Changes in Microbial Safety and Quality of High-Pressure Processed Camel Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Preparation
2.2. Camel Milk
2.3. Sample Preparation
2.4. Treatment
2.5. Microbial Enumeration
2.6. Calculation of D-Values
2.7. Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.8. Statistical Analysis
3. Results and Discussion
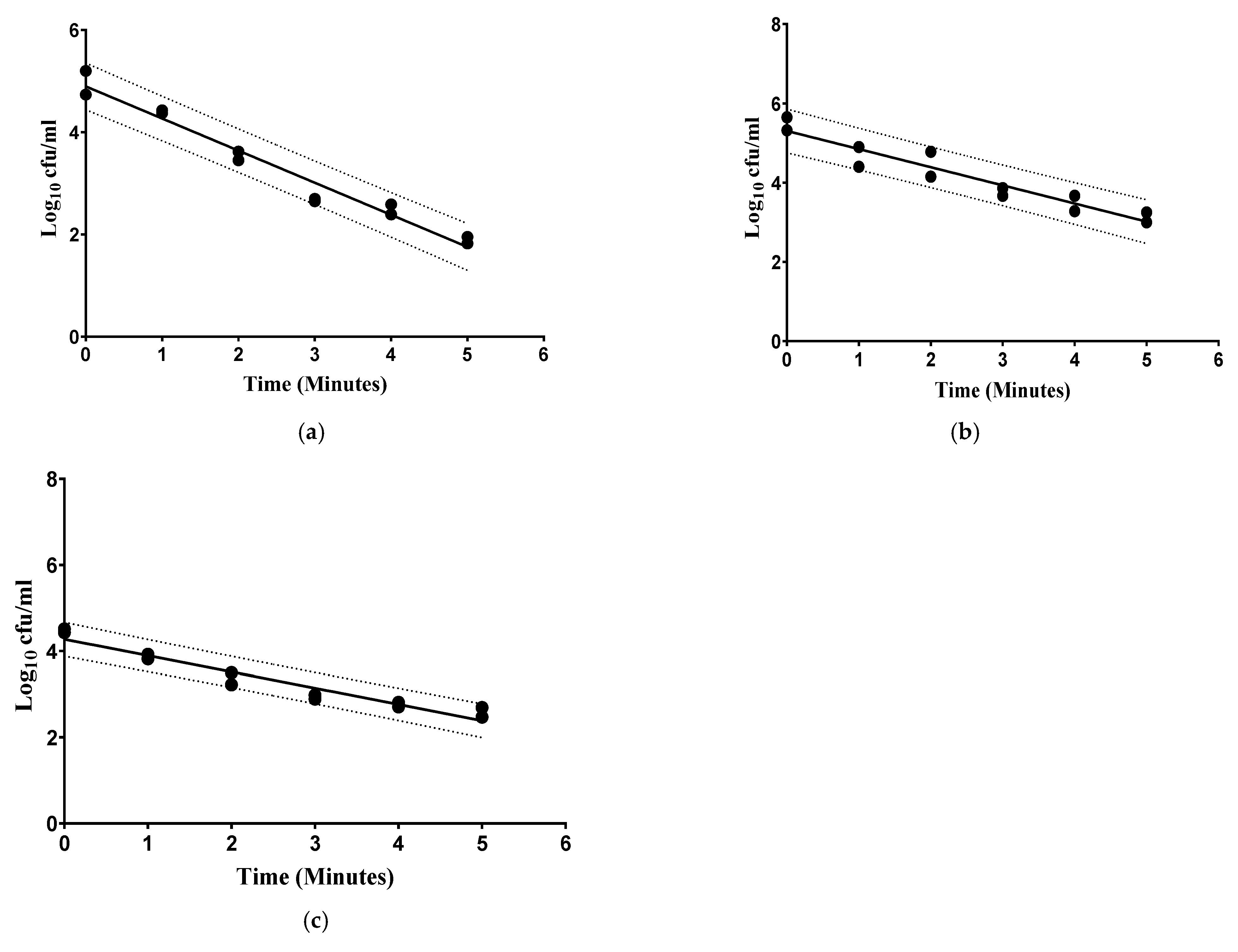
3.1. Effect of HPP Activity on Food Borne Pathogenic Bacteria
3.2. Effect of HPP Activity on Spoilage Causing Bacteria
3.3. Effect of HPP Activity on Proteolytic Profile and Protein Separation in Camel Milk
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zibaee, S.; Yousefi, M.; Taghipour, A.; Kiani, M.A.; Noras, M.R. Nutritional and Therapeutic Characteristics of Camel Milk in Children: A Systematic Review. Electron. Physician 2015, 7, 1523. [Google Scholar] [CrossRef] [PubMed]
- El-Aziz, A.; Kassem, J.M.; Aasem, F.M.; Abbas, H.M. Physicochemical Properties and Health Benefits of Camel Milk and Its Applications in Dairy Products: A Review. Egypt. J. Chem. 2022, 65, 101–118. [Google Scholar] [CrossRef]
- Imarc UAE Camel Dairy Market Report by Product Type. Available online: https://www.imarcgroup.com/uae-camel-dairy-market (accessed on 5 August 2024).
