The Addition of Pumpkin Flour Impacts the Functional and Bioactive Properties of Soft Wheat Composite Flour Blends
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Blend Preparation
2.2. Flour Blend Preparation
2.3. Particle Size Distribution—Granulometric Analysis
2.4. Techno-Functional Properties of Blends
2.5. Pasting Properties
2.6. Gel Texture
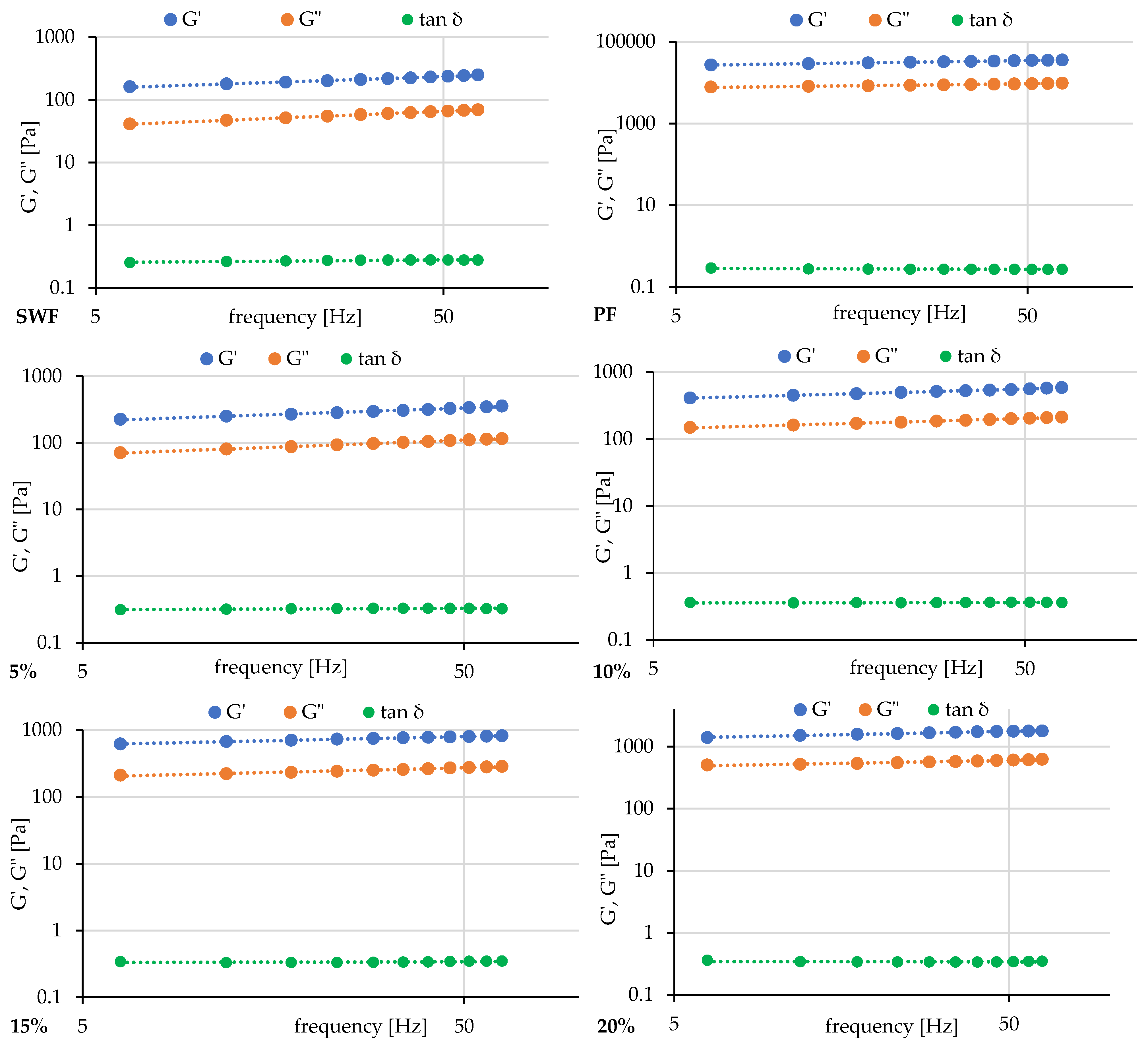
2.7. Frequency and Amplitude Sweep
2.8. Colour Measurement
2.9. Extract Preparation
2.10. Determination of Total Phenolic Compounds
2.11. Determination of Antioxidant and Oxidoreductive Activities
2.11.1. DPPH Assay
2.11.2. ABTS Assay
2.11.3. FRAP Assay
2.12. Determination of Reducing Sugar Content
2.13. Statistical Analyses
3. Results and Discussion
3.1. Granulometric Analysis and Functional Characteristic
3.2. Bioactive Characteristics of Pumpkin Flour/Soft Wheat Blends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, A.; Kausar, T.; Din, A.; Murtaza, M.A.; Jamil, M.A.; Noreen, S.; Rehman, H.; Shabbir, H.; Ramzan, M.A. Determination of total phenolic, flavonoid, carotenoid, and mineral contents in peel, flesh, and seeds of pumpkin (Cucurbita maxima). J. Food Process. Preserv. 2021, 45, e15542. [Google Scholar] [CrossRef]
- Hussain, A.; Kausar, T.; Din, A.; Murtaza, M.A.; Jamil, M.A.; Iqbal, M.A.; Majeed, M.A.; Rafique, A.; Iftikhar, A.; Noreen, S.; et al. Production, characterization, food application and biological study of powder of pumpkin (Cucurbita maxima) parts (peel, flesh and seeds). Pure Appl. Biol. 2023, 12, 48–60. [Google Scholar] [CrossRef]
- Anitha, S.; Hn, R.; Ashwini, A. Effect of mixing pumpkin powder with wheat flour on physical, nutritional and sensory characteristics of cookies. Int. J. Chem. Stud. 2020, 8, 1030–1035. [Google Scholar] [CrossRef]
- Khatib, S.; Muhieddine, M. Nutritional profile and medicinal properties of pumpkin fruit pulp. In The Health Benefits of Foods-Current Knowledge and Further Development; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Černiauskienė, J.; Kulaitienė, J.; Danilčenko, H.; Jarienė, E.; Juknevičienė, E. Pumpkin fruit flour as a source for food enrichment in dietary fiber. Not. Bot. Horti Agrobot. 2014, 42, 19–23. [Google Scholar] [CrossRef]
- Zahra, N.; Hina, S.; Masood, S.; Kalim, I.; Saeed, M.K.; Ahmad, I.; Arshad, M. Exploration of locally grown yellow and green pumpkin as a potential source of b-carotene and vitamin A. Biol. Sci.—PJSIR 2020, 63, 238–241. [Google Scholar] [CrossRef]
