In Vitro and In Ovo Evaluation of Oenothera biennis L. Oil as an Alternative Preservative for Oil-Based Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oil Samples
2.2. Sample Preparation
2.3. Determination of Peroxide Value (PV)
2.4. Determination of p-Anisidine Value (p-AV)
2.5. Total Oxidation Value (TOTOX)
2.6. In Ovo Evaluation Using the CAM Assay
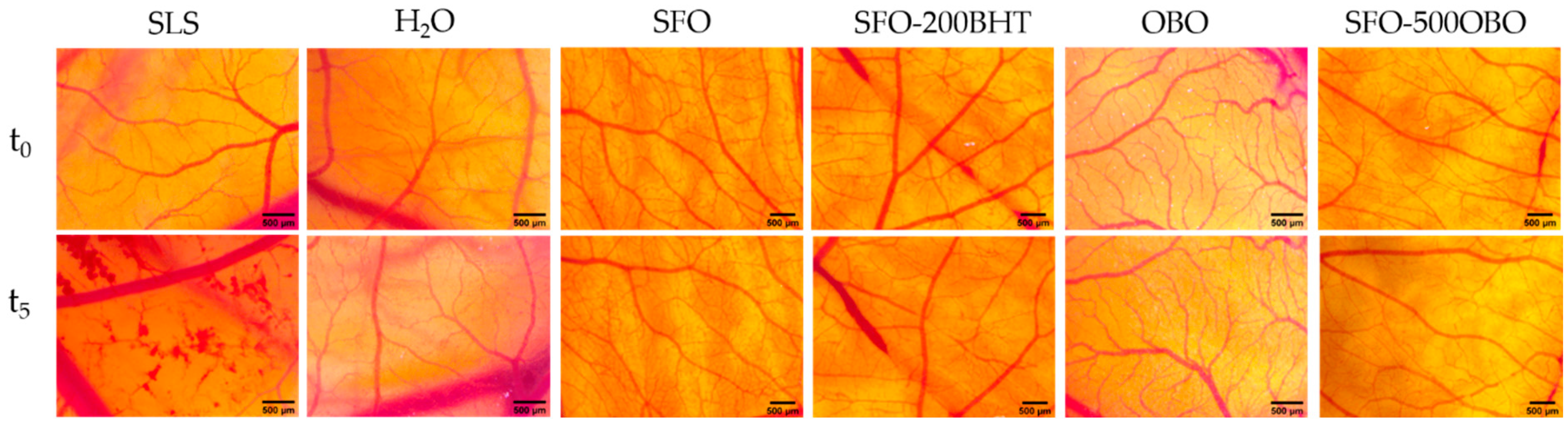
2.7. Irritation Assessment Using HET-CAM Assay
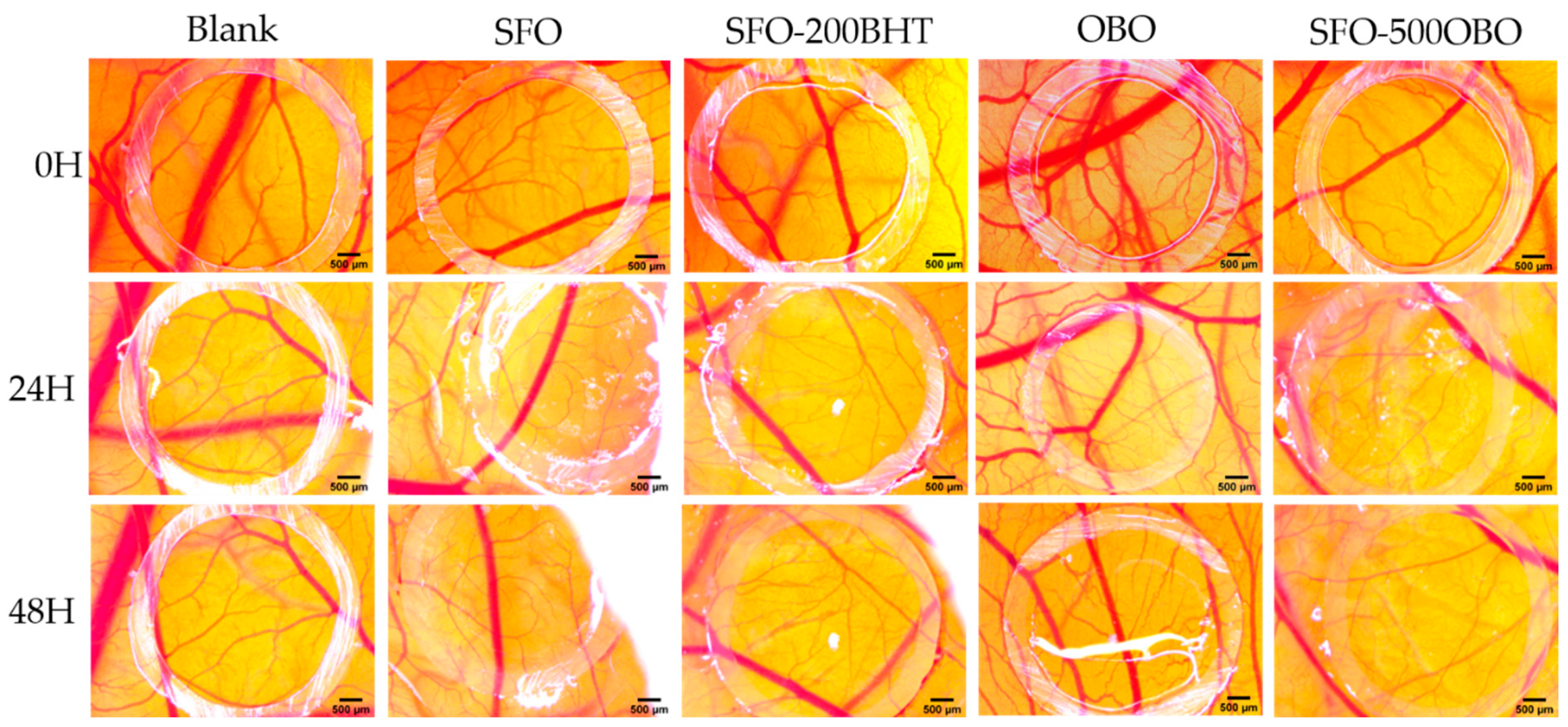
2.8. Angiogenesis Evaluation Using the CAM Assay
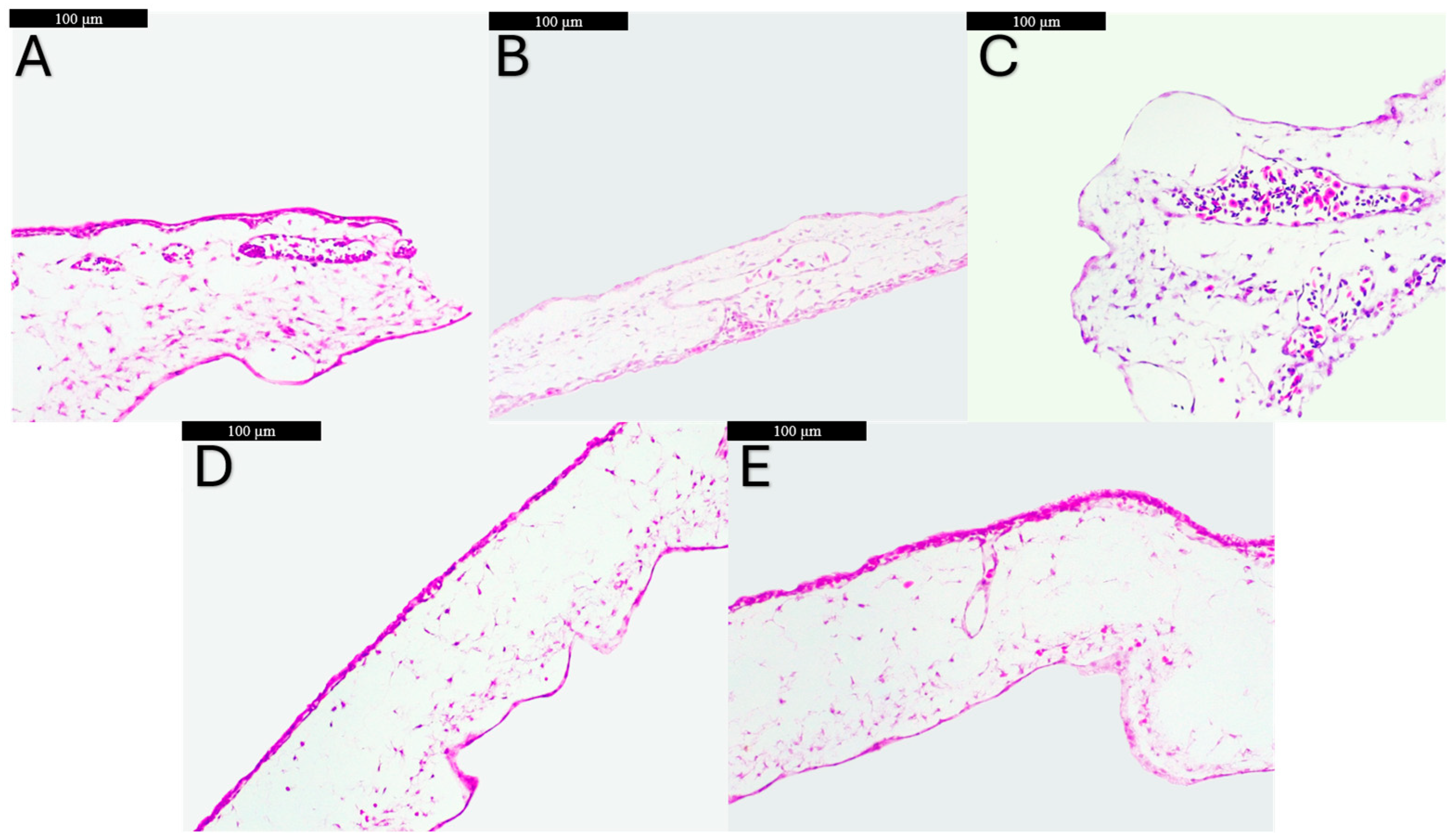
2.9. Histological Processing of CAMs
2.10. Statistical Analysis
3. Results
3.1. PV Assessment
3.2. p-AV Assessment
3.3. Determination of TOTOX Values
3.4. Irritative Potential In Ovo Using HET-CAM Assay
3.5. Effects on the CAM
3.6. Histological Assessment of CAMs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timoszuk, M.; Bielawska, K.; Skrzydlewska, E. Evening Primrose (Oenothera biennis) Biological Activity Dependent on Chemical Composition. Antioxidants 2018, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Barnes, J.; Gibbons, S.; Williamson, E. Fundamentals of Pharmacognosy and Phytotherapy, 2nd ed.; Elsevier Ltd.: Edinburgh, UK, 2012. [Google Scholar]
- Steckel, L.E.; Sosnoskie, L.M.; Steckel, S.J. Common Evening-Primrose (Oenothera biennis L.). Weed Technol. 2019, 33, 757–760. [Google Scholar] [CrossRef]
