Antimicrobial Effectiveness of Clove Oil in Decontamination of Ready-to-Eat Spinach (Spinacia oleracea L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Antimicrobial Compounds
2.2. Microorganisms
2.3. Zone of Inhibition
2.4. Bacterial Death Curve
2.5. RTE Washing Experiment
2.6. RTE Total Bacteria Survival in CO Wash Water
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results
3.1. Zone of Inhibition
3.2. Bacterial Death Curve
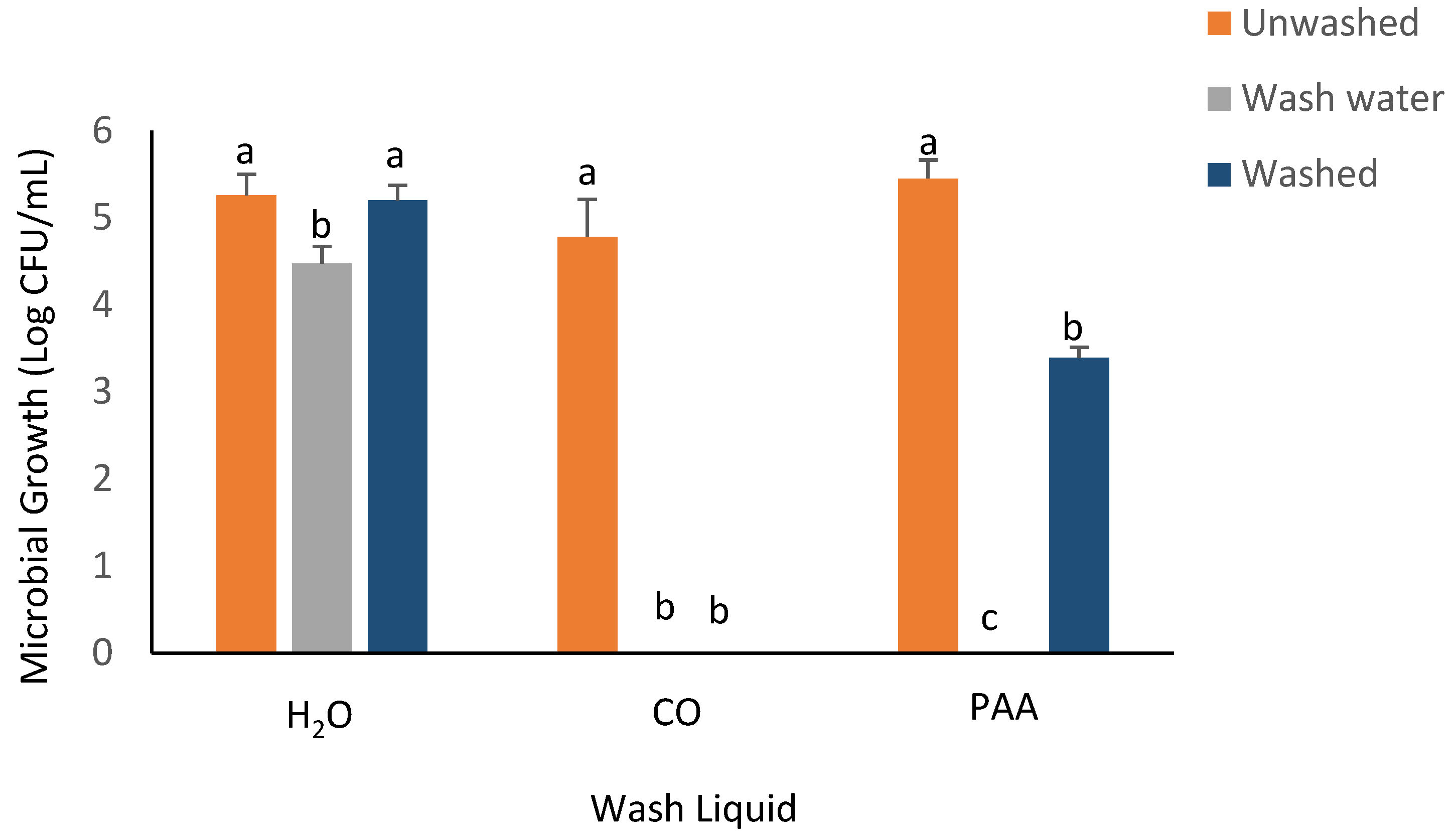
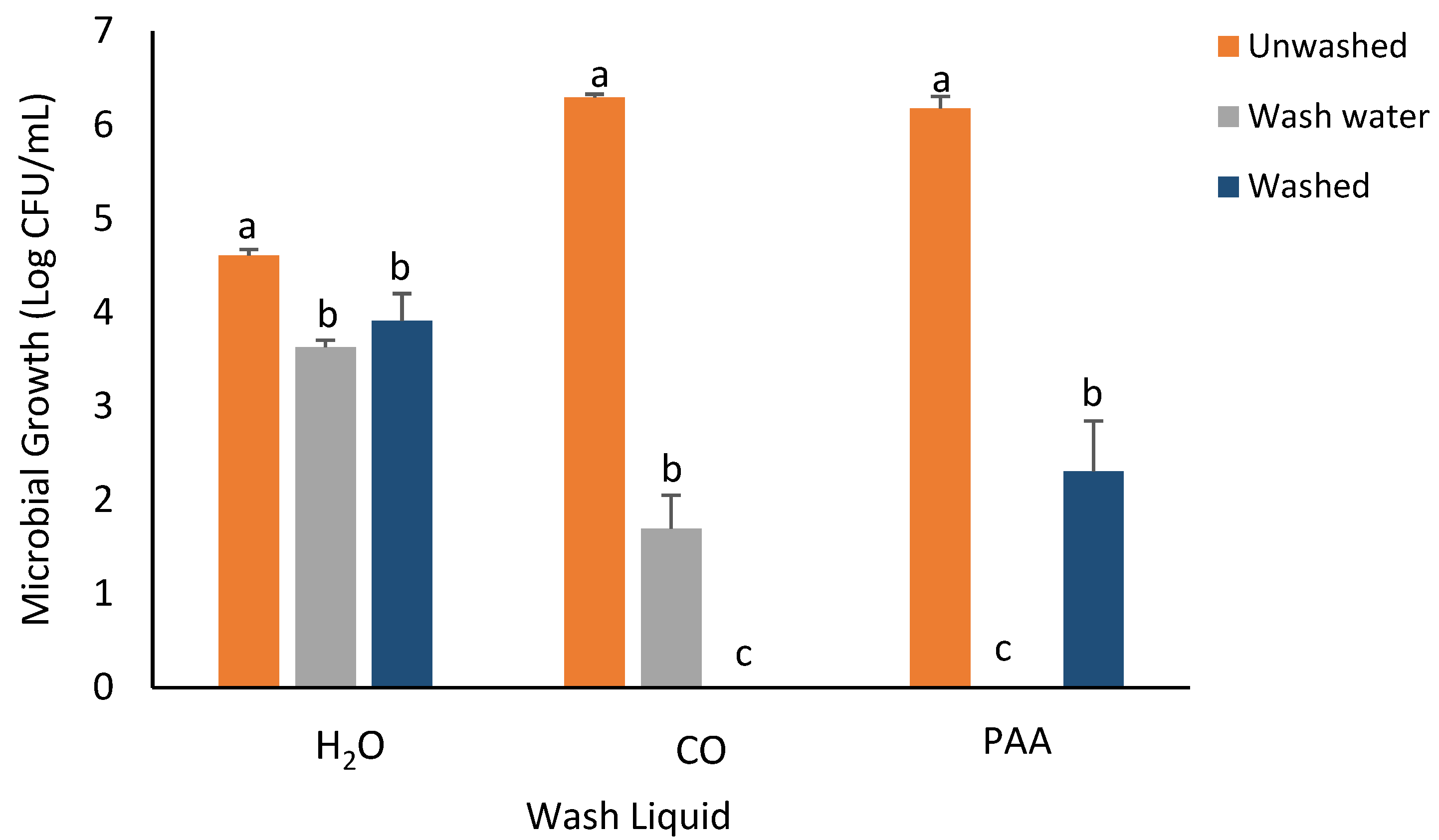
3.3. RTE Washing Experiment
3.4. Nomad IoT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
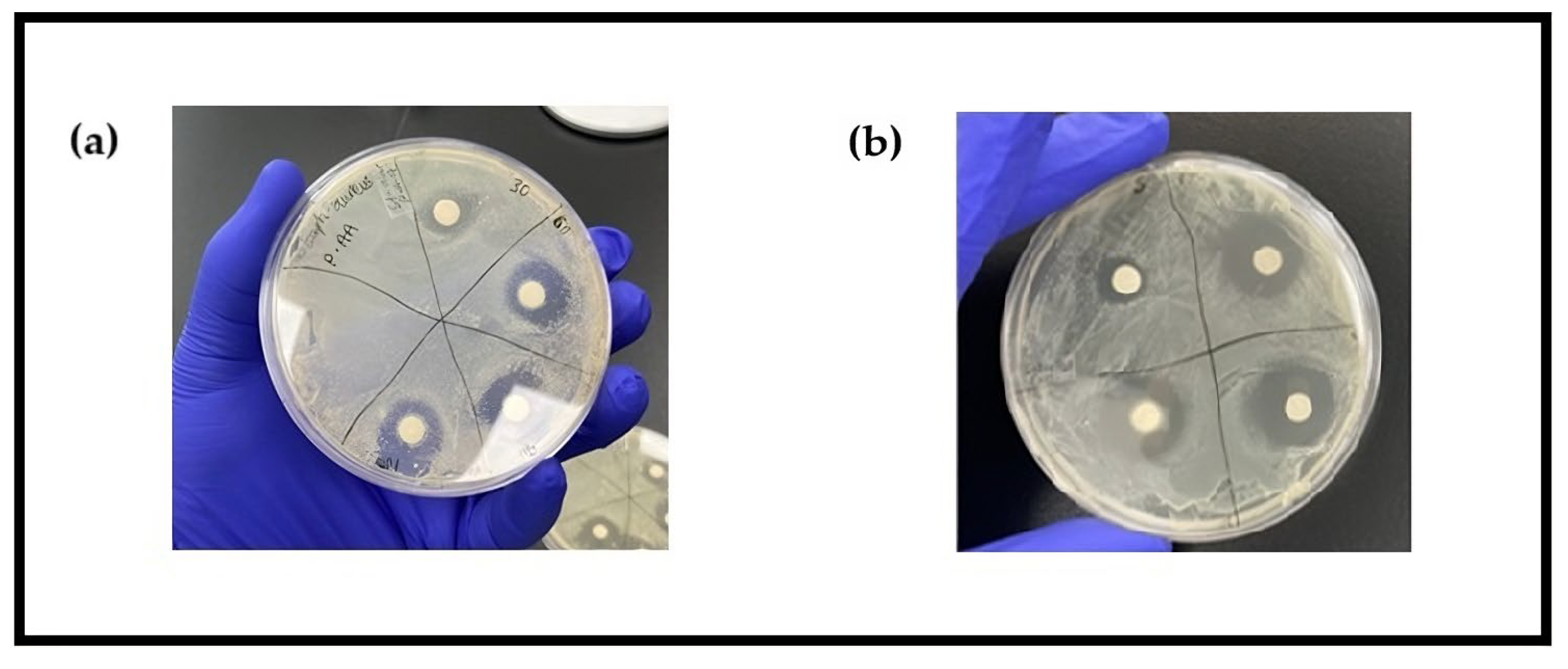
References
- WHO. Food Safety—World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 1 December 2024).