- Gavril, R.N.; Stoica, F.; Lipșa, F.D.; Constantin, O.E.; Stănciuc, N.; Aprodu, I.; Râpeanu, G. Pumpkin and Pumpkin By-Products: A Comprehensive Overview of Phytochemicals, Extraction, Health Benefits, and Food Applications. Foods 2024, 13, 2694. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, G.; Kehinde, B.A.; Chhikara, N.; Panghal, A.; Kaur, H. Pharmacological and biomedical uses of extracts of pumpkin and its relatives and applications in the food industry: A review. Int. J. Veg. Sci. 2019, 26, 79–95. [Google Scholar] [CrossRef]
- Pereira, A.M.; Krumreich, F.D.; Ramos, A.H.; Krolow, A.C.R.; Santos, R.B.; Gularte, M.A. Physicochemical characterization, carotenoid content and protein digestibility of pumpkin access flours for food application. Food Sci. Technol. 2020, 40 (Suppl. S2), 691–698. [Google Scholar] [CrossRef]
- Shajan, A.E.; Dash, K.K.; Hamid; Bashir, O.; Shams, R. Comprehensive comparative insights on physico-chemical characteristics, bioactive components, and therapeutic potential of pumpkin fruit. Future Foods 2024, 9, 100312. [Google Scholar] [CrossRef]
- Nurgozhina, Z.; Shansharova, D.; Umirzakova, G.; Maliktayeva, P.; Yakiyayeva, M. The influence of grain mixtures on the quality and nutritional value of bread. Potravin. Slovak J. Food Sci. 2022, 16, 320–340. [Google Scholar] [CrossRef]
- Alashi, A.; Taiwo, K.; Oyedele, D.; Adebooye, O.; Aluko, R. Polyphenol composition and antioxidant properties of vegetable leaf-fortified bread. J. Food Biochem. 2018, 43, e12625. [Google Scholar] [CrossRef] [PubMed]
- Minarovičová, L.; Lauková, M.; Karovičová, J.; Kohajdová, Z. Utilization of pumpkin powder in baked rolls. Potravin. Slovak J. Food Sci. 2018, 12, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Waryat, W.; Sunarmani, S.; Kurniasih, T. Chemical characteristics and sensory analysis of cake enriched pumpkin flour to improve food security. E3S Web Conf. 2023, 444, 04005. [Google Scholar] [CrossRef]
- Davis, K.F.; Chhatre, A.; Rao, N.D.; Singh, D.; Ghosh-Jerath, S.; Mridul, A.; Poblete-Cazenave, M.; Pradhan, N.; DeFries, R. Assessing the sustainability of post-green revolution cereals in India. Proc. Natl. Acad. Sci. USA 2019, 116, 25034–25041. [Google Scholar] [CrossRef]
- Fernández Peláez, J.; Guerra, P.; Gallego, C.; Gomez, M. Physical Properties of Flours Obtained from Wasted Bread Crusts and Crumbs. Foods 2021, 10, 282. [Google Scholar] [CrossRef]
- Carpentieri, S.; Orkusz, A.; Ferrari, G.; Harasym, J. Effect of replacing durum wheat semolina with Tenebrio molitor larvae powder on the techno-functional properties of the binary blends. Curr. Res. Food Sci. 2024, 8, 100672. [Google Scholar] [CrossRef]
