Edible Insects as an Alternative Source of Nutrients: Benefits, Risks, and the Future of Entomophagy in Europe—A Narrative Review
Abstract
:1. Introduction
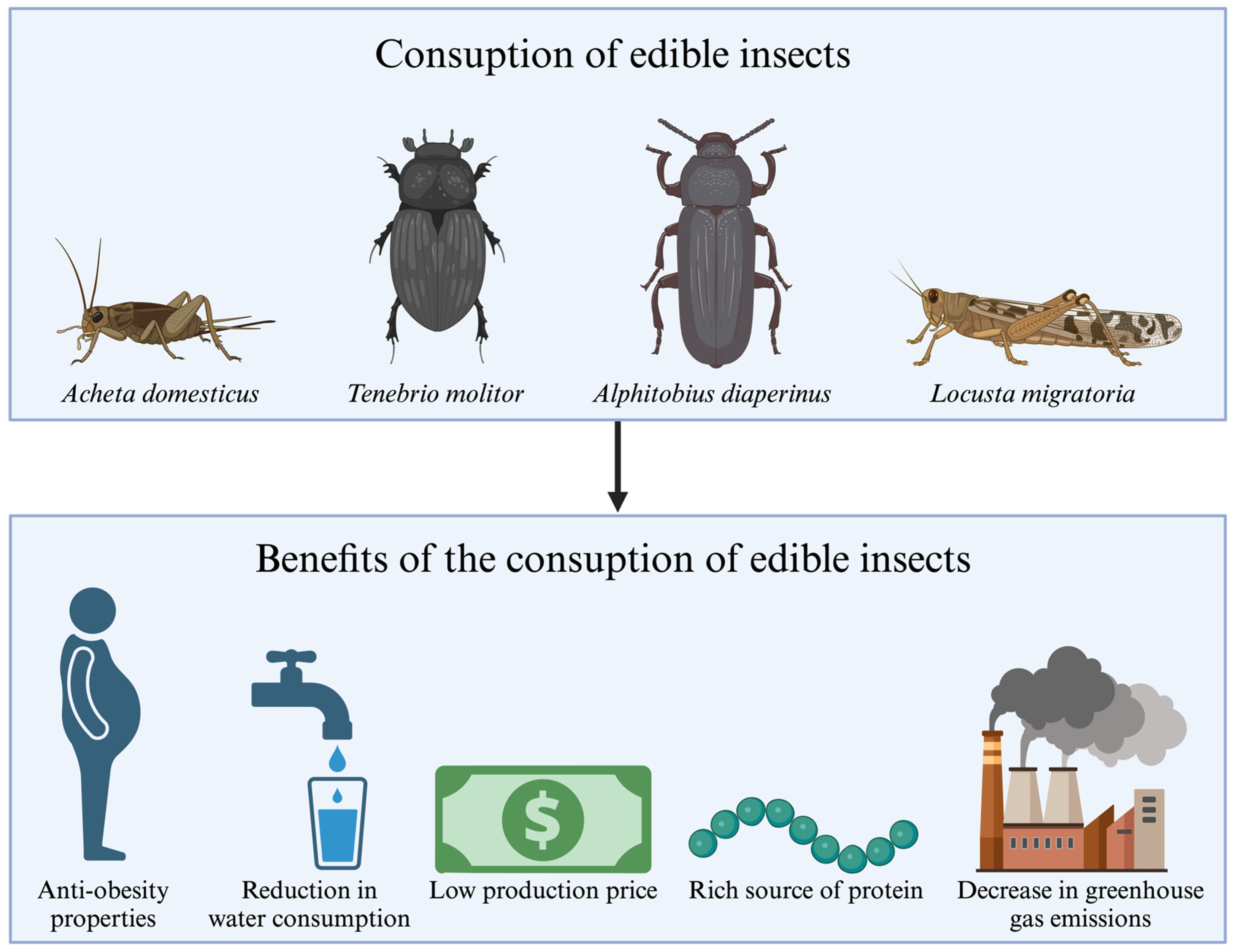
2. Edible Insects and Their Allergens—General Aspects
3. Methodology
4. Edible Insects and Their Allergens—Detailed Characteristics
4.1. Acheta Domesticus (The House Cricket)
4.2. Tenebrio Molitor (The Yellow Mealworm)
4.3. Locusta Migratoria (The Migratory Locust)
4.4. Alphitobius Diaperinus (The Lesser Mealworm)
5. Edible Insects Outside Europe
6. Epidemiology of Allergy to Edible Insects
7. Major Allergens in Case of Food Allergy to Edible Insects
7.1. Tropomyosin
7.2. Arginine Kinase (AK)
7.3. Larval Cuticle Protein (LCP)
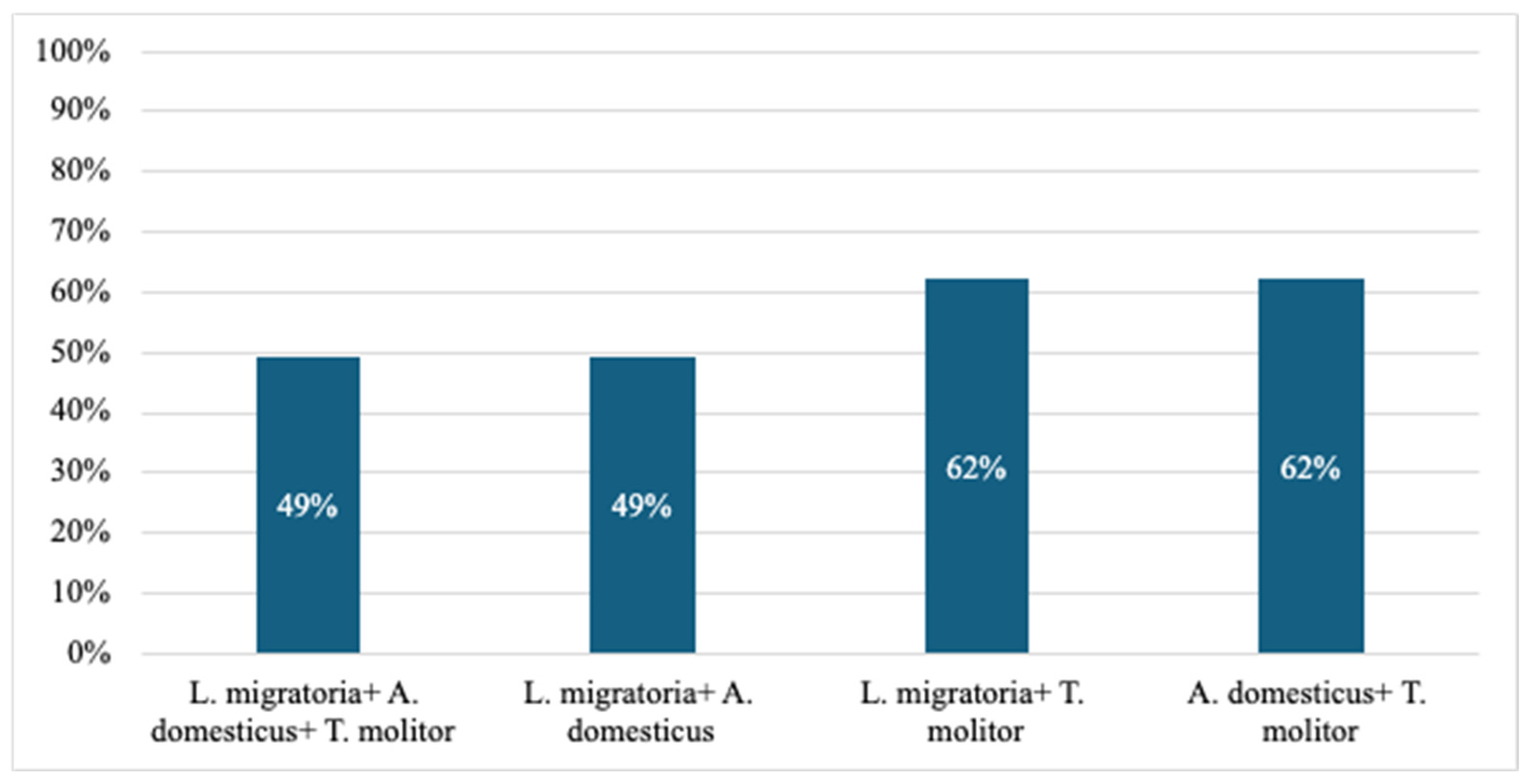
8. Cross-Reactivity of Edible Insects’ Allergens
8.1. Tropomyosin
8.2. Arginine Kinase (AK)
9. Diagnosis of Allergy to Edible Insects
10. Diagnosis of Edible Insect Allergens’ Cross-Reactivity
11. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Gier, S.; Verhoeckx, K. Insect (Food) Allergy and Allergens. Mol. Immunol. 2018, 100, 82–106. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Anania, C.; Caffarelli, C.; Martelli, A.; del Giudice, M.M.; Cravidi, C.; Duse, M.; Manti, S.; Tosca, M.A.; Cardinale, F.; et al. Food Allergy: An Updated Review on Pathogenesis, Diagnosis, Prevention and Management. Acta Biomed. 2020, 91, e2020012. [Google Scholar]
- Bartha, I.; Almulhem, N.; Santos, A.F. Feast for Thought: A Comprehensive Review of Food Allergy 2021–2023. J. Allergy Clin. Immunol. 2024, 153, 576–594. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Hochwallner, H.; Linhart, B.; Pahr, S. Food Allergies: The Basics. Gastroenterology 2015, 148, 1120–1131.e4. [Google Scholar] [CrossRef]
- Waserman, S.; Watson, W. Food Allergy. Allergy Asthma Clin. Immunol. 2011, 7, S7. [Google Scholar] [CrossRef]
- Palmer, L. Edible Insects as a Source of Food Allergens. Ph.D. Thesis, University of Nebraska-Lincoln, Lincoln, NE, USA, 2016. Available online: http://digitalcommons.unl.edu/foodscidiss/78 (accessed on 8 January 2025).