- Castro-Ibáñez, I.; Gil, M.I.; Allende, A. Ready-to-Eat Vegetables: Current Problems and Potential Solutions to Reduce Microbial Risk in the Production Chain. LWT Food Sci. Technol. 2017, 85, 284–292. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M.; Dar, B.N.; Greiner, R.; Roohinejad, S. Microbiological Contamination of Ready-to-Eat Vegetable Salads in Developing Countries and Potential Solutions in the Supply Chain to Control Microbial Pathogens. Food Control 2018, 85, 235–244. [Google Scholar] [CrossRef]
- Ritchie, H.; Rosado, P.; Roser, M. Data Page: Per Capita Consumption of Vegetables. Agricultural Production. 2023. Available online: https://ourworldindata.org/grapher/vegetable-consumption-per-capita (accessed on 30 November 2024).
- Wang, D.D.; Li, Y.; Bhupathiraju, S.N.; Rosner, B.A.; Sun, Q.; Giovannucci, E.L.; Rimm, E.B.; Manson, J.A.E.; Willett, W.C.; Stampfer, M.J.; et al. Fruit and Vegetable Intake and Mortality Results From 2 Prospective Cohort Studies of US Men and Women and a Meta-Analysis of 26 Cohort Studies. Circulation 2021, 143, 1642–1654. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and Vegetable Intake and the Risk of Cardiovascular Disease, Total Cancer and All-Cause Mortality-A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Hung, H.C.; Joshipura, K.J.; Jiang, R.; Hu, F.B.; Hunter, D.; Smith-Warner, S.A.; Willett, W.C. Fruit and Vegetable Intake and Risk of Major Chronic Disease. J. Natl. Cancer Inst. 2004, 96, 1577–1584. [Google Scholar] [CrossRef]
- Thomas, G.A.; Paradell Gil, T.; Müller, C.T.; Rogers, H.J.; Berger, C.N. From Field to Plate: How Do Bacterial Enteric Pathogens Interact with Ready-to-Eat Fruit and Vegetables, Causing Disease Outbreaks? Food Microbiol. 2024, 117, 104389. [Google Scholar] [CrossRef]
- Bennett, S.D.; Sodha, S.V.; Ayers, T.L.; Lynch, M.F.; Gould, L.H.; Tauxe, R.V. Produce-Associated Foodborne Disease Outbreaks, USA, 1998–2013. Epidemiol. Infect. 2018, 146, 1397–1406. [Google Scholar] [CrossRef]
- Azimirad, M.; Nadalian, B.; Alavifard, H.; Panirani, S.N.; Bonab, S.M.V.; Azimirad, F.; Gholami, F.; Jabbari, P.; Yadegar, A.; Busani, L.; et al. Microbiological Survey and Occurrence of Bacterial Foodborne Pathogens in Raw and Ready-to-Eat Green Leafy Vegetables Marketed in Tehran, Iran. Int. J. Hyg. Environ. Health 2021, 237, 113824. [Google Scholar] [CrossRef]
- Faour-Klingbeil, D.; Murtada, M.; Kuri, V.; Todd, E.C.D. Understanding the Routes of Contamination of Ready-to-Eat Vegetables in the Middle East. Food Control 2016, 62, 125–133. [Google Scholar] [CrossRef]
- Toe, E.; Attien, P.; Moroh, A.J.-L.; Sina, H.; Kouame, D.N.; Kambire, O.; Baba-Moussa, L.; Guessennd, N.; Dako, E.; Dadie, A.T. Prevalence and Characterization of Salmonella Isolated from Vegetables Salads and Ready to Eat Raw Mixed Vegetable Salads in Abidjan, Cte DIvoire. J. Microbiol. Antimicrob. 2022, 14, 15–25. [Google Scholar] [CrossRef]
- Shieh, Y.C.; Tortorello, M.L.; Fleischman, G.J.; Li, D.; Schaffner, D.W. Tracking and Modeling Norovirus Transmission during Mechanical Slicing of Globe Tomatoes. Int. J. Food Microbiol. 2014, 180, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, A.L.; Davidson, G.R.; Marks, B.P.; Todd, E.C.D.; Ryser, E.T. Tracking an Escherichia Coli O157:H7-Contaminated Batch of Leafy Greens through a Pilot-Scale Fresh-Cut Processing Line. J. Food Prot. 2014, 77, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry: Guide to Minimize Microbial Food Safety Hazards of Fresh-Cut Fruits and Vegetables; FDA: Washington, DC, USA, 2008. [Google Scholar]
- Wang, H.; Ryser, E.T. Microbiological Safety of Fresh-Cut Produce from the Processor to Your Plate. Food Safety Magazine, 1 August 2014. [Google Scholar]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate Outbreaks of Foodborne Illness in the United States Associated With Fresh Produce From 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef]
- Bondi, M.; Lauková, A.; De Niederhausern, S.; Messi, P.; Papadopoulou, C. Natural Preservatives to Improve Food Quality and Safety. J. Food Qual. 2017, 2017, 1–3. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Ullah, H.; Hussain, Y.; Santarcangelo, C.; Baldi, A.; Di Minno, A.; Khan, H.; Xiao, J.; Daglia, M. Natural Polyphenols for the Preservation of Meat and Dairy Products. Molecules 2022, 27, 1906. [Google Scholar] [CrossRef]