- Nedviha, S.; Harasym, J. Functional and Antioxidative Characteristics of Soft Wheat and Tiger Nut (Cyperus esculentus) Flours Binary Blends. Foods 2024, 13, 596. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Klymenko, S.; Kucharska, A.Z.; Sokół-Łętowska, A.; Piórecki, N. Antioxidant activities and phenolic compounds in fruits of cultivars of cornelian cherry (Cornus mas L.). Agrobiodivers. Improv. Nutr. Health Life Qual. 2019, 3, 484–499. [Google Scholar]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Batista, J.; Braga, L.; Oliveira, R.; Silva, E.; Damiani, C. Partial replacement of wheat flour by pumpkin seed flour in the production of cupcakes filled with carob. Food Sci. Technol. 2018, 38, 250–254. [Google Scholar] [CrossRef]
- Aljahani, A.H. Wheat-Yellow Pumpkin Composite Flour: Physico-Functional, Rheological, Antioxidant Potential and Quality Properties of Pan and Flat Bread. Saudi J. Biol. Sci. 2022, 29, 3432–3439. [Google Scholar] [CrossRef]
- Adubofuor, J.; Anomah, J.W.; Amoah, I. Anti—Nutritional Factors and Mineral Composition of Pumpkin Pulp and Functional Properties of Pumpkin—Wheat Composite Flour for Bread Preparation. Int. J. Innov. Food Sci. Technol. 2018, 1, 1–9. [Google Scholar] [CrossRef]
- Hoxha, I.; Hoxha, B.; Xhabiri, G.; Shala, N.; Dreshaj, A.; Durmishi, N. The Effect of the Addition of Pumpkin Flour on the Rheological, Nutritional, Quality, and Sensory Properties of Bread. Ecol. Eng. Environ. Technol. 2023, 24, 178–185. [Google Scholar] [CrossRef]
- Pasha, I.; Ain, Q.; Khan, B.; Butt, S.; Saeed, M. Rheological and Functional Properties of Pumpkin Wheat Composite Flour. Pak. J. Food Sci. 2013, 23, 100–104. [Google Scholar]
- Van Toan, N.; Thi Thanh Thuy, N.; City, M.; Trung Ward, L.; Duc District, T.; Chi Minh City, H. Production of High-Quality Flour and the Made Biscuits from Pumpkin. Int. J. Food Sci. Nutr. 2018, 3, 157–166. [Google Scholar]
- Eke-Ejiofor, J.; Victor-Uku, E.C.; Akusu, M.O. Physicochemical and Functional Properties of Pumpkin (Cucurbita pepo) Pulp Flour and Acceptability of Its Inclusion in Cake. Asian Food Sci. J. 2021, 20, 57–71. [Google Scholar] [CrossRef]
- Bekele, D.W.; Emire, S.A. Effects of Pre-Drying Treatment and Particle Sizes on Physicochemical and Structural Properties of Pumpkin Flour. Heliyon 2023, 9, e21609. [Google Scholar] [CrossRef]
- Mittal, S.; Dhiman, A.K.; Sharma, A.; Attri, S.; Kathuria, D. Standardization of Recipes for Preparation of Pumpkin (Cucurbita moschata) Flour and Its Quality Evaluation during Storage. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 3224–3235. [Google Scholar] [CrossRef]
- Saeleaw, M.; Schleining, G. Composition, Physicochemical and Morphological Characterization of Pumpkin Flour. Agric. Food Sci. 2011. Available online: https://kmweb.moa.gov.tw/files/document/391216/6d72ed7b3912ce1b2099e0e31f8238b1_v1.pdf (accessed on 7 January 2025).