- De Marchi, L.; Wangorsch, A.; Zoccatelli, G. Allergens from Edible Insects: Cross-Reactivity and Effects of Processing. Curr. Allergy Asthma Rep. 2021, 21, 35. [Google Scholar] [CrossRef]
- Lin, X.; Wang, F.; Lu, Y.; Wang, J.; Chen, J.; Yu, Y.; Tao, X.; Xiao, Y.; Peng, Y. A Review on Edible Insects in China: Nutritional Supply, Environmental Benefits, and Potential Applications. Curr. Res. Food Sci. 2023, 7, 100596. [Google Scholar] [CrossRef]
- Lange, K.W.; Nakamura, Y. Edible Insects and Their Potential Anti-Obesity Effects: A Review. Food Sci. Anim. Prod. 2023, 1, 9240008. [Google Scholar] [CrossRef]
- Wangorsch, A.; Jamin, A.; Spiric, J.; Vieths, S.; Scheurer, S.; Mahler, V.; Hofmann, S.C. Allergic Reaction to a Commercially Available Insect Snack Caused by House Cricket (Acheta domesticus) Tropomyosin. Mol. Nutr. Food Res. 2024, 68, e2300420. [Google Scholar] [CrossRef]
- Conway, A.; Jaiswal, S.; Jaiswal, A.K. The Potential of Edible Insects as a Safe, Palatable, and Sustainable Food Source in the European Union. Foods 2024, 13, 387. [Google Scholar] [CrossRef]
- Ganseman, E.; Goossens, J.; Blanter, M.; Jonckheere, A.C.; Bergmans, N.; Vanbrabant, L.; Gouwy, M.; Ronsmans, S.; Vandenbroeck, S.; Dupont, L.J.; et al. Frequent Allergic Sensitization to Farmed Edible Insects in Exposed Employees. J. Allergy Clin. Immunol. Pract. 2023, 11, 3732–3741.e10. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Rochow, V.B.; Gahukar, R.T.; Ghosh, S.; Jung, C. Chemical Composition, Nutrient Quality and Acceptability of Edible Insects Are Affected by Species, Developmental Stage, Gender, Diet, and Processing Method. Foods 2021, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.; Sousa-Pinto, B.; Fonseca, J.; Fonseca, S.C.; Cunha, L.M. Edible Insects and Food Safety: Allergy. J. Insects Food Feed. 2021, 7, 833–847. [Google Scholar] [CrossRef]
- Downs, M.; Johnson, P.; Zeece, M. Insects and Their Connection to Food Allergy. In Insects as Sustainable Food Ingredients: Production, Processing and Food Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 255–272. ISBN 9780128028568. [Google Scholar]
- Commission Implementing Regulation (EU) 2023/5 of 3 January 2023 Authorising the Placing on the Market of Acheta Domesticus (House Cricket) Partially Defatted Powder as a Novel Food and Amending Implementing Regulation (EU) 2017/2470 (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/reg_impl/2023/5/oj (accessed on 21 October 2024).
- Commission Implementing Regulation (EU) 2022/169 of 8 February 2022 Authorising the Placing on the Market of Frozen, Dried and Powder Forms of Yellow Mealworm (Tenebrio Molitor Larva) as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU) 2017/2470 (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/reg_impl/2022/169/oj (accessed on 21 October 2024).
- Comission Implementing Regulation (EU) 2021/1975 of 12 November 2021 Authorising the Placing on the Market of Frozen, Dried and Powder Forms of Locusta Migratoria as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and Amending Commission Implementing Regulation (EU) 2017/2470 (Text with EEA Relevance). Available online: http://data.europa.eu/eli/reg_impl/2021/1975/oj (accessed on 21 October 2024).
- Commission Implementing Regulation (EU) 2023/58 of 5 January 2023 Authorising the Placing on the Market of the Frozen, Paste, Dried and Powder Forms of Alphitobius Diaperinus Larvae (Lesser Mealworm) as a Novel Food and Amending Implementing Regulation (EU) 2017/2470 (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/reg_impl/2023/58/oj (accessed on 21 October 2024).