- Seifu, E. Camel Milk Products: Innovations, Limitations and Opportunities. Food Prod. Process. Nutr. 2023, 5, 15. [Google Scholar] [CrossRef]
- Swelum, A.A.; El-Saadony, M.T.; Abdo, M.; Ombarak, R.A.; Hussein, E.O.S.; Suliman, G.; Alhimaidi, A.R.; Ammari, A.A.; Ba-Awadh, H.; Taha, A.E. Nutritional, Antimicrobial and Medicinal Properties of Camel’s Milk: A Review. Saudi J. Biol. Sci. 2021, 28, 3126–3136. [Google Scholar] [CrossRef] [PubMed]
- Abera, T.; Legesse, Y.; Mummed, B.; Urga, B. Bacteriological Quality of Raw Camel Milk along the Market Value Chain in Fafen Zone, Ethiopian Somali Regional State. BMC Res. Notes 2016, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Saeed, E.; Amer, A.A.E.M.; Keshta, H.G.; Hafez, E.E.; Sultan, R.M.; Khalifa, E. Prevalence, antibiotic sensitivity profile, and phylogenetic analysis of Escherichia coli isolated from raw dromedary camel milk in Matrouh Governorate, Egypt. J. Adv. Vet. Anim. Res. 2022, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Saeed, E.; Amer, A.A.; Keshta, H.G.; Khalifa, E. Prevalence and Antibiogram of Salmonella enterica Isolated from Raw Dromedary Camel Milk in Matrouh Governorate, Egypt. Int. J. Vet. Sci. 2022, 11, 168–174. [Google Scholar]
- Lezzoum-Atek, S.; Belhout, C.; Bouchenafa, H.; Bouayad, L. Assessment of Contamination of Raw Camel Milk by Listeria spp. and Staphylococcus spp. Biol. Life Sci. Forum. 2023, 22, 9. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; El Zubeir, I.Y.M.; El, Y.M. Effect of Heat Treatment on Keeping Quality of Camel Milk. Ann. Food Sci. Technol. 2014, 15, 239–245. [Google Scholar]
- Moltó-Puigmartí, C.; Permanyer, M.; Castellote, A.I.; López-Sabater, M.C. Effects of Pasteurisation and High-Pressure Processing on Vitamin C, Tocopherols and Fatty Acids in Mature Human Milk. Food Chem. 2011, 124, 697–702. [Google Scholar] [CrossRef]
- Delgado, F.J.; Cava, R.; Delgado, J.; Ramírez, R. Tocopherols, Fatty Acids and Cytokines Content of Holder Pasteurised and High-Pressure Processed Human Milk. Dairy Sci. Technol. 2014, 94, 145–156. [Google Scholar] [CrossRef]
- López-Fandiño, R. High Pressure-Induced Changes in Milk Proteins and Possible Applications in Dairy Technology. Int. Dairy J. 2006, 16, 1119–1131. [Google Scholar] [CrossRef]
- Ho, T.M.; Zou, Z.; Bansal, N. Camel Milk: A Review of Its Nutritional Value, Heat Stability, and Potential Food Products. Food Res. Int. 2022, 153, 110870. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Abu-Jdayil, B.; Bamigbade, G.; Kamal-Eldin, A.; Hamed, F.; Huppertz, T.; Liu, S.-Q.; Ayyash, M. Properties of Low-Fat Cheddar Cheese Prepared from Bovine-Camel Milk Blends: Chemical Composition, Microstructure, Rheology and Volatile Compounds. J. Dairy Sci. 2024, 107, 2706–2720. [Google Scholar] [CrossRef] [PubMed]
- Naveena, B.; Nagaraju, M. Review on principles, effects, advantages, and disadvantages of high-pressure processing of food. Int. J. Chem. Stud. 2020, 8, 2964–2967. [Google Scholar] [CrossRef]
- Silva, F.V. Heat assisted HPP for the inactivation of bacteria, moulds and yeasts spores in foods: Log reductions and mathematical models. Trends Food Sci. Technol. 2019, 88, 143–156. [Google Scholar]
- ISO 16654; Microbiology of Food and Animalfeeding Stuffs–Horizontal Method for the Detection of Escherichia coli 0157. European Standard, 1st ed.; BSI: London, UK, 2001.