- Aktas, N.; Gerçekaslan, K.E. Pumpkin (Cucurbita pepo L.) Pulp Flour as a Source of Dietary Fiber: Chemical, Physicochemical and Technological Properties. Akad. Gida 2024, 22, 14–22. [Google Scholar] [CrossRef]
- Promsakha na Sakon Nakhon, P.; Jangchud, K.; Jangchud, A.; Prinyawiwatkul, W. Comparisons of Physicochemical Properties and Antioxidant Activities among Pumpkin (Cucurbita moschata L.) Flour and Isolated Starches from Fresh Pumpkin or Flour. Int. J. Food Sci. Technol. 2017, 52, 2436–2444. [Google Scholar] [CrossRef]
- Mardiah, M.; Fitriandini, S.; Hafiani, N.; Fitrilia, T.; Widowati, S. Effect of Drying Method on Physicochemical Properties of Pumpkin Flour. Int. J. Adv. Sci. Technol. 2020, 29, 3174–3189. [Google Scholar]
- Slamet, A.; Praseptiangga, D.; Rofandi, H. Physicochemical and Sensory Properties of Pumpkin (Cucurbita moschata D) and Arrowroot (Marantha arundinaceae L.) starch-based instant porridge. Int. J. Adv. Sci. Eng. Inf. Technol. 2019, 9, 412–421. [Google Scholar] [CrossRef]
- Davoudi, Z.; Shahedi, M.; Kadivar, M. Effects of pumpkin powder addition on the rheological, sensory, and quality attributes of taftoon bread. Cereal Chem. 2020, 97, 904–911. [Google Scholar] [CrossRef]
- Ge, F.; Wu, P.; Chen, X. Evolutions of rheology, microstructure and starch hydrolysis of pumpkin-enriched bread during simulated gastrointestinal digestion. Int. J. Food Sci. Technol. 2021, 56, 6000–6010. [Google Scholar] [CrossRef]
- Weldeyohanis Gebremariam, F.; Tadesse Melaku, E.; Sundramurthy, V.P.; Woldemichael Woldemariam, H. Development offunctional cookies form wheat-pumpkin seed based composite flour. Heliyon 2024, 10, e24443. [Google Scholar] [CrossRef]
- Tedom, W.; Fombang, E.; Ngaha, W.; Ejoh, R. Optimal conditions for production of fermented flour from pumpkin (Cucurbita pepo L.) for infant foods. Eur. J. Nutr. Food Saf. 2019, 10, 125–136. [Google Scholar] [CrossRef]
- Mahmood, K.; Alamri, M.S.; Abdellatif, M.A.; Hussain, S.; Qasem, A.A.A. Wheat flour and gum cordia composite system:Pasting, rheology and texture studies. Food Sci. Technol. 2018, 38, 691–697. [Google Scholar] [CrossRef]
- Lukinac, J.; Čuljak, J.; Pavlović, M.; Šubarić, D.; Komlenić, D. Quality evaluation of biscuits produced from composite blends of pumpkin seed oil press cake and wheat flour. Int. J. Food Sci. Technol. 2018, 54, 602–609. [Google Scholar] [CrossRef]