- Karnaneedi, S.; Johnston, E.B.; Bose, U.; Juhász, A.; Broadbent, J.A.; Ruethers, T.; Jerry, E.M.; Kamath, S.D.; Limviphuvadh, V.; Stockwell, S.; et al. The Allergen Profile of Two Edible Insect Species—Acheta domesticus and Hermetia iIllucens. Mol. Nutr. Food Res. 2024, 68, e2300811. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Zhao, T.; Fitriani, A.; Rahmadhia, S.N.; Alirezalu, K.; Fernando, I. Acheta domesticus (House Cricket) as Human Foods—An Approval of the European Commission—A Systematic Review. Food Front. 2024, 5, 435–473. [Google Scholar] [CrossRef]
- Scala, E.; Abeni, D.; Villella, V.; ViIlalta, D.; Cecchi, L.; Caprini, E.; Asero, R. Investigating Novel Food Sensitization: A Real-Life Prevalence Study of Cricket, Locust, and Mealworm IgE-Reactivity in Naïve Allergic Individuals. J. Investig. Allergol. Clin. Immunol. 2024; ahead of print. [Google Scholar] [CrossRef]
- Barre, A.; Pichereaux, C.; Velazquez, E.; Maudouit, A.; Simplicien, M.; Garnier, L.; Bienvenu, F.; Bienvenu, J.; Burlet-Schiltz, O.; Auriol, C.; et al. Insights into the Allergenic Potential of the Edible Yellow Mealworm (Tenebrio molitor). Foods 2019, 8, 515. [Google Scholar] [CrossRef]
- Villa, C.; Moura, M.B.M.V.; Teixeira, C.S.S.; Costa, J.; Mafra, I. Monitoring Yellow Mealworm (Tenebrio Molitor) as a Potential Novel Allergenic Food: Effect of Food Processing and Matrix. Nutrients 2023, 15, 482. [Google Scholar] [CrossRef]
- Segú, H.; Jalševac, F.; Sierra-Cruz, M.; Feliu, F.; Movassat, J.; Rodríguez-Gallego, E.; Terra, X.; Pinent, M.; Ardévol, A.; Blay, M.T. Assessing the Impact of Insect Protein Sources on Intestinal Health and Disease: Insights from Human Ex Vivo and Rat In Vivo Models. Food Funct. 2024, 15, 4552–4563. [Google Scholar] [CrossRef]
- Egonyu, J.P.; Subramanian, S.; Tanga, C.M.; Dubois, T.; Ekesi, S.; Kelemu, S. Global Overview of Locusts as Food, Feed and Other Uses. Glob. Food Secur. 2021, 31, 100574. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kwak, K.W.; Park, J.Y.; Park, E.S.; Nam, C.J.; An, K.S.; Kim, H.J.; Yoon, H.J.; Kim, Y.S.; Park, K.; et al. Evaluation of Subchronic Oral Dose Toxicity and Allergen of Freeze-Dried Powder of Locusta Migratoria (Orthoptera: Acrididae) as a Novel Food Source. Toxicol. Res. 2023, 39, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Frozen and Dried Formulations from Migratory Locust (Locusta migratoria) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06667. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Wu, Y.S.; Vijeepallam, K.; Batumalaie, K.; Hatta, M.H.M.; Lutuf, H.; Castro-Muñoz, R.; Fernando, I.; Ibrahim, S.A. Alphitobius Diaperinus Larvae (Lesser Mealworm) as Human Food—An Approval of the European Commission—A Critical Review. J. Insects Food Feed. 2025, 11, 61–100. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Athanassiou, C.G. The Lesser Mealworm Alphitobius Diaperinus: A Noxious Pest or a Promising Nutrient Source? Rev. Aquac. 2019, 11, 1418–1437. [Google Scholar] [CrossRef]
- Leni, G.; Tedeschi, T.; Faccini, A.; Pratesi, F.; Folli, C.; Puxeddu, I.; Migliorini, P.; Gianotten, N.; Jacobs, J.; Depraetere, S.; et al. Shotgun Proteomics, in-Silico Evaluation and Immunoblotting Assays for Allergenicity Assessment of Lesser Mealworm, Black Soldier Fly and Their Protein Hydrolysates. Sci. Rep. 2020, 10, 1228. [Google Scholar] [CrossRef]
- Andrić, A.; Miličić, M.; Bojanić, M.; Obradović, V.; Zorić, L.Š.; Petrović, M.; Gadjanski, I. Survey on Public Acceptance of Insects as Novel Food in a Non-EU Country: A Case Study of Serbia. J. Insects Food Feed. 2023, 10, 91–106. [Google Scholar] [CrossRef]
- Rehman, N.; Ogrinc, N. Consumer Perceptions and Acceptance of Edible Insects in Slovenia. Foods 2024, 13, 2629. [Google Scholar] [CrossRef]
- Orkusz, A.; Wolańska, W.; Harasym, J.; Piwowar, A.; Kapelko, M. Consumers’ Attitudes Facing Entomophagy: Polish Case Perspectives. Int. J. Environ. Res. Public Health 2020, 17, 2427. [Google Scholar] [CrossRef]