- Gadelha, J.R.; Allende, A.; López-Gálvez, F.; Fernández, P.; Gil, M.I.; Egea, J.A. Chemical Risks Associated with Ready-to-Eat Vegetables: Quantitative Analysis to Estimate Formation and/or Accumulation of Disinfection Byproducts during Washing. EFSA J. 2019, 17, e170913. [Google Scholar] [CrossRef]
- Joshi, K.; Mahendran, R.; Alagusundaram, K.; Norton, T.; Tiwari, B.K. Novel Disinfectants for Fresh Produce. Trends Food Sci. Technol. 2013, 34, 54–61. [Google Scholar] [CrossRef]
- Gil, M.I.; Marín, A.; Andujar, S.; Allende, A. Should Chlorate Residues Be of Concern in Fresh-Cut Salads? Food Control 2016, 60, 416–421. [Google Scholar] [CrossRef]
- Bornhorst, E.R.; Luo, Y.; Park, E.; Vinyard, B.T.; Nou, X.; Zhou, B.; Turner, E.; Millner, P.D. Immersion-Free, Single-Pass, Commercial Fresh-Cut Produce Washing System: An Alternative to Flume Processing. Postharvest Biol. Technol. 2018, 146, 124–133. [Google Scholar] [CrossRef]
- Garrido, Y.; Marín, A.; Tudela, J.A.; Allende, A.; Gil, M.I. Chlorate Uptake during Washing Is Influenced by Product Type and Cut Piece Size, as Well as Washing Time and Wash Water Content. Postharvest Biol. Technol. 2019, 151, 45–52. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Risks for Public Health Related to the Presence of Chlorate in Food. EFSA J. 2015, 13, 4135. [Google Scholar] [CrossRef]
- Amy, G.L. Disinfectants and Disinfectant By-Products; World Health Organization: Geneva, Switzerland, 2000; ISBN 9241572167. [Google Scholar]
- Lee, W.N.; Huang, C.H. Formation of Disinfection Byproducts in Wash Water and Lettuce by Washing with Sodium Hypochlorite and Peracetic Acid Sanitizers. Food Chem. X 2019, 1, 100003. [Google Scholar] [CrossRef]
- Alvaro, J.E.; Moreno, S.; Dianez, F.; Santos, M.; Carrasco, G.; Urrestarazu, M. Effects of Peracetic Acid Disinfectant on the Postharvest of Some Fresh Vegetables. J. Food Eng. 2009, 95, 11–15. [Google Scholar] [CrossRef]
- Bai, J.; Li, J.; Chen, Z.; Bai, X.; Yang, Z.; Wang, Z.; Yang, Y. Antibacterial Activity and Mechanism of Clove Essential Oil against Foodborne Pathogens. LWT 2023, 173, 114249. [Google Scholar] [CrossRef]
- Kumar Pandey, V.; Shams, R.; Singh, R.; Dar, A.H.; Pandiselvam, R.; Rusu, A.V.; Trif, M. A Comprehensive Review on Clove (Caryophyllus aromaticus L.) Essential Oil and Its Significance in the Formulation of Edible Coatings for Potential Food Applications. Front. Nutr. 2022, 9, 987674. [Google Scholar] [CrossRef]
- Mittal, R.P.; Rana, A.; Jaitak, V. Essential Oils: An Impending Substitute of Synthetic Antimicrobial Agents to Overcome Antimicrobial Resistance. Curr. Drug Targets 2018, 20, 605–624. [Google Scholar] [CrossRef]
- Nuñez, L.; Aquino, M.D’. Microbicide Activity of Clove Essential Oil (Eugenia caryophyllata). Braz. J. Microbiol. 2012, 43, 1255–1260. [Google Scholar] [CrossRef]
- Thosar, N.; Basak, S.; Bahadure, R.N.; Rajurkar, M. Antimicrobial Efficacy of Five Essential Oils against Oral Pathogens: An In Vitro Study. Eur. J. Dent. 2013, 7, S071–S077. [Google Scholar] [CrossRef]
- Barbosa, L.N.; Rall, V.L.M.; Fernandes, A.A.H.; Ushimaru, P.I.; Da Silva Probst, I.; Fernandes, A. Essential Oils against Foodborne Pathogens and Spoilage Bacteria in Minced Meat. Foodborne Pathog. Dis. 2009, 6, 725–728. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, M.; Wei, S. Progress on the Antimicrobial Activity Research of Clove Oil and Eugenol in the Food Antisepsis Field. J. Food Sci. 2018, 83, 1476–1483. [Google Scholar] [CrossRef]
- Liñán-Atero, R.; Aghababaei, F.; García, S.R.; Hasiri, Z.; Ziogkas, D.; Moreno, A.; Hadidi, M. Clove Essential Oil: Chemical Profile, Biological Activities, Encapsulation Strategies, and Food Applications. Antioxidants 2024, 13, 488. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Srivastava, S.; Ashish; Dash, K.K.; Singh, R.; Dar, A.H.; Singh, T.; Farooqui, A.; Shaikh, A.M.; Kovacs, B. Bioactive Properties of Clove (Syzygium aromaticum) Essential Oil Nanoemulsion: A Comprehensive Review. Heliyon 2024, 10, e22437. [Google Scholar] [CrossRef]
- Ginting, E.V.; Retnaningrum, E.; Widiasih, D.A. Antibacterial Activity of Clove (Syzygium aromaticum) and Cinnamon (Cinnamomum burmannii) Essential Oil against Extended-Spectrum β-Lactamase-Producing Bacteria. Vet. World 2021, 14, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Rehab, M.A.E.-B.; Zeinab, S.H. Eugenol and Linalool: Comparison of Their Antibacterial and Antifungal Activities. Afr. J. Microbiol. Res. 2016, 10, 1860–1872. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Rabiaee, S.; Lotfalian, S.; Habibian, S. Effect of Clove Essential Oil (Syzygium aromaticum) on Some Virulence Factors of Staphylococcus Aureus. Med. Lab. J. 2020, 14, 13–19. [Google Scholar] [CrossRef]