- ISO 6579: 2002; Microbiology of Food and Animal Feeding Stuffs: Horizontal Method for the Detection of Salmonella spp. ISO: Geneva, Switzerland, 2002.
- ISO. 11290–1: 2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp. Part 1: Detection Method. International Organization for Standardization:: Geneva, Switzerland, 2017.
- Osaili, T.; Al-Nabulsi, A.A.; Hasan, F.; Dhanasekaran, D.K.; Hussain, A.Z.S.; Ismail, L.C.; Naja, F.; Radwan, H.; Faris, M.E.; Olaimat, A.N. Effect of Eugenol, Vanillin and β-Resorcylic Acid on Foodborne Pathogen Survival in Marinated Camel Meat. J. Food Prot. 2023, 86, 100038. [Google Scholar] [CrossRef]
- Osaili, T.M.; Hasan, F.; Dhanasekaran, D.K.; Obaid, R.S.; Al-Nabulsi, A.A.; Rao, S.; Fatima, H.; Ayyash, M.; Savvaidis, I.; Holley, R. Thermal Inactivation of Escherichia coli O157:H7 Strains and Salmonella Spp. in Camel Meat Burgers. LWT 2020, 120, 108914. [Google Scholar] [CrossRef]
- Huang, H.-W.; Wu, S.-J.; Lu, J.-K.; Shyu, Y.-T.; Wang, C.-Y. Current Status and Future Trends of High-Pressure Processing in Food Industry. Food Control 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Oey, I.; Van der Plancken, I.; Van Loey, A.; Hendrickx, M. Does High Pressure Processing Influence Nutritional Aspects of Plant Based Food Systems? Trends Food Sci. Technol. 2008, 19, 300–308. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, Y.; Zhang, Y.; Pang, X.; Wang, Y.; Lv, J.; Zhang, S. Effects of Different Heat Treatments on Maillard Reaction Products and Volatile Substances of Camel Milk. Front. Nutr. 2023, 10, 1072261. [Google Scholar] [CrossRef] [PubMed]
- Shahein, M.H.; Amr, A.S.; Sadder, M.; Al-Qadiri, H.M.; Albawarshi, Y.; Al-khamaiseh, A.M.; Kanaan, O. Lethality of High Hydrostatic Pressure Processing on Listeria Monocytogenes, Staphylococcus Aureus and Escherichia Coli in Low Salt White Brined Cheese: D-Value. Int. Dairy J. 2023, 143, 105675. [Google Scholar] [CrossRef]
- Dogan, C.; Erkmen, O. High Pressure Inactivation Kinetics of Listeria Monocytogenes Inactivation in Broth, Milk, and Peach and Orange Juices. J. Food Eng. 2004, 62, 47–52. [Google Scholar] [CrossRef]
- Sehrawat, R.; Kaur, B.P.; Nema, P.K.; Tewari, S.; Kumar, L. Microbial Inactivation by High Pressure Processing: Principle, Mechanism and Factors Responsible. Food Sci. Biotechnol. 2021, 30, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, H.S.; Zaman, S.U.; Smith, J.P. High Pressure Destruction Kinetics of Escherichia Coli (O157: H7) and Listeria Monocytogenes (Scott A) in a Fish Slurry. J. Food Eng. 2008, 87, 99–106. [Google Scholar] [CrossRef]