- Gere, A.; Székely, G.; Kovács, S.; Kókai, Z.; Sipos, L. Readiness to Adopt Insects in Hungary: A Case Study. Food Qual. Prefer. 2017, 59, 81–86. [Google Scholar] [CrossRef]
- Hartmann, C.; Siegrist, M. Science & Research | Overview Insects as Food: Perception and Acceptance Findings from Current Research. Ernahr. Umsch. 2017, 64, 44–50. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Osei-Owusu, J.; Asante, K.; Dofuor, A.K.; Boateng, B.O.; Debrah, S.K.; Ninsin, K.D.; Siddiqui, S.A.; Chia, S.Y. Insects as Food and Medicine: A Sustainable Solution for Global Health and Environmental Challenges. Front. Nutr. 2023, 10, 1113219. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mao, C.; Li, X.; Jiang, L.; Zhang, W.; Li, M.; Liu, H.; Fang, Y.; Liu, S.; Yang, G.; et al. Edible Insects: A New Sustainable Nutritional Resource Worth Promoting. Foods 2023, 12, 4073. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.; Wang, N. Entomophagy and Allergies: A Study of the Prevalence of Entomophagy and Related Allergies in a Population Living in North-Eastern Thailand. Biosci. Horiz. 2018, 11, hzy003. [Google Scholar] [CrossRef]
- Pali-Schöll, I.; Meinlschmidt, P.; Larenas-Linnemann, D.; Purschke, B.; Hofstetter, G.; Rodríguez-Monroy, F.A.; Einhorn, L.; Mothes-Luksch, N.; Jensen-Jarolim, E.; Jäger, H. Edible Insects: Cross-Recognition of IgE from Crustacean- and House Dust Mite Allergic Patients, and Reduction of Allergenicity by Food Processing. World Allergy Organ. J. 2019, 12, 100006. [Google Scholar] [CrossRef]
- Lamberti, C.; Nebbia, S.; Cirrincione, S.; Brussino, L.; Giorgis, V.; Romito, A.; Marchese, C.; Manfredi, M.; Marengo, E.; Giuffrida, M.G.; et al. Thermal Processing of Insect Allergens and IgE Cross-Recognition in Italian Patients Allergic to Shrimp, House Dust Mite and Mealworm. Food Res. Int. 2021, 148, 110567. [Google Scholar] [CrossRef]
- Suresh, S.; Mohd Zaini, N.S.; Rahim, M.H.A.; Ahmad, N.H. Insects and Worms as an Alternative Protein Source in the Halal Food Industry. In Innovation of Food Products in Halal Supply Chain Worldwide; Nizar, N.N.A., Abidin, S.A.S.Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 127–148. ISBN 9780323916622. [Google Scholar]
- Ribeiro, J.C.; Cunha, L.M.; Sousa-Pinto, B.; Fonseca, J. Allergic Risks of Consuming Edible Insects: A Systematic Review. Mol. Nutr. Food Res. 2018, 62, 1700030. [Google Scholar] [CrossRef]
- Caraballo, L.; Valenta, R.; Acevedo, N.; Zakzuk, J. Are the Terms Major and Minor Allergens Useful for Precision Allergology? Front. Immunol. 2021, 12, 651500. [Google Scholar] [CrossRef]
- Reese, G.; Ayuso, R.; Lehrer, S.B. Tropomyosin: An invertebrate pan-allergen. Int. Arch. Allergy Immunol. 1999, 119, 247–258. [Google Scholar] [CrossRef]
- Francis, F.; Doyen, V.; Debaugnies, F.; Mazzucchelli, G.; Caparros, R.; Alabi, T.; Blecker, C.; Haubruge, E.; Corazza, F. Limited Cross Reactivity among Arginine Kinase Allergens from Mealworm and Cricket Edible Insects. Food Chem. 2019, 276, 714–718. [Google Scholar] [CrossRef]
- Broekman, H.C.H.P.; Knulst, A.C.; den Hartog Jager, C.F.; van Bilsen, J.H.M.; Raymakers, F.M.L.; Kruizinga, A.G.; Gaspari, M.; Gabriele, C.; Bruijnzeel-Koomen, C.A.F.M.; Houben, G.F.; et al. Primary Respiratory and Food Allergy to Mealworm. J. Allergy Clin. Immunol. 2017, 140, 600–603. [Google Scholar] [CrossRef]
- Dramburg, S.; Hilger, C.; Santos, A.F.; de las Vecillas, L.; Aalberse, R.C.; Acevedo, N.; Aglas, L.; Altmann, F.; Arruda, K.L.; Asero, R.; et al. EAACI Molecular Allergology User’s Guide 2.0. Pediatr. Allergy Immunol. 2023, 34, e13854. [Google Scholar] [CrossRef] [PubMed]
- Maillot, A.; Mathelin, C.; Cazanove, G.; Marteau, A. Anaphylaxis after Consumption of Wasp Larvae in Reunion Island: A Case Report. Front. Allergy 2023, 4, 1213879. [Google Scholar] [CrossRef] [PubMed]