- Fecker, R.; Buda, V.; Alexa, E.; Avram, S.; Pavel, I.Z.; Muntean, D.; Cocan, I.; Watz, C.; Minda, D.; Dehelean, C.A.; et al. Phytochemical and Biological Screening of Oenothera biennis L. Hydroalcoholic Extract. Biomolecules 2020, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.; Reda, A.; Nabil, M.; Elimam, D.; Zayed, A. Evening Primrose Oil: A Comprehensive Review of Its Bioactives, Extraction, Analysis, Oil Quality, Therapeutic Merits, and Safety. Food Funct. 2023, 14, 8049–8070. [Google Scholar] [CrossRef]
- Hadidi, M.; Ibarz, A.; Pouramin, S. Optimization of Extraction and Deamidation of Edible Protein from Evening Primrose (Oenothera biennis L.) Oil Processing by-Products and Its Effect on Structural and Techno-Functional Properties. Food Chem. 2021, 334, 127613. [Google Scholar] [CrossRef]
- Ghatas, Y.; Mohamed, Y. Influence of Some Phosphorus Sources and Biofertilizers (Em and Phosphorein) on Vegetative Growth, Fixed Oil Productivity and Chemical Constituents of Oenothera biennis L. Plant. Sci. J. Flowers Ornam. Plants 2020, 7, 247–268. [Google Scholar] [CrossRef]
- Montserrat-De La Paz, S.; Fernández-Arche, M.A.; Ángel-Martín, M.; García-Giménez, M.D. Phytochemical Characterization of Potential Nutraceutical Ingredients from Evening Primrose Oil (Oenothera biennis L.). Phytochem. Lett. 2014, 8, 158–162. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Zuo, G.; Lim, S.S.; Yan, H. Defatted Seeds of Oenothera biennis as a Potential Functional Food Ingredient for Diabetes. Foods 2021, 10, 538. [Google Scholar] [CrossRef]