- Ma, K.; Mahesh, C.; Vineeta, P.; Gk, S.; Ad, S. Effect of pumpkin flour on the rheological characteristics of wheat flour and on biscuit quality. J. Food Process. Technol. 2019, 10, 1–6. [Google Scholar] [CrossRef]
- Badia-Olmos, C.; Laguna, L.; Haros, C.M.; Tárrega, A. Techno-Functional and Rheological Properties of Alternative Plant-Based Flours. Foods 2023, 12, 1411. [Google Scholar] [CrossRef]
- Litvynchuk, S.; Galenko, O.; Cavicchi, A.; Ceccanti, C.; Mignani, C.; Guidi, L.; Shevchenko, A. Conformational Changes in the Structure of Dough and Bread Enriched with Pumpkin Seed Flour. Plants 2022, 11, 2762. [Google Scholar] [CrossRef]
- Ke-Xue, Z.; Cai-Xia, L.; Xiao-Na, G.; Wei, P.; Hui-Ming, Z. Antioxidant activities and total phenolic contents of various extracts from defatted wheat germ. Food Chem. 2011, 126, 1122–1126. [Google Scholar] [CrossRef]
- Pinna, N.; Abbou, S.B.; Ianni, F.; Flores, G.A.; Pietercelie, A.; Perretti, G.; Blasi, F.; Angelini, P.; Cossignani, L. Phenolic compounds from pumpkin pulp: Extraction optimization and biological properties. Food Chem. X 2024, 23, 101628. [Google Scholar] [CrossRef]
- Oloyede, F.M.; Adebooye, O.C.; Obuotor, E.M. Planting date and fertilizer affect antioxidants in pumpkin fruit. Sci. Hortic. 2014, 168, 46–50. [Google Scholar] [CrossRef]
- Bonoli, M.; Marconi, E.; Caboni, M.F. Free and bound phenolic compounds in barley (Hordeum vulgare L.) flours—Evaluation of the extraction capability of different solvent mixtures and pressurized liquid methods by micellar electrokinetic chromatography and spectrophotometry. J. Chromatogr. A 2004, 1057, 1–12. [Google Scholar] [CrossRef]
- Batool, M.; Ranjha, M.M.A.N.; Roobab, U.; Manzoor, M.F.; Farooq, U.; Nadeem, H.R.; Nadeem, M.; Kanwal, R.; AbdElgawad, H.; Al Jaouni, S.K.; et al. Nutritional Value, Phytochemical Potential, and Therapeutic Benefits of Pumpkin (Cucurbita sp.). Plants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A.; Królczyk, J.B. Optimization of Extraction Conditions for the Antioxidant Potential of Different Pumpkin Varieties (Cucurbita maxima). Sustainability 2020, 12, 1305. [Google Scholar] [CrossRef]
- Stryjecka, M.; Krochmal-Marczak, B.; Cebulak, T.; Kiełtyka-Dadasiewicz, A. Assessment of Phenolic Acid Content and Antioxidant Properties of the Pulp of Five Pumpkin Species Cultivated in Southeastern Poland. Int. J. Mol. Sci. 2023, 24, 8621. [Google Scholar] [CrossRef] [PubMed]
- Kostecka-Gugała, A.; Kruczek, M.; Ledwożyw-Smoleń, I.; Kaszycki, P. Antioxidants and Health-Beneficial Nutrients in Fruits of Eighteen Cucurbita Cultivars: Analysis of Diversity and Dietary Implications. Molecules 2020, 25, 1792. [Google Scholar] [CrossRef] [PubMed]