- Hwang, C.-C.; Lin, C.-S.; Hsiao, Y.-T.; Huang, Y.-L.; Yen, F.-L.; Lee, Y.-C.; Tsai, Y.-H. Inactivation Kinetics of Foodborne Pathogens in Carrot Juice by High-Pressure Processing. Biology 2023, 12, 1383. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Garcia-Hernandez, R.; Driedger, D.; McMullen, L.M.; Gänzle, M. Effect of the Food Matrix on Pressure Resistance of Shiga-Toxin Producing Escherichia Coli. Food Microbiol. 2016, 57, 96–102. [Google Scholar] [CrossRef]
- Salazar, J.K.; Natarajan, V.; Stewart, D.; Warren, J.; Gonsalves, L.J.; Mhetras, T.; Tortorello, M. Lou Fate of Listeria Monocytogenes in Ready-to-Eat Refrigerated Dips Treated with High Pressure Processing. J. Food Prot. 2019, 82, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Palou, E.; Lopez-Malo, A.; Barbosa-Canovas, G.V.; Swanson, B.G. High-Pressure Treatment in Food Preservation. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2007; pp. 833–872. [Google Scholar]
- Vasanthakumari, R. Textbook of Microbiology; Wolters Kluwer India Pvt Ltd.: Gurugram, India, 2016; ISBN 9351296504. [Google Scholar]
- Yang, D.; Zhang, Y.; Zhao, L.; Wang, Y.; Rao, L.; Liao, X. Pressure-Resistant Acclimation of Lactic Acid Bacteria from a Natural Fermentation Product Using High Pressure. Innov. Food Sci. Emerg. Technol. 2021, 69, 102660. [Google Scholar] [CrossRef]
- Black, E.P.; Setlow, P.; Hocking, A.D.; Stewart, C.M.; Kelly, A.L.; Hoover, D.G. Response of Spores to High-Pressure Processing. Compr. Rev. Food Sci. Food Saf. 2007, 6, 103–119. [Google Scholar] [CrossRef]
- Rozali, S.N.; Milani, E.A.; Deed, R.C.; Silva, F.V. Bacteria, Mould and Yeast Spore Inactivation Studies by Scanning Electron Microscope Observations. Int. J. Food Microbiol. 2017, 263, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Lee, Y.S.; Li, J.; Li, C. Resistance Mechanisms and Reprogramming of Microorganisms for Efficient Biorefinery under Multiple Environmental Stresses. Synth. Syst. Biotechnol. 2019, 4, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.G.; Delgadillo, I.; Saraiva, J.A. Effect of Thermal Pasteurisation and High-Pressure Processing on Immunoglobulin Content and Lysozyme and Lactoperoxidase Activity in Human Colostrum. Food Chem. 2014, 151, 79–85. [Google Scholar] [CrossRef]
- Dubey, U.S.; Lal, M.; Mittal, A.; Kapur, S. Therapeutic Potential of Camel Milk. Emir. J. Food Agric. 2016, 28, 164. [Google Scholar] [CrossRef]
- Maryniak, N.Z.; Hansen, E.B.; Ballegaard, A.R.; Sancho, A.I.; Bøgh, K.L. Comparison of the Allergenicity and Immunogenicity of Camel and Cow’s Milk—A Study in Brown Norway Rats. Nutrients 2018, 10, 1903. [Google Scholar] [CrossRef]
