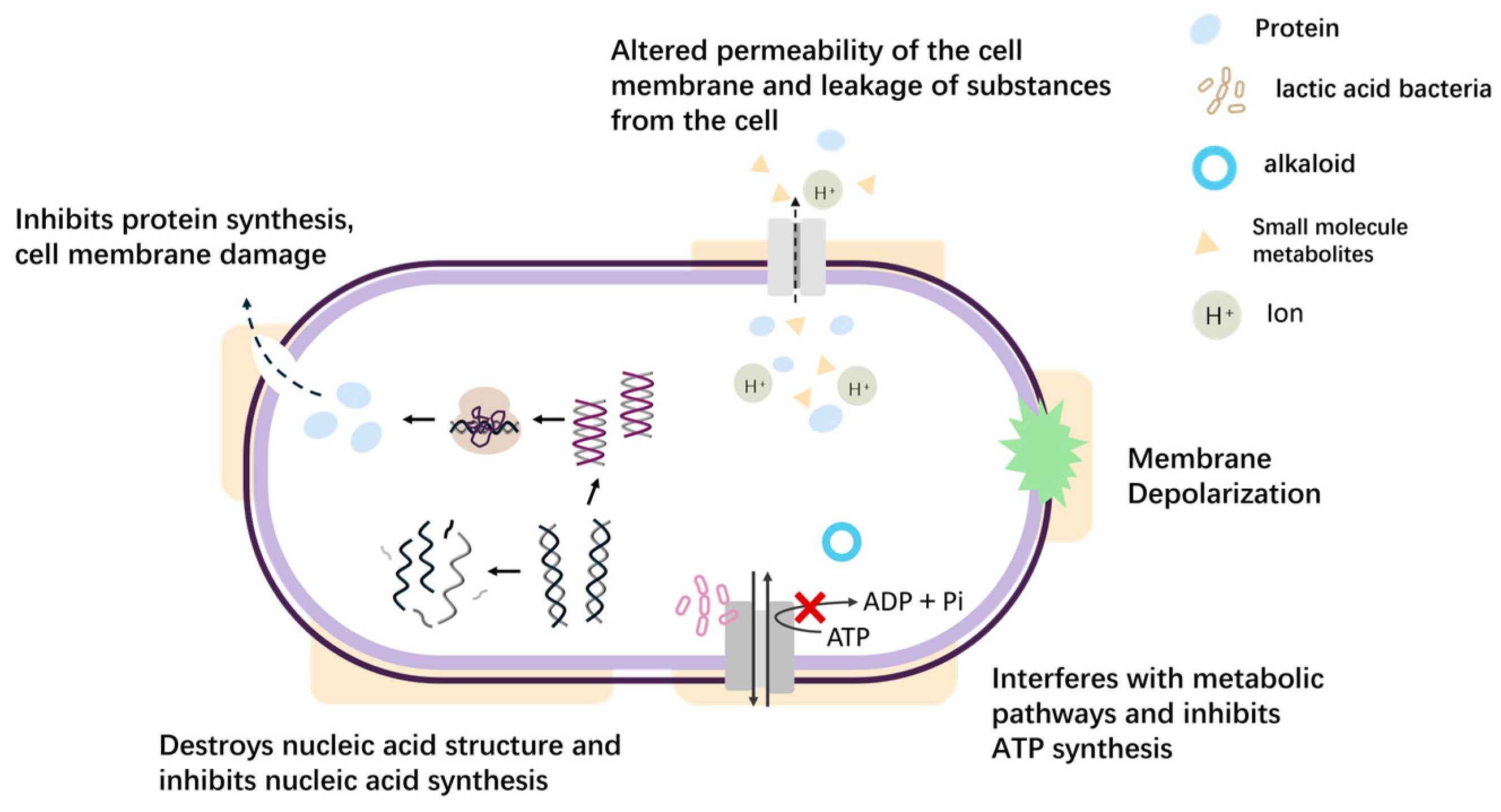
| Antibacterial Method | Culture Condition | Target of an Action | Consequences of Action | Medium of Action | Bibliography | |
|---|---|---|---|---|---|---|
| The amount of chitosan and tea polyphenols added is 3:1. | 4 °C | Mesophiles | Growth was reduced by approximately 2.0 log CFU/g. | The expiry date extends the shelf life by 6 days. | Pork | [92] |
| Novel bioactive sponge mats composed of oxidized bacterial cellulose and chitosan-gum Arabic microcapsules loaded with cinnamon essential oil | 4 °C | S. Staphylococcus aureus, Escherichia coli | Day 10 TVC less than 6 log CFU/g. | Shelf life extended from 4 to 10 days. | Meat | [93] |
| Highly absorbent antibacterial chitosan-based aerogels | 4 °C | Listeria monocytogenes, Staphylococcus aureus, E. coli, and S. typhimurium | The diameters of the inhibition zones of Escherichia coli, S. typhimurium Salmonella, Listeria monocytogenes, and Staphylococcus aureus increased to 21.65 ± 0.58, 23.35 ± 0.64, 21.86 ± 0.89, and 22.15 ± 0.53 at CuNPs solution (60 μL), respectively, mm. | Shelf life is 14 days. | Pork | [94] |
| Tannic acid-grafted chitosan coating on the quality (TA-g-CH) | 4 °C | Pseudomonas | The TVC of pork coated with TA + CH and TA-g-CH at the end of the storage period did not exceed acceptable limits. | Microbiological growth extended the shelf life of pork samples by 6 to 9 days. | Pork | [95] |
| Effects of chitosan coating with green tea aqueous extract | 0 °C | Mesophilic, psychrotrophic | There was a 49.4% and 41.4% reduction in mesophilic and psychrophilic growth, respectively, in the treated group compared to the blank group on day 25 (p < 0.05). | - | Pork chops | [96] |
| Psyllium EmF + 1.0% chitosan | 4 °C | Listeria monocytogenes | - | Shelf life extended to 15 days | Beef | [97] |
| Ethylcellulose/gelatin-carboxymethyl chitosan bilayer films doped with Euryale ferox seed shell polyphenol | 3~5 °C | L. monocytogenes | - | Still has food value after 9 days | Cooked beef and cooked chicken | [98] |
| Thymol and carvacrol at 0.4 percent and 0.8 percent (w/w). | 4 °C | Pseudomonas, Brochothrix thermosphacta | Pseudomonas reduced to 0.9−1 log; Brochothrix thermosphacta reduced by 1.1–1.6 logs. | Shelf life extended by 6 days | Corned beef | [99] |
| 0.1% ZEO (Artemisia multiflora essential oil) + 0.2% GSE (grape seed extract) | 8 °C | Mesophiles, lactic acid bacteria | Mesophiles and lactic acid bacteria were the most sensitive and membrane-tolerant groups, with reductions of 0.1–1.1 and 0.1–0.7 log cycles, respectively, and a 1.23 log reduction in TVC on day 9 of refrigeration. | - | Instant salami | [100] |