- Liubych, V.; Novikov, V.; Pushka, O.; Pushka, I.; Cherchel, V.; Kyrpa, M.; Kolibabchuk, T.; Kirian, V.; Moskalets, V.; Moskalets, T. Development of the recipe of pasta with pumpkin flour. Eureka Life Sci. 2023, 1, 57–65. [Google Scholar] [CrossRef]

| Mesh Size | SWF | PF |
|---|---|---|
| [µm] | [%] | [%] |
| >200 | 72.96 ± 10.94 d | 60.76 ± 9.11 c |
| 200< >180 | 6.70 ± 1.01 c | 3.78 ± 0.57 a |
| 180< >150 | 7.74 ± 1.16 c | 5.30 ± 0.80 a |
| 150< >125 | 4.28 ± 0.64 b | 4.86 ± 0.73 a |
| 125< >106 | 4.00 ± 0.60 b | 4.94 ± 0.74 a |
| 106< >80 | 3.86 ± 0.58 b | 4.60 ± 0.69 a |
| <80 | 1.10 ± 0.17 a | 13.26 ± 1.99 b |
| Sample | WHC | WAC | WAI | SP | WSI | OAC | HLI |
|---|---|---|---|---|---|---|---|
| g H2O/g DM | g H2O/100 g DM | g Oil/g DM | |||||
| SFW | 2.52 ± 0.00 a | 1.83 ± 0.03 a | 5.62 ± 0.03 a | 5.84 ± 0.01 a | 3.80 ± 0.36 a | 1.14 ± 0.02 a | 1.80 ± 0.00 a |
| 5% | 2.52 ± 0.02 a | 1.90 ± 0.07 ab | 5.68 ± 0.10 a | 5.91 ± 0.15 a | 4.62 ± 0.14 ab | 1.18 ± 0.02 b | 1.79 ± 0.01 a |
| 10% | 2.78 ± 0.11 b | 2.04 ± 0.07 ab | 5.80 ± 0.03 ab | 6.15 ± 0.03 ab | 5.82 ± 0.06 ab | 1.26 ± 0.01 c | 1.79 ± 0.03 a |
| 15% | 3.07 ± 0.04 c | 2.14 ± 0.00 ab | 5.94 ± 0.07 b | 6.38 ± 0.00 b | 6.84 ± 1.10 bc | 1.33 ± 0.00 d | 1.78 ± 0.02 a |
| 20% | 3.56 ± 0.15 d | 2.24 ± 0.04 b | 6.88 ± 0.15 c | 7.53 ± 0.16 c | 8.60 ± 0.00 c | 1.41 ± 0.01 e | 1.75 ± 0.03 a |
| PF | 10.89 ± 0.14 e | 8.01 ± 0.33 c | 8.64 ± 0.14 d | 12.22 ± 0.23 d | 29.27 ± 2.47 d | 1.78 ± 0.02 f | 4.98 ± 0.04 b |
| Sample | Peak Viscosity [mPa·s] | Trough Viscosity [mPa·s] | Breakdown [mPa·s] | Final Viscosity [mPa·s] | Setback [mPa·s] | Pasting Temp [°C] | Peak Time [s] |
|---|---|---|---|---|---|---|---|
| SWF | 2444.0 ± 4.2 e | 1386.5 ± 0.7 d | 1057.5 ± 3.5 c | 2920.0 ± 12.7 c | 1533.5 ± 13.4 d | 87.6 ± 0.53 b | 6.42 ± 0.40 a |
| 5% | 2342.5 ± 27.6 de | 1273.0 ± 1.4 c | 1069.5 ± 29.0 c | 2706.0 ± 26.9 bc | 1433.0 ± 28.3 cd | 87.7 ± 0.57 b | 6.00 ± 0.00 a |
| 10% | 2219.0 ± 25.5 cd | 1146.5 ± 12.0 b | 1072.5 ± 13.4 c | 2501.0 ± 38.2 b | 1354.5 ± 26.2 bc | 87.5 ± 0.48 b | 5.40 ± 0.57 a |
| 15% | 1978.5 ± 92.6 b | 981.0 ± 46.7 a | 997.5 ± 46.0 bc | 2184.0 ± 84.9 a | 1203.0 ± 38.2 ab | 87.2 ± 0.04 b | 5.67 ± 0.00 a |
| 20% | 1859.5 ± 13.4 a | 901.0 ± 4.2 a | 958.5 ± 9.2 c | 2000.5 ± 36.1 a | 1099.5 ± 31.8 ab | 87.3 ± 0.04 b | 5.64 ± 0.05 a |