- Okezie, O.A.; Kgomotso, K.K.; Letswiti, M.M. Mopane Worm Allergy in a 36-Year-Old Woman: A Case Report. J. Med. Case Rep. 2010, 4, 42. [Google Scholar] [CrossRef]
- Jankowski, W.M.; Przychodniak, D.; Kurowski, M. LTP Syndrome and Edible Insect Allergy in a Patient with Recurrent Anaphylaxis Incidents. Pol. J. Allergol. 2024; online. [Google Scholar] [CrossRef]
- Majsiak, E.; Choina, M.; Gromek, W.; Wykrota, J.; Kozłowska, D.; Swadźba, J.; Cukrowska, B.; Kowal, K. IgE-based analysis of sensitization and cross-reactivity to yellow mealworm and edible insect allergens before their widespread dietary introduction. Sci. Rep. 2025, 15, 1466. [Google Scholar] [CrossRef]
- Ganseman, E.; Ieven, T.; Frans, G.; Coorevits, L.; Pörtner, N.; Martens, E.; Bullens, D.M.; Schrijvers, R.; Breynaert, C.; Proost, P. Alpha-Amylase as the Culprit in an Occupational Mealworm Allergy Case. Front. Allergy 2022, 3, 992195. [Google Scholar] [CrossRef]
- Broekman, H.; Verhoeckx, K.C.; den Hartog Jager, C.F.; Kruizinga, A.G.; Pronk-Kleinjan, M.; Remington, B.C.; Bruijnzeel-Koomen, C.A.; Houben, G.F.; Knulst, A.C. Majority of Shrimp-Allergic Patients Are Allergic to Mealworm. J. Allergy Clin. Immunol. 2016, 137, 1261–1263. [Google Scholar] [CrossRef]
- Miraglia del Giudice, M.; Dinardo, G.; Klain, A.; D’Addio, E.; Bencivenga, C.L.; Decimo, F.; Indolfi, C. Anaphylaxis after Shrimp Intake in a European Pediatric Population: Role of Molecular Diagnostics and Implications for Novel Foods. Children 2023, 10, 1583. [Google Scholar] [CrossRef]
- Verhoeckx, K.C.M.; van Broekhoven, S.; den Hartog-Jager, C.F.; Gaspari, M.; de Jong, G.A.H.; Wichers, H.J.; van Hoffen, E.; Houben, G.F.; Knulst, A.C. House Dust Mite (Der p 10) and Crustacean Allergic Patients May React to Food Containing Yellow Mealworm Proteins. Food Chem. Toxicol. 2014, 65, 364–373. [Google Scholar] [CrossRef]
- Binder, M.; Mahler, V.; Hayek, B.; Sperr, W.R.; Schöller, M.; Prozell, S.; Wiedermann, G.; Valent, P.; Valenta, R.; Duchêne, M. Molecular and Immunological Characterization of Arginine Kinase from the Indianmeal Moth, Plodia Interpunctella, a Novel Cross-Reactive Invertebrate Pan-Allergen. J. Immunol. 2001, 167, 5470–5477. [Google Scholar] [CrossRef]
- Galindo, P.A.; Lombardero, M.; Borja, J.; Gomez, E.; Feo, F.; Barber, D.; García, R. A New Arthropod Panallergen? Allergy 2001, 56, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Broekman, H.C.H.P.; Knulst, A.C.; de Jong, G.; Gaspari, M.; den Hartog Jager, C.F.; Houben, G.F.; Verhoeckx, K.C.M. Is Mealworm or Shrimp Allergy Indicative for Food Allergy to Insects? Mol. Nutr. Food Res. 2017, 61, 1601061. [Google Scholar] [CrossRef]
- Hustinx-Broekman, H.C.H.P. Allergenic Risks of Mealworm and Other Insects: An Approach to Assess the Risks of New Food Proteins in Allergic Patients = Allergene Risico’s van Meelworm En Andere Insecten: Een Aanpak Voor de Benadering van de Risico’s van Nieuwe Voedingseiwitten in Allergische Patiënten. Ph.D. Thesis, Universiteit Utrecht, Utrecht, The Netherlands, 2017. ISBN 9789462956087. Available online: https://dspace.library.uu.nl/handle/1874/349295 (accessed on 30 November 2024).
- De Marchi, L.; Mainente, F.; Leonardi, M.; Scheurer, S.; Wangorsch, A.; Mahler, V.; Pilolli, R.; Sorio, D.; Zoccatelli, G. Allergenicity Assessment of the Edible Cricket Acheta Domesticus in Terms of Thermal and Gastrointestinal Processing and IgE Cross-Reactivity with Shrimp. Food Chem. 2021, 359, 129878. [Google Scholar] [CrossRef] [PubMed]
- Kamemura, N.; Sugimoto, M.; Tamehiro, N.; Adachi, R.; Tomonari, S.; Watanabe, T.; Mito, T. Cross-Allergenicity of Crustacean and the Edible Insect Gryllus Bimaculatus in Patients with Shrimp Allergy. Mol. Immunol. 2019, 106, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Delfino, D.; Prandi, B.; Ridolo, E.; Dellafiora, L.; Pedroni, L.; Nicoletta, F.; Cavazzini, D.; Sforza, S.; Tedeschi, T.; Folli, C. Allergenicity of Tropomyosin Variants Identified in the Edible Insect Hermetia Illucens (Black Soldier Fly). Food Chem. 2024, 437, 137849. [Google Scholar] [CrossRef]