- Jeyakumar, G.E.; Lawrence, R. Mechanisms of Bactericidal Action of Eugenol against Escherichia coli. J. Herb. Med. 2021, 26, 100406. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an Essential Oil of Clove) Acts as an Antibacterial Agent against Salmonella Typhi by Disrupting the Cellular Membrane. J. Ethnopharmacol. 2010, 1, 107–115. [Google Scholar] [CrossRef]
- Costa, L.V.; Moreira, J.M.A.R.; de Godoy Menezes, I.; Dutra, V.; do Bom Parto Ferreira de Almeida, A. Antibiotic Resistance Profiles and Activity of Clove Essential Oil (Syzygium aromaticum) against Pseudomonas Aeruginosa Isolated of Canine Otitis. Vet. World 2022, 15, 2499–2505. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Shi, C.; Aliakbarlu, J.; Cui, H.; Lin, L. Antibacterial Mechanisms of Clove Essential Oil against Staphylococcus Aureus and Its Application in Pork. Int. J. Food Microbiol. 2022, 380, 109864. [Google Scholar] [CrossRef] [PubMed]
- Elbestawy, M.K.M.; El-Sherbiny, G.M.; Moghannem, S.A. Antibacterial, Antibiofilm and Anti-Inflammatory Activities of Eugenol Clove Essential Oil against Resistant Helicobacter Pylori. Molecules 2023, 28, 2448. [Google Scholar] [CrossRef]
- Ebirim, R.I.; Long, W. Evaluation of Antimicrobial and Preservative Effects of Cinnamaldehyde and Clove Oil in Catfish (Ictalurus punctatus) Fillets Stored at 4 °C. Foods 2024, 13, 1445. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Sokorai, K.; Ukuku, D.O.; Fan, X.; Olanya, M.; Juneja, V. Effects of Pulsed Light and Sanitizer Wash Combination on Inactivation of Escherichia Coli O157: H7, Microbial Loads and Apparent Quality of Spinach Leaves. Food Microbiol. 2019, 82, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Li, X.; Liu, X.; Xing, Z.; Su, R.; Wang, Y.; Xia, X.; Shi, C. Antibacterial Effect of Eugenol on Shigella Flexneri and Its Mechanism. Foods 2022, 11, 2565. [Google Scholar] [CrossRef]
- Sohilait, H.J.; Kainama, H.; Nindatu, M. Chemical Composition and Antibacterial Activity of the Essential Oils from Different Parts of Eugenia caryophylata, Thunb Grown in Amboina Island. Int. J. Org. Chem. 2018, 8, 229–239. [Google Scholar] [CrossRef][Green Version]
- Dulal, S.; Chaudhary, S.; Dangi, C.; Sah, S.N. Antibacterial Effect of Essential Oils (Clove Oil, Castor Oil and Ginger Oil) Against Human Pathogenic Bacteria. Int. J. Appl. Sci. Biotechnol. 2021, 9, 250–255. [Google Scholar] [CrossRef]
- Beveridge, T.J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef]
- Lambert, P.A. Cellular Impermeability and Uptake of Biocides and Antibiotics in Gram-Positive Bacteria and Mycobacteria. J. Appl. Microbiol. 2002, 92, 46S–54S. [Google Scholar] [CrossRef]
- Saxena, D.; Maitra, R.; Bormon, R.; Czekanska, M.; Meiers, J.; Titz, A.; Verma, S.; Chopra, S. Tackling the Outer Membrane: Facilitating Compound Entry into Gram-Negative Bacterial Pathogens. npj Antimicrob. Resist. 2023, 1, 17. [Google Scholar] [CrossRef]
- Niu, D.; Wang, Q.-Y.; Ren, E.-F.; Zeng, X.-A.; Wang, L.-H.; He, T.-F.; Wen, Q.-H.; Brennan, C.S. Multi-Target Antibacterial Mechanism of Eugenol and Its Combined Inactivation with Pulsed Electric Fields in a Hurdle Strategy on Escherichia coli. Food Control 2019, 106, 106742. [Google Scholar]
- Kong, J.; Wang, Y.; Yao, Z.; Lin, Y.; Zhang, Y.; Han, Y.; Zhou, T.; Ye, J.; Cao, J. Eugenol Works Synergistically with Colistin against Colistin-Resistant Pseudomonas Aeruginosa and Klebsiella Pneumoniae Isolates by Enhancing Membrane Permeability. Microbiol. Spectr. 2023, 11, e0366622. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry Guide to Minimize Microbial Food Safety Hazards for Fresh Fruits and Vegetables; FDA: Washington, DC, USA, 1998. [Google Scholar]
- Gragg, S.E.; Brooks, J.C.; Brashears, M.M. Reduction of Escherichia Coli O157:H7 in Fresh Spinach Using Bovamine® Meat Cultures as a Post-Harvest Intervention and Its Impact on Sensory Properties. Foods Prot. Trends 2010, 30, 72–77. [Google Scholar]
- Guentzel, J.L.; Liang Lam, K.; Callan, M.A.; Emmons, S.A.; Dunham, V.L. Reduction of Bacteria on Spinach, Lettuce, and Surfaces in Food Service Areas Using Neutral Electrolyzed Oxidizing Water. Food Microbiol. 2008, 25, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Rapid Microbiology Nomad Smart Microbiology IoT Test Devices. Available online: https://www.rapidmicrobiology.com/products/nomad-smart-microbiology-iot-test-devices (accessed on 1 December 2024).