| PF | 2265.0 ± 14.1 cd | 2098.0 ± 89.1 e | 167 ± 75.0 a | 3549.5 ± 231.2 d | 1451.5 ± 142.1 cd | 50.2 ± 0.00 a | 5.17 ± 2.60 a |
| Sample | Hardness [N] | Cohesiveness | Springiness | Gumminess [N] | Resilience |
|---|---|---|---|---|---|
| SWF | 0.46 ± 0.01 c | 0.815 ± 0.04 c | 0.842 ± 0.01 e | 0.312 ± 0.01 c | 0.576 ± 0.02 a |
| 5% | 0.44 ± 0.01 c | 0.777 ± 0.00 c | 0.792 ± 0.00 d | 0.272 ± 0.01 b | 0.507 ± 0.02 a |
| 10% | 0.40 ± 0.06 bc | 0.778 ± 0.02 c | 0.763 ± 0.02 d | 0.236 ± 0.04 b | 0.549 ± 0.02 a |
| 15% | 0.34 ± 0.04 b | 0.482 ± 0.10 b | 0.165 ± 0.03 c | 0.028 ± 0.01 a | 0.419 ± 0.10 a |
| 20% | 0.35 ± 0.02 b | 0.378 ± 0.02 b | 0.123 ± 0.00 b | 0.014 ± 0.00 a | 0.452 ± 0.09 a |
| PF | 0.04 ± 0.00 a | 0.000 ± 0.00 a | 0.000 ± 0.00 a | 0.000 ± 0.00 a | 1.251 ± 0.15 b |
| Sample | G′ [Pa] | a | G″ [Pa] | b | tan δ | c | G′ = G″ [Pa] |
|---|---|---|---|---|---|---|---|
| SWF | 112.7 ± 11.5 a | 0.1876 ± 0.0151 c | 26.7 ± 1.6 a | 0.2298 ± 0.0125 c | 0.2379 ± 0.0103 a | 0.0418 ± 0.0025 d | 182.3 ± 5.3 d |
| 5% | 154.7 ± 5.4 a | 0.1969 ± 0.0107 c | 47.5 ± 8.4 a | 0.2150 ± 0.0100 c | 0.3064 ± 0.0427 bc | 0.0184 ± 0.0199 c | 160.2 ± 1.3 c |
| 10% | 309.0 ± 15.9 b | 0.1515 ± 0.0054 b | 109.7 ± 2.1 b | 0.1570 ± 0.0008 b | 0.3556 ± 0.0247 bc | 0.0055 ± 0.0045 bc | 122.7 ± 10.8 b |
| 15% | 497.4 ± 22.5 c | 0.1215 ± 0.0001 a | 160.1 ± 14.7 b | 0.1365 ± 0.0088 b | 0.3215 ± 0.0154 c | 0.0149 ± 0.0093 c | 96.3 ± 16.4 ab |
| 20% | 1151.0 ± 84.6 d | 0.1071 ± 0.0025 a | 412.6 ± 47.9 c | 0.0939 ± 0.0017 a | 0.3582 ± 0.0153 c | −0.0130 ± 0.0042 ab | 120.5 ± 1.3 b |
| PF | 21,570.0 ± 1576.9 e | 0.1205 ± 0.0024 a | 6362.1 ± 445.6 d | 0.0979 ± 0.0052 a | 0.2949 ± 0.0001 b | −0.0225 ± 0.0035 a | 103.151 ± 6.417 ab |
| Sample | L* | a* | b* | C* | hº |
|---|---|---|---|---|---|
| Control | 91.00 ± 0.95 d | 1.03 ± 0.10 a | 10.13 ± 0.62 a | 10.18 ± 0.62 a | 84.3 ± 0.29 e |
| 5% | 89.23 ± 0.59 d | 1.98 ± 0.30 b | 14.28 ± 0.39 b | 14.41 ± 0.42 b | 82.14 ± 0.95 d |
| 10% | 87.00 ± 0.37 c | 3.43 ± 0.31 c | 19.98 ± 0.46 c | 20.27 ± 0.48 c | 80.27 ± 0.79 c |
| 15% | 85.55 ± 0.60 bc | 4.00 ± 0.12 d | 22.60 ± 0.47 a | 22.95 ± 0.48 d | 79.96 ± 0.15 c |
| 20% | 84.28 ± 0.46 b | 5.68 ± 0.30 e | 27.13 ± 0.74 e | 27.71 ± 0.76 e | 78.18 ± 0.51 b |
| PF | 69.50 ± 3.18 a | 16.05 ± 0.42 f | 43.50 ± 2.64 f | 46.37 ± 2.52 f | 69.71 ± 1.11 a |
| Sample | GE mg/1 g DM |
|---|---|
| SWF | 2.60 ± 0.18 a |
| 5% | 3.51 ± 0.14 b |
| 10% | 4.08 ± 0.09 c |
| 15% | 5.13 ± 0.07 d |
| 20% | 5.85 ± 0.05 e |
| PF | 8.55 ± 0.03 f |