- Edwards, S.; Rocha, I.; Williamson, E.; Heinrich, M. Phytopharmacy: An Evidence-Based Guide to Herbal Medical Products; Wiley-Blackwell: Hoboken, NJ, USA, 2015. [Google Scholar]
- Frankel, E.N. Lipid Oxidation, 2nd ed.; Woodhead Publishing: Sawston, UK, 2005. [Google Scholar]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Controversy around Parabens: Alternative Strategies for Preservative Use in Cosmetics and Personal Care Products. Environ. Res. 2021, 198, 110488. [Google Scholar] [CrossRef]
- Tinello, F.; Lante, A. Accelerated Storage Conditions Effect on Ginger- and Turmeric-Enriched Soybean Oils with Comparing a Synthetic Antioxidant BHT. LWT 2020, 131, 109797. [Google Scholar] [CrossRef]
- Fecker, R.; Magyari-Pavel, I.Z.; Cocan, I.; Alexa, E.; Popescu, I.M.; Lombrea, A.; Bora, L.; Dehelean, C.A.; Buda, V.; Folescu, R.; et al. Oxidative Stability and Protective Effect of the Mixture between Helianthus annuus L. and Oenothera biennis L. Oils on 3D Tissue Models of Skin Irritation and Phototoxicity. Plants 2022, 11, 2977. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The Chicken Chorioallantoic Membrane Model in Biology, Medicine and Bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef] [PubMed]
- Tamanoi, F. Recent Excitements in the Study of the CAM Assay. In The Enzymes; Academic Press: Cambridge, MA, USA, 2019; pp. 1–9. [Google Scholar] [CrossRef]
- Avram, Ș.; Bora, L.; Vlaia, L.L.; Muț, A.M.; Olteanu, G.-E.; Olariu, I.; Magyari-Pavel, I.Z.; Minda, D.; Diaconeasa, Z.; Sfirloaga, P.; et al. Cutaneous Polymeric-Micelles-Based Hydrogel Containing Origanum Vulgare L. Essential Oil: In Vitro Release and Permeation, Angiogenesis, and Safety Profile In Ovo. Pharmaceuticals 2023, 16, 940. [Google Scholar] [CrossRef] [PubMed]