- BioMire. BioMire. Available online: https://www.rapidmicrobiology.com/supplier/biomire (accessed on 30 November 2024).
- Yuwono, M.; Fuad Hafid, A.; Toto Poemorno, A.; Agil, M.; Indrayanto, G.; Ebel, S. Eugenol. Anal. Profiles Drug Subst. Excip. 2002, 29, 149–177. [Google Scholar] [CrossRef]
- Nagaraju, P.G.; Sengupta, P.; Poornima Priyadarshini, C.G.; Rao, P.J. Nanoencapsulation of Clove Oil and Study of Physico-Chemical Properties, Cytotoxic, Haemolytic and Antioxidant Activities. J. Food Process Eng. 2020, 44, e13645. [Google Scholar]
- Huang, X.; Lao, Y.; Pan, Y.; Chen, Y.; Zhao, H.; Gong, L.; Xie, N.; Mo, C.H. Synergistic Antimicrobial Effectiveness of Plant Essential Oil and Its Application in Seafood Preservation: A Review. Molecules 2021, 26, 307. [Google Scholar] [CrossRef]
- Yu, H.H.; Chin, Y.W.; Paik, H.D. Application of Natural Preservatives for Meat and Meat Products against Food-borne Pathogens and Spoilage Bacteria: A Review. Foods 2021, 10, 2418. [Google Scholar] [CrossRef]

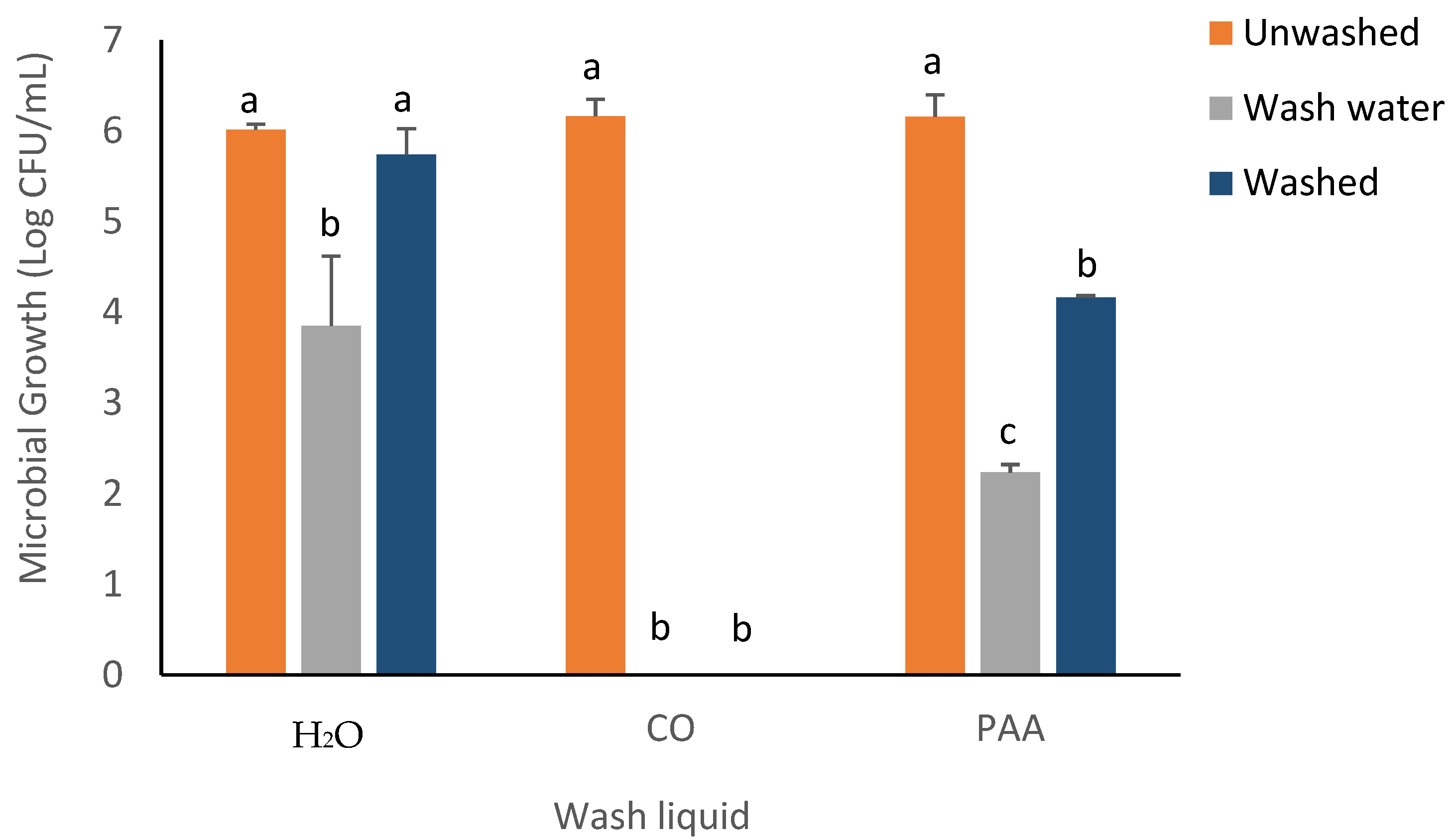
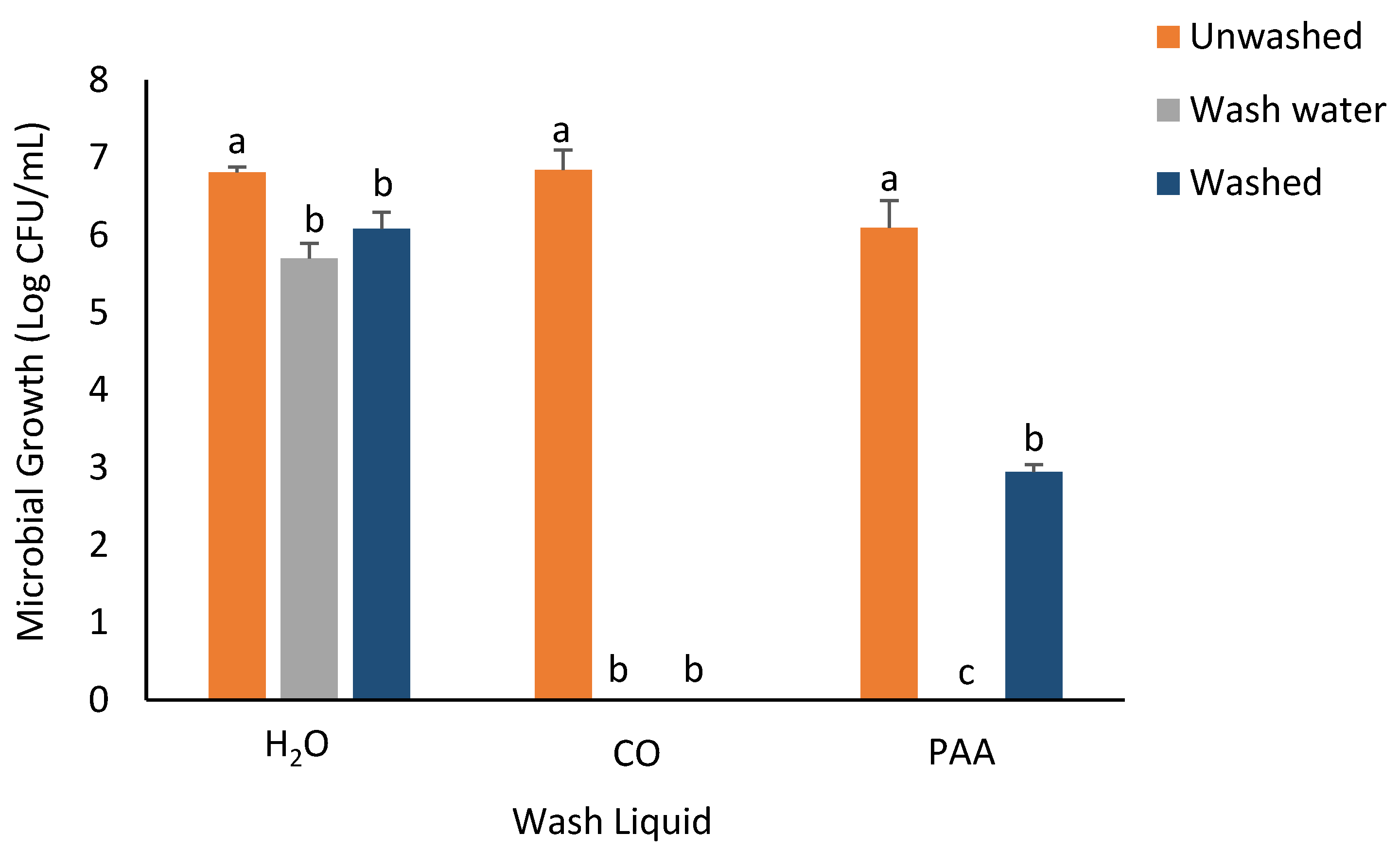