- Broekman, H.; Knulst, A.; Den Hartog Jager, S.; Monteleone, F.; Gaspari, M.; De Jong, G.; Houben, G.; Verhoeckx, K. Effect of Thermal Processing on Mealworm Allergenicity. Mol. Nutr. Food Res. 2015, 59, 1855–1864. [Google Scholar] [CrossRef]
- Srinroch, C.; Srisomsap, C.; Chokchaichamnankit, D.; Punyarit, P.; Phiriyangkul, P. Identification of Novel Allergen in Edible Insect, Gryllus Bimaculatus and Its Cross-Reactivity with Macrobrachium spp. Allergens. Food Chem. 2015, 184, 160–166. [Google Scholar] [CrossRef]
- Phiriyangkul, P.; Srinroch, C.; Srisomsap, C.; Chokchaichamnankit, D.; Punyarit, P. Effect of Food Thermal Processing on Allergenicity Proteins in Bombay Locust (Patanga succincta). ETP Int. J. Food Eng. 2015, 1, 23–28. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, L.; Wu, Y.; Xia, Q.; Chen, J.; Roux, K.H. Identification and Characterization of an Arginine Kinase as a Major Allergen from Silkworm (Bombyx mori) Larvae. Int. Arch. Allergy Immunol. 2009, 150, 8–14. [Google Scholar] [CrossRef]
- Zhao, X.; Li, L.; Kuang, Z.; Luo, G.; Li, B. Proteomic and Immunological Identification of Two New Allergens from Silkworm (Bombyx mori L.) Pupae. Centr. Eur. J. Immunol. 2015, 40, 30–34. [Google Scholar] [CrossRef]
- Hăbeanu, M.; Gheorghe, A.; Mihalcea, T. Nutritional Value of Silkworm Pupae (Bombyx Mori) with Emphases on Fatty Acids Profile and Their Potential Applications for Humans and Animals. Insects 2023, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Pascal, M.; Grishina, G.; Yang, A.C.; Sánchez-García, S.; Lin, J.; Towle, D.; Ibañez, M.D.; Sastre, J.; Sampson, H.A.; Ayuso, R. Molecular Diagnosis of Shrimp Allergy: Efficiency of Several Allergens to Predict Clinical Reactivity. J. Allergy Clin. Immunol. Pr. 2015, 3, 521–529.e10. [Google Scholar] [CrossRef] [PubMed]
- Ukleja-Sokołowska, N.; Lis, K.; Żbikowska-Gotz, M.; Adamczak, R.; Kuźmiński, A.; Bartuzi, Z. Clinical Utility of Immunological Methods Based on the Singleplex and Multiplex ImmunoCap Systems for Diagnosis of Shrimp Allergy. J. Int. Med. Res. 2021, 49, 3000605211006597. [Google Scholar] [CrossRef] [PubMed]
- Ukleja-Sokołowska, N.; Lis, K.; Bartuzi, M.; Żbikowska-Gotz, M.; Adamczak, R.; Bartuzi, Z. Extended IgE Profile of Shrimp-Sensitized Patients Based on Multiplex Examination ALEX2—Allergy Explorer. Postep. Dermatol. Alergol. 2023, 40, 661–669. [Google Scholar] [CrossRef]
- Scala, E.; Abeni, D.; Aruanno, A.; Boni, E.; Brusca, I.; Cappiello, F.; Caprini, E.; Buzzulini, F.; Deleonardi, G.; Demonte, A.; et al. Mollusk Allergy in Shrimp-Allergic Patients: Still a Complex Diagnosis. An Italian Real-Life Cross-Sectional Multicenter Study. World Allergy Organ. J. 2022, 15, 100685. [Google Scholar] [CrossRef]
- Giovannini, M.; Beken, B.; Buyuktiryaki, B.; Barni, S.; Liccioli, G.; Sarti, L.; Lodi, L.; Pontone, M.; Bartha, I.; Mori, F.; et al. IgE-Mediated Shellfish Allergy in Children. Nutrients 2023, 15, 2714. [Google Scholar] [CrossRef]
- Pedro, B.S.d.S.; Guerrero, A.L.; Del Pino, M.N.; Villar, M.A.; Álvarez, F.; López-Matas, M.A.; Carnés, J. Myosin Heavy Chain: An Allergen Involved in Anaphylaxis to Shrimp Head. J. Investig. Allergol. Clin. Immunol. 2023, 33, 66–68. [Google Scholar]
- Rydzyńska, M.; Rosada, T.; Grześk-Kaczyńska, M.; Lis, K.; Bartuzi, Z.; Ukleja-Sokołowska, N. Sarcoplasmic Calcium Binding Protein as a Cause of Anaphylaxis after Eating Shrimp in a Patient with Pollen Food Allergy Syndrome—Case Report. Alerg. Astma Immunol. 2024, 29, 18–25. [Google Scholar]
- ThermoFisher ImmunoCAPTM Specific IgE Tests. Available online: https://www.thermofisher.com/phadia/wo/en/our-solutions/immunocap-allergy-solutions/specific-ige-single-allergens.html (accessed on 21 October 2024).