- Kumar, D.; Verma, A.K.; Chatli, M.K.; Singh, R.; Kumar, P.; Mehta, N.; Malav, O.P. Camel Milk: Alternative Milk for Human Consumption and Its Health Benefits. Nutr. Food Sci. 2016, 46, 217–227. [Google Scholar] [CrossRef]
- Mazri, C.; Sánchez, L.; Ramos, S.J.; Calvo, M.; Pérez, M.D. Effect of High-Pressure Treatment on Denaturation of Bovine Lactoferrin and Lactoperoxidase. J. Dairy Sci. 2012, 95, 549–557. [Google Scholar] [CrossRef]
- Garcia-Graells, C.; Valckx, C.; Michiels, C.W. Inactivation of Escherichia coli and Listeria innocua in Milk by Combined Treatment with High Hydrostatic Pressure and the Lactoperoxidase System. Appl. Environ. Microbiol. 2000, 66, 4173–4179. [Google Scholar] [CrossRef]
- Mbye, M.; Ayyash, M.; Abu-Jdayil, B.; Kamal-Eldin, A. The Texture of Camel Milk Cheese: Effects of Milk Composition, Coagulants, and Processing Conditions. Front. Nutr. 2022, 9, 868320. [Google Scholar] [CrossRef]
- Mbye, M.; Ayyash, M.; Mohamed, H.; Abu-Jdayil, B.; Kamleh, R.; Kamal-Eldin, A. Effects of Ultrafiltration Followed by Heat or High-Pressure Treatment on Camel and Bovine Milk Cheeses. NFS J. 2023, 31, 123–132. [Google Scholar] [CrossRef]
- Khaliq, A.; Mishra, A.K.; Niroula, A.; Baba, W.; Shaukat, M.N.; Rabbani, A. An Updated Comprehensive Review of Camel Milk: Composition, Therapeutic Properties, and Industrial Applications. Food Biosci. 2024, 62, 105531. [Google Scholar] [CrossRef]
- Omar, A.; Harbourne, N.; Oruna-Concha, M.J. Effects of Industrial Processing Methods on Camel Skimmed Milk Properties. Int. Dairy J. 2018, 84, 15–22. [Google Scholar] [CrossRef]

| Temp | Days | CNL | 1 min | 2 min | 3 min | 4 min | 5 min | p-Value |
|---|---|---|---|---|---|---|---|---|
| 4 °C | 0 | 0.00 abcde ± 0.0 | −0.45 cdef ± 0.3 | −0.24 bcdef ± 0.1 | −0.25 bcdef ± 0.5 | −0.36 bcdef ± 0.5 | −0.63 def ± 0.3 | 0.001 |
| 1 | 0.42 ab ± 0.2 | −0.61 def ± 0.4 | −0.59 def ± 0.4 | −0.27 bcdef ± 0.6 | −0.26 bcdef ± 0.4 | −0.81 f ± 0.5 | ||
| 4 | 0.63 a ± 0.1 | 0.01 abcde ± 0.5 | −0.02 abcde ± 0.5 | 0.13 abcd ± 0.4 | −0.35 bcdef ± 0.5 | −0.71 ef ± 0.8 | ||
| 7 | −0.01 abcde ± 0.3 | −0.21 bcdef ± 0.2 | −0.5 cdef ± 0.2 | −0.37 cdef ± 0.5 | −0.15 abcdef ± 0.6 | −0.76 ef ± 0.5 | ||
| 10 | 0.23 abc ± 0.5 | −0.22 bcdef ± 0.6 | −0.39 cdef ± 0.4 | −0.16 bcdef ± 0.4 | −0.4 cdef ± 0.6 | −0.25 bcdef ± 0.6 | ||
| 10 °C | 0 | 0.00 hijklm ± 0.0 | −0.6 lm ± 0.2 | −0.72 m ± 0.3 | −0.48 klm ± 0.6 | −0.45 klm ± 0.6 | −0.63 lm ± 0.4 | 0.001 |