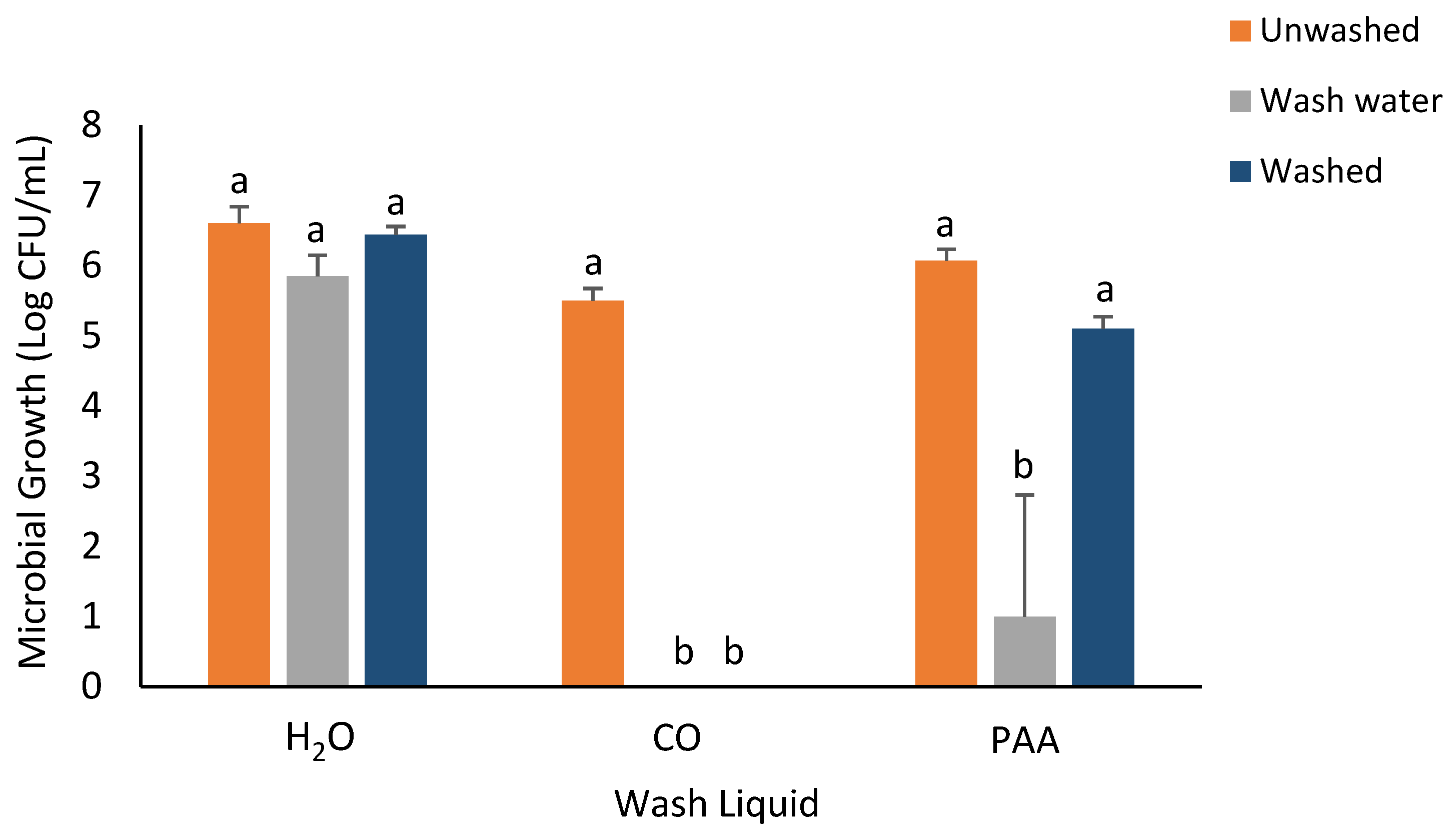


| ZIB for Microbial Strain | |||||
|---|---|---|---|---|---|
| Treatment/Conc. | Escherichia coli | Staphylococcus aureus | Shigella flexneri | Salmonella enterica | Salmonella Typhimurium |
| H2O | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| PAA (mg/L) | |||||
| 60 | 8.00 ± 0.00 b | 16.67 ± 4.16 c | 8.33 ± 0.58 b | 8.00 ± 0.00 b | 8.33 ± 1.53 b |
| 80 | 8.33 ± 0.58 b | 19.00 ± 3.60 c | 9.00 ± 1.00 b | 8.33 ± 0.58 b | 9.33 ± 1.53 b |
| CO (% v/v) | |||||
| 5 | 9.67 ± 1.15 bc | 7.67 ± 0.58 b | 8.33 ± 0.58 b | 10.00 ± 1.15 bc | 9.67 ± 4.62 b |
| 10 | 11.33 ± 0.58 c | 9.00 ± 1.00 b | 10.33 ± 0.58 bc | 11.33 ± 0.58 c | 9.33 ± 1.53 b |
| Quality Attribute | PAA | H2O | CO |
|---|---|---|---|
| Color | 3.77 ± 1.15 a | 3.77 ± 1.12 a | 3.48 ± 1.18 a |
| Texture | 3.94 ± 0.96 a | 3.61 ± 1.20 a | 2.39 ± 1.31 b |
| Aroma | 3.45 ± 1.15 a | 3.77 ± 1.12 a | 2.45 ± 1.57 b |