| 0.4% chitosan + 0.02% ε-polylysine + 0.2% ascorbic acid | 3 °C | Total viable count (TVC) | After 12 days of storage did not exceed the standard. | Shelf life extended by 6 days | Pork chunks | [101] |
| Sea buckthorn pomace extract SPF-6–esterified potato starch film | 25 °C | Escherichia coli, Listeria monocytogenes, Staphylococcus aureus, Salmonella | After 13 months the TVC value reached only 3.67 log CFU/g | - | Beef jerky | [102] |
| Olive leaf extract (OLE)-gelatin films | 23 °C | L. monocytogenes | Reduced L. monocytogenes growth. | - | smoked salmon | [103] |
| Chitosan nanoparticles of cinnamon essential oils (CE-NPs) 527 nm | 4 °C | Pseudomonas | Pseudomonas, Lac-tic Acid Bacteria, and Enterobacteriaceae end-of-treatment colony counts were 5.48, 5.15, and 3.20 log CFU/g. | Shelf life can be extended to 15 days. | Pork | [104] |
| Sea tangle extract | 10 °C | Enterobacteriaceae | Eight weeks of storage without exceeding limits. | - | Pork ham | [105] |
| Lysozyme and Chinese liquor | 25 ± 2 °C | S. aureus | Total Staphylococcus aureus reduced to 2.8 log CFU/g. | - | Dry fermented sausage | [106] |
| Paeonia japonica (Makino) Miyabe, Takeda, Rhus chinensis Mill, Paeomia suffruticosa, Psidium guajava, Nelumbo nucifera, and Ecklonia cava | 21 °C | E. coli, Listeria monocytogenes, and Salmonella spp. | Reduced E. coli levels by more than 99.9% after 8 days of storage and slowed the growth of Listeria monocytogenes and Salmonella spp. by more than 80% after 14 days. | - | Sausages | [107] |
| Tapioca starch active nanocomposite films and their antimicrobial effectiveness | 4 °C | Listeria monocytogenes | A reduction of 1 to 2 log CFU/cm2 was observed over 10 days | Shelf life is 10 days. | Ready-to-eat chicken meat | [108] |
| 3% chitosan/low-density polyethylene composite film with essential oil of Xiaquan grass | 4 °C | Staphylococcus aureus, Bacillus cereus, Escherichia coli, and Salmonella enteritidis | - | Shelf life extended to 13 days. | Chicken breast | [109] |
| Konjac glucan/chitosan antimicrobial composite film with oregano essential oil microcapsules | 0–4 °C | Pseudomonas putida, P. fluorescens, and Pseudomonas aeruginosa | 10% KGM/CTS inhibition zone size was 10.7 ± 0.58 mm for Pseudomonas putida, 9.00 ± 0.00 mm for P. fluorescens, and 13.7 ± 0.58 mm for Pseudomonas aeruginosa. | The shelf life was extended by 3 days. | Pork | [110] |
| Nano-encapsulated chitosan film with garlic essential oil | 4 °C | Pseudomonas | The value is 3.3 colony forming units/g for 50 days. | Storage up to 7 days. | Sausages | [111] |