| 1 | 0.76 cdefg ± 0.1 | 0.39 defghij ± 0.4 | 0.08 ghijkl ± 0.6 | −0.11 ijklm ± 0.7 | −0.26 jklm ± 0.7 | −0.04 ijklm ± 0.6 | ||
| 4 | 0.85 cdef ± 0.6 | 0.18 fghijk ± 0.7 | 0.3 efghij ± 0.4 | 0.14 fghijk ± 0.3 | 0.14 fghijk ± 0.6 | 0.15 fghijk ± 0.4 | ||
| 7 | 1.61 ab ± 0.5 | 0.42 defghij ± 0.5 | 0.58 cdefghi ± 0.5 | 0.69 cdefgh ± 0.2 | 0.38 defghij ± 0.6 | 0.3 efghij ± 0.3 | ||
| 10 | 2.17 a ± 0.4 | 1.23 bc ± 0.5 | 1.1 bcd ± 0.6 | 0.96 bcde ± 0.5 | 0.73 cdefg ± 0.7 | 0.86 cdef ± 0.4 | ||
| 4 °C vs. 10 °C | 0 | NS | 0.218 | * | 0.305 | 0.688 | 0.985 | |
| 1 | * | * | * | 0.525 | 0.985 | * | ||
| 4 | 0.187 | 0.453 | 0.086 | 0.937 | * | * | ||
| 7 | * | * | * | * | * | * | ||
| 10 | * | * | * | * | * | * |
| Temp | Days | CNL | 1 min | 2 min | 3 min | 4 min | 5 min | p-Value |
|---|---|---|---|---|---|---|---|---|
| 4 °C | 0 | 0.00 bcd ± 0.0 | −0.48 bcde ± 0.3 | −0.22 bcde ± 0.2 | −0.14 bcd ± 0.5 | −0.29 bcde ± 0.4 | −0.65 de ± 0.3 | 0.001 |
| 1 | −0.05 bcd ± 0.3 | −0.61 cde ± 0.3 | −0.63 de ± 0.4 | −0.40 bcde ± 0.5 | −0.38 bcde ± 0.4 | −0.95 e ± 0.4 | ||
| 4 | 0.30 ab ± 0.1 | −0.40 bcde ± 0.3 | −0.29 bcde ± 0.3 | −0.02 bcd ± 0.3 | 0.05 bcd ± 0.3 | −0.54 cde ± 0.3 | ||
| 7 | 0.17 bc ± 0.4 | −0.53 cde ± 0.4 | −0.31 bcde ± 0.2 | −0.31 bcde ± 0.2 | 0.10 bcd ± 0.3 | −0.51 cde ± 0.4 | ||
| 10 | 1.00 a ± 0.7 | 0.08 bcd ± 0.5 | −0.15 bcde ± 0.5 | 0.01 bcd ± 0.6 | −0.08 bcd ± 0.7 | 0.04 bcd ± 0.7 | ||
| 10 °C | 0 | 0.00 defghi ± 0.0 | −1.14 kl ± 0.4 | −1.05 jkl ± 0.4 | −0.64 hijkl ± 0.9 | −0.83 ijkl ± 0.7 | −1.17 l ± 0.5 | 0.001 |
| 1 | 0.17 bcdefghi ± 0.5 | −0.38 efghijkl ± 0.3 | −0.62 ghijkl ± 0.4 | −0.5 fghijkl ± 0.4 | −0.7 ijkl ± 0.5 | −0.82 ijkl ± 0.7 | ||
| 4 | 0.58 abcde ± 0.7 | −0.01 defghi ± 0.7 | 0.03 defghi ± 0.4 | −0.04 defghij ± 0.3 | −0.15 defghijk ± 0.8 | −0.09 defghij ± 0.3 | ||
| 7 | 1.06 abc ± 0.7 | 0.65 abcd ± 1.1 | 0.34 bcdefgh ± 0.8 | 0.39 bcdefg ± 0.9 | 0.09 cdefghi ± 0.7 | −0.13 defghijk ± 0.4 | ||
| 10 | 1.56 a ± 1.0 | 1.18 ab ± 0.8 | 0.61 abcde ± 0.7 | 0.45 bcdef ± 0.7 | 0.37 bcdefgh ± 0.7 | 0.48 bcdef ± 0.7 | ||
| 4 °C vs. 10 °C | 0 | NS | 0.101 | * | 0.145 | 0.053 | * | |
| 1 | 0.181 | 0.216 | 0.948 | 0.661 | 0.084 | 0.597 | ||
| 4 | 0.327 | 0.227 | 0.108 | 0.859 | 0.482 | * | ||
| 7 | * | * | * | * | 0.951 | 0.058 | ||
| 10 | 0.175 | * | * | 0.102 | 0.107 | 0.156 |
| Temp | Days | CNL | 1 min | 2 min | 3 min | 4 min | 5 min | p-Value |
|---|---|---|---|---|---|---|---|---|
| 4 °C | 0 | 0.00 abcd ± 0.0 | −0.09 abcd ± 0.3 | 0.14 abc ± 0.2 | 0.1 abc ± 0.5 | −0.15 abcd ± 0.3 | −0.19 abcd ± 0.3 | 0.001 |