- Madx Array Diagnostics Alex Allergy Xplorer. Available online: https://www.macroarraydx.com/products/alex (accessed on 21 October 2024).
| References | Protein | Functions of the Protein in Insect | Effects of Thermal Processing |
|---|---|---|---|
| [7] | Tropomyosin | Muscle protein | Extremely resistant to thermal processing. Tropomyosin remains immunoreactive after heat treatments such as boiling and baking. |
| [7] | Arginine kinase | Energy metabolism enzyme | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [7] | Hexamerin 1B | Transport and storage protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [7] | Glyceraldehyde 3-phosphate dehydrogenase | Enzyme involved in glycolysis | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [7] | Chitinase | Chitin-degrading enzyme | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [7,22] | Paramyosin | Muscle protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [20] | Hemocyanin | Respiratory protein | Relatively resistant to thermal processing. It may retain partial allergenicity after heat treatment. |
| [20] | Vitellogenin | Yolk protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [20] | Troponin I | Muscle protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [20,22] | Myosin light chain | Muscle protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [20] | Apolipophorin-III | Transport protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [22] | Troponin C | Calcium-binding protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [22] | Sarcoplasmic calcium-binding protein | Calcium-binding protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| References | Protein | Functions of Protein in Insect | Effects of Thermal Processing |
|---|---|---|---|
| [23] | Tropomyosin | Muscle protein | Extremely resistant to thermal processing. Tropomyosin remains immunoreactive after heat treatments such as boiling and baking. |
| [23] | Arginine kinase | Energy metabolism enzyme | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [23] | Heat shock protein 70 | Heat shock protein | Relatively resistant to thermal processing. It may retain partial allergenicity after heat treatment. |
| [23] | α-Amylase | Enzyme that breaks down starches | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [23] | Apolipophorin-III | Transport protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [23] | Hemolymph protein 12 kDa | Storage protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [23] | Protein of the larval cuticle | Structural protein of the cuticle | Relatively resistant to thermal processing. It may retain partial allergenicity after heat treatment. |
| [24] | Hexamerin 1B | Transport and storage protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [25] | Chitinase | Chitin-degrading enzyme | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [25] | Glutathione S-transferase | Detoxification enzyme | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [22] | Troponin C | Calcium-binding protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [22] | Paramyosin | Muscle protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [22] | Myosin light chain | Muscle protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [22] | Sarcoplasmic calcium-binding protein | Calcium-binding protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| References | Protein | Functions of Protein in Insect | Effects of Thermal Processing |
|---|---|---|---|
| [27] | Tropomyosin | Muscle protein | Extremely resistant to thermal processing. Tropomyosin remains immunoreactive after heat treatments such as boiling and baking. |
| [27] | Arginine kinase | Energy metabolism enzyme | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [27] | S-glutathione transferase | Detoxification enzyme | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [27] | Chitinase | Chitin-degrading enzyme | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [27] | Cross-reactive carbohydrate determinants | Structural elements of glycoproteins | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [17] | Hemocyanin | Respiratory protein | Relatively resistant to thermal processing. It may retain partial allergenicity after heat treatment. |
| [28] | Heat shock protein 70 | Heat shock protein | Relatively resistant to thermal processing. It may retain partial allergenicity after heat treatment. |
| [28] | Hexamerin | Transport and storage protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [28] | Serine protease | Protein degrading enzyme | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [28] | Trypsin | Protein degrading enzyme | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [22] | Troponin C | Calcium-binding protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [22] | Paramyosin | Muscle protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [22] | Myosin light chain | Muscle protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [22] | Sarcoplasmic calcium-binding protein | Calcium-binding protein | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| References | Protein | Functions of Protein in Insect | Effects of Thermal Processing |
|---|---|---|---|
| [25,29] | Tropomyosin | Muscle protein | Extremely resistant to thermal processing. Tropomyosin remains immunoreactive after heat treatments such as boiling and baking. |
| [25,29] | Arginine kinase | Energy metabolism enzyme | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [25] | Chitinase | Chitin-degrading enzyme | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
| [25] | Glutathione S-transferase | Detoxification enzyme | Intense processing methods, such as prolonged boiling and high-temperature frying, may reduce its allergenicity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankowski, W.M.; Przychodniak, D.; Gromek, W.; Majsiak, E.; Kurowski, M. Edible Insects as an Alternative Source of Nutrients: Benefits, Risks, and the Future of Entomophagy in Europe—A Narrative Review. Foods 2025, 14, 270. https://doi.org/10.3390/foods14020270
Jankowski WM, Przychodniak D, Gromek W, Majsiak E, Kurowski M. Edible Insects as an Alternative Source of Nutrients: Benefits, Risks, and the Future of Entomophagy in Europe—A Narrative Review. Foods. 2025; 14(2):270. https://doi.org/10.3390/foods14020270
Chicago/Turabian StyleJankowski, Wojciech Michał, Dominik Przychodniak, Weronika Gromek, Emilia Majsiak, and Marcin Kurowski. 2025. "Edible Insects as an Alternative Source of Nutrients: Benefits, Risks, and the Future of Entomophagy in Europe—A Narrative Review" Foods 14, no. 2: 270. https://doi.org/10.3390/foods14020270
APA StyleJankowski, W. M., Przychodniak, D., Gromek, W., Majsiak, E., & Kurowski, M. (2025). Edible Insects as an Alternative Source of Nutrients: Benefits, Risks, and the Future of Entomophagy in Europe—A Narrative Review. Foods, 14(2), 270. https://doi.org/10.3390/foods14020270