| Bacterial cellulose membranes containing streptococcal lactate | 4 °C | Listeria monocytogenes | The 14 day final count was significantly (p < 0.05) lower by approximately 3.4 log CFU/g. | - | Frankfurter sausage | [112] |
| 25 μg/mL Streptococcus lactis and 62.5 μg/mL carvacrol. | 4 °C | Listeria monocytogenes | Inhibited bacterial growth and increased the doubling time from 15.01 to 23.35 h. | - | Bolognese sausage | [113] |
| Fabrication of high-stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO | 4 °C, 25 °C | Escherichia coli O157:H7 | The number was reduced to 2.96 and 5.80 log CFU/g, respectively. | - | Beef | [114] |
| A novel fish gelatin film incorporated with protocatechuic acid | 4 °C | Total viable counts | Reduced TVC by approx. 0.40–1.68 log10 CFU/cm2 | - | Beef | [115] |
| Chitosan film containing green tea extracts (CGT-film) | 4 °C | aerobic bacteria, yeasts, mold, and lactic acid bacteria | The 12 day total viable count was 5.24 log CFU/g. | - | Pork sausage | [116] |
| Encapsulation of Phlorotannin in alginate/PEO blended nanofibers mixing ratio 50:50:10 (SA/PEO/Ph) | 4 °C, 25 °C | streptococcus enteritis | Decreased from 2.92 log CFU/g at 4 °C to 6.27 log CFU/g at 25 °C. | - | Chicken meat | [117] |
| Chitosan coatings incorporated with free or nano-encapsulated paulownia tomentosa essential oil | 4 °C | Pseudomonas spp. | Pseudomonas spp. counts were maintained below 5 log CFU/g and LAB counts were reduced by 2.61 log CFU/g (p < 0.05). | Storage 16 days. | Ready-to-cook pork chops | [118] |
| Curcumin—cinnamon oil nano emulsion/pectin coating | 4 °C | Aerobic bacteria, Psychrophiles | The counts of CCNC-coated aerobic bacteria and Psychrophiles were reduced by 97.8% and 99.5% at 12 days, respectively. | Shelf life extended to 12 days. | Chicken fillet | [119] |
| 0.5% Cinnamaldehyde (CV) or muscimol (TM) teriyaki sauce | 4 °C | Escherichia coli, Salmonella, and Listeria monocytogenes | The strain was completely inactivated by cold pickling and could not be recovered | - | Corned beef | [120] |
| Effects of nanoemulsion-based active coatings with a composite mixture of star anise essential oil, polylysine, and nisin | 4 °C | Escherichia coli | - | Shelf life extended from 8 to 16 days. | Ready-to-eat Yao meat products | [77] |
| Chitosan-silver nanoparticles | 4 °C | Escherichia coli, S. typhimurium | 1000 and 2500 mg/g antimicrobial activity 14.47 and 9.00 × 104 log CFU/g. | - | Minced meat | [121] |
| Nanoemulsion with star anise essential oil, polylysine, and nisin | 4 °C | E. coli | E. coli growth was reduced by approximately 1 log CFU/g. | Shelf life extended from 8 to 16 days | Ready-to-eat Yao meat | [77] |