| 1 | 0.13 abc ± 0.5 | −0.36 abcd ± 0.3 | −0.44 bcd ± 0.4 | −0.12 abcd ± 0.5 | 0.01 abcd ± 0.4 | −0.56 cd ± 0.5 | ||
| 4 | 0.12 abc ± 1.2 | −0.31 abcd ± 0.6 | −0.27 abcd ± 0.9 | −0.05 abcd ± 0.7 | 0.43 ab ± 0.3 | −0.91 d ± 0.9 | ||
| 7 | 0.35 abc ± 0.6 | 0.03 abcd ± 0.1 | 0.1 abc ± 0.4 | 0.31 abc ± 0.3 | 0.42 ab ± 0.4 | −0.21 abcd ± 0.4 | ||
| 10 | 0.38 abc ± 0.5 | 0.01 abcd ± 0.5 | −0.22 abcd ± 0.4 | 0.21 abc ± 0.4 | 0.38 abc ± 0.7 | 0.54 a ± 0.5 | ||
| 10 °C | 0 | 0.00 g ± 0.0 | −0.08 g ± 0.2 | −0.02 g ± 0.3 | 0.37 fg ± 0.7 | 0.28 fg ± 0.7 | −0.04 g ± 0.3 | 0.001 |
| 1 | 0.55 cdefg ± 0.3 | 0.56 cdefg ± 0.4 | 0.25 fg ± 0.4 | 0.41 efg ± 0.4 | 0.29 fg ± 0.7 | 0.24 fg ± 0.7 | ||
| 4 | 0.7 bcdefg ± 1.0 | 0.67 bcdefg ± 0.5 | 0.53 defg ± 0.7 | 0.55 cdefg ± 0.5 | 0.74 bcdefg ± 0.6 | 0.33 fg ± 1.1 | ||
| 7 | 1.51 ab ± 0.4 | 1.47 abc ± 0.4 | 1.46 abc ± 0.4 | 1.34 abcd ± 0.1 | 1.32 abcde ± 0.2 | 1.12 abcdef ± 0.1 | ||
| 10 | 1.39 abcd ± 0.2 | 1.8 a ± 0.4 | 1.6 ab ± 0.5 | 2.02 a ± 1.0 | 1.56 ab ± 0.6 | 1.41 abcd ± 0.6 | ||
| 4 °C vs. 10 °C | 0 | NS | 0.939 | 0.238 | 0.407 | 0.227 | 0.369 | |
| 1 | * | * | * | * | 0.229 | * | ||
| 4 | 0.212 | * | 0.023 | * | 0.127 | * | ||
| 7 | * | * | * | * | * | * | ||
| 10 | * | * | * | * | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osaili, T.M.; Dhanasekaran, D.K.; Hasan, F.; Obaid, R.S.; Al-Nabulsi, A.A.; Olaimat, A.N.; Ismail, L.C.; Alkalbani, N.; Ayyash, M.; Bamigbade, G.B.; et al. Changes in Microbial Safety and Quality of High-Pressure Processed Camel Milk. Foods 2025, 14, 320. https://doi.org/10.3390/foods14020320
Osaili TM, Dhanasekaran DK, Hasan F, Obaid RS, Al-Nabulsi AA, Olaimat AN, Ismail LC, Alkalbani N, Ayyash M, Bamigbade GB, et al. Changes in Microbial Safety and Quality of High-Pressure Processed Camel Milk. Foods. 2025; 14(2):320. https://doi.org/10.3390/foods14020320
Chicago/Turabian StyleOsaili, Tareq M., Dinesh Kumar Dhanasekaran, Fayeza Hasan, Reyad S. Obaid, Anas A. Al-Nabulsi, Amin N. Olaimat, Leila Cheikh Ismail, Nadia Alkalbani, Mutamed Ayyash, Gafar Babatunde Bamigbade, and et al. 2025. "Changes in Microbial Safety and Quality of High-Pressure Processed Camel Milk" Foods 14, no. 2: 320. https://doi.org/10.3390/foods14020320
APA StyleOsaili, T. M., Dhanasekaran, D. K., Hasan, F., Obaid, R. S., Al-Nabulsi, A. A., Olaimat, A. N., Ismail, L. C., Alkalbani, N., Ayyash, M., Bamigbade, G. B., Holley, R., Cheema, A. S., Bani Odeh, W. A., Mohd, K. A., & Kamal, A. K. H. (2025). Changes in Microbial Safety and Quality of High-Pressure Processed Camel Milk. Foods, 14(2), 320. https://doi.org/10.3390/foods14020320