| Overall Acceptability | 3.74 ± 0.93 a | 3.84 ± 1.16 a | 2.42 ± 1.38 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armah, A.A.; Ofori, K.F.; Sutherland, K.; Otchere, E.; Lewis, W.A.; Long, W., III. Antimicrobial Effectiveness of Clove Oil in Decontamination of Ready-to-Eat Spinach (Spinacia oleracea L.). Foods 2025, 14, 249. https://doi.org/10.3390/foods14020249
Armah AA, Ofori KF, Sutherland K, Otchere E, Lewis WA, Long W III. Antimicrobial Effectiveness of Clove Oil in Decontamination of Ready-to-Eat Spinach (Spinacia oleracea L.). Foods. 2025; 14(2):249. https://doi.org/10.3390/foods14020249
Chicago/Turabian StyleArmah, Abigail A., Kelvin F. Ofori, Kenisha Sutherland, Emmanuel Otchere, Winter A. Lewis, and Wilbert Long, III. 2025. "Antimicrobial Effectiveness of Clove Oil in Decontamination of Ready-to-Eat Spinach (Spinacia oleracea L.)" Foods 14, no. 2: 249. https://doi.org/10.3390/foods14020249
APA StyleArmah, A. A., Ofori, K. F., Sutherland, K., Otchere, E., Lewis, W. A., & Long, W., III. (2025). Antimicrobial Effectiveness of Clove Oil in Decontamination of Ready-to-Eat Spinach (Spinacia oleracea L.). Foods, 14(2), 249. https://doi.org/10.3390/foods14020249