| Physical Technology | Technical Parameters | Culture Condition | Target of an Action | Consequences of Action | Medium of Action | Bibliography | |
|---|---|---|---|---|---|---|---|
| LEEB irradiation with superchilled | 0.2 MeV;8 kGy | −1.0 ± 0.5 °C | Weissella, Carnobacterium, and Lactobacillus | On the 10, 20, and 30 day of storage Weissella, Carnobacterium, and Lactobacillus abundance decreased by 9.29%, 29.98%, and 14.02%, respectively. | Shelf-life of pork to (at least) 30 days | Pork | [122] |
| Ozone treatment and vacuum packaging | 2 mg/L, 5, 10 mg/L | 4 ± 1 °C | Lactic acid bacteria | Lactic acid bacteria reached 2 log CFU/g on days 14–16 for the 2 mg/L treated samples and did not reach 7 log CFU/g within 16 days of the 5 or 10 mg/L ozone treatments. | The shelf life of chicken thigh samples treated with 2 mg/L, 5, or 10 mg/L ozone was extended by 4, 6, and 6 days, respectively (p < 0.001). | Drumstick | [123] |
| Electron beam irradiation and air conditioning | 40% CO2/60% N2, 4 kGy irradiation | 4 °C | - | - | Shelf life extended by 28 days. | Duck in sauce | [124] |
| High Pressure Vacuum | 600 MPa, 3 min | 4 °C | TAC | The TAC of the treated product remained below 0.5 log CFU/g for a 30 day storage period. | - | Frankfurters | [125] |
| Pulsed intense light with UV irradiation | Cured meat thickness 3 mm, 10 cm from the pulsed light source, 11 cm from the UV light source, and irradiated for 5 min. | 10 °C | - | The total colony count decreased from 1.5 × 107 log CFU/g to 5.6 × 104 log CFU/g. | - | Preserved meat | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Na, F.; Zhang, M.; Yang, W. Microbial Control in the Processing of Low-Temperature Meat Products: Non-Thermal Sterilization and Natural Antimicrobials. Foods 2025, 14, 225. https://doi.org/10.3390/foods14020225
Zhang X, Na F, Zhang M, Yang W. Microbial Control in the Processing of Low-Temperature Meat Products: Non-Thermal Sterilization and Natural Antimicrobials. Foods. 2025; 14(2):225. https://doi.org/10.3390/foods14020225
Chicago/Turabian StyleZhang, Xiaoyang, Feng Na, Min Zhang, and Wei Yang. 2025. "Microbial Control in the Processing of Low-Temperature Meat Products: Non-Thermal Sterilization and Natural Antimicrobials" Foods 14, no. 2: 225. https://doi.org/10.3390/foods14020225
APA StyleZhang, X., Na, F., Zhang, M., & Yang, W. (2025). Microbial Control in the Processing of Low-Temperature Meat Products: Non-Thermal Sterilization and Natural Antimicrobials. Foods, 14(2), 225. https://doi.org/10.3390/foods14020225