| Sample | TPC (GAE mg/1 g DM) | DPPH (TE mg/1 g DM) | ABTS (TE mg/1 g DM) | FRAP (FeSO4 mM/1 g DM) | ||||
|---|---|---|---|---|---|---|---|---|
| H2O | EtOH | H2O | EtOH | H2O | EtOH | H2O | EtOH | |
| SWF | 0.19 ± 0.00 a | 0.05 ± 0.01 a | 76.2 ± 3.3 a | 205.8 ± 7.5 a | 3399.8 ± 149.1 a | 754.8 ± 42.8 a | 0.87 ± 0.03 a | 5.87 ± 0.12 a |
| 5% | 0.28 ± 0.02 b | 0.13 ± 0.00 b | 110.0 ± 3.5 b | 415.6 ± 7.8 b | 3927.7 ± 17.1 b | 866.0 ± 26.8 b | 1.06 ± 0.08 a | 6.54 ± 0.02 b |
| 10% | 0.42 ± 0.01 c | 0.21 ± 0.01 c | 166.7 ± 6.9 c | 457.7 ± 18.5 c | 4451.3 ± 10.6 c | 987.5 ± 13.2 c | 1.85 ± 0.05 a | 6.82 ± 0.24 bc |
| 15% | 0.48 ± 0.01 d | 0.25 ± 0.01 d | 200.3 ± 3.0 d | 486.5 ± 1.8 c | 4928.3 ± 72.6 d | 1176.1 ± 3.1 d | 2.98 ± 0.29 b | 7.35 ± 0.02 cd |
| 20% | 0.57 ± 0.01 e | 0.34 ± 0.01 e | 237.6 ± 7.5 e | 557.6 ± 7.8 d | 5158.6 ± 112.6 d | 1389.5 ± 27.4 e | 3.69 ± 0.14 b | 7.69 ± 0.06 d |
| PF | 1.22 ± 0.02 f | 0.98 ± 0.03 f | 752.9 ± 6.7 f | 1042.7 ± 23.8 e | 7685.1 ± 136.3 e | 2484.4 ± 22.8 f | 20.24 ± 1.00 c | 32.42 ± 0.51 e |
| sample | *** | *** | *** | *** | ||||
| solvent | *** | *** | *** | *** | ||||
| Sample × solvent | *** | *** | *** | *** | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alija, D.; Olędzki, R.; Nikolovska Nedelkoska, D.; Wojciechowicz-Budzisz, A.; Xhabiri, G.; Pejcz, E.; Alija, E.; Harasym, J. The Addition of Pumpkin Flour Impacts the Functional and Bioactive Properties of Soft Wheat Composite Flour Blends. Foods 2025, 14, 243. https://doi.org/10.3390/foods14020243
Alija D, Olędzki R, Nikolovska Nedelkoska D, Wojciechowicz-Budzisz A, Xhabiri G, Pejcz E, Alija E, Harasym J. The Addition of Pumpkin Flour Impacts the Functional and Bioactive Properties of Soft Wheat Composite Flour Blends. Foods. 2025; 14(2):243. https://doi.org/10.3390/foods14020243
Chicago/Turabian StyleAlija, Durim, Remigiusz Olędzki, Daniela Nikolovska Nedelkoska, Agata Wojciechowicz-Budzisz, Gafur Xhabiri, Ewa Pejcz, Eljesa Alija, and Joanna Harasym. 2025. "The Addition of Pumpkin Flour Impacts the Functional and Bioactive Properties of Soft Wheat Composite Flour Blends" Foods 14, no. 2: 243. https://doi.org/10.3390/foods14020243
APA StyleAlija, D., Olędzki, R., Nikolovska Nedelkoska, D., Wojciechowicz-Budzisz, A., Xhabiri, G., Pejcz, E., Alija, E., & Harasym, J. (2025). The Addition of Pumpkin Flour Impacts the Functional and Bioactive Properties of Soft Wheat Composite Flour Blends. Foods, 14(2), 243. https://doi.org/10.3390/foods14020243