- Avram, S.; Ghiulai, R.; Pavel, I.; Mioc, M.; Babuta, R.; Voicu, M.; Coricovac, D.; Danciu, C.; Dehelean, C.; Soica, C. Phytocompounds Targeting Cancer Angiogenesis Using the Chorioallantoic Membrane Assay. In Natural Products and Cancer Drug Discovery; InTechOpen: London, UK, 2017; pp. 45–66. [Google Scholar] [CrossRef]
- Minda, D.; Ghiulai, R.; Banciu, C.D.; Pavel, I.Z.; Danciu, C.; Racoviceanu, R.; Soica, C.; Budu, O.D.; Muntean, D.; Diaconeasa, Z.; et al. Phytochemical Profile, Antioxidant and Wound Healing Potential of Three Artemisia Species: In Vitro and In Ovo Evaluation. Appl. Sci. 2022, 12, 1359. [Google Scholar] [CrossRef]
- AOCS Official Method Cd 8b-90, Peroxide Value Acetic Acid-Isooctane Method. In Official Methods and Recommended Practices of the AOCS; AOCS Press: Urbana, IL, USA, 2009.
- AOCS Official Method Cd 18-90, p-Anisidine Value. In Official Methods and Recommended Practices of the AOCS; AOCS Press: Urbana, IL, USA, 2017.
- Cocan, I.; Negrea, M.; Cozma, A.; Alexa, E.; Poiana, M.; Raba, D.; Danciu, C.; Popescu, I.; Cadariu, A.I.; Obistioiu, D.; et al. Chili and Sweet Pepper Seed Oil Used as a Natural Antioxidant to Improve the Thermo-Oxidative Stability of Sunflower Oil. Agronomy 2021, 11, 2579. [Google Scholar] [CrossRef]
- Ghiulai, R.; Avram, S.; Stoian, D.; Pavel, I.Z.; Coricovac, D.; Oprean, C.; Vlase, L.; Farcas, C.; Mioc, M.; Minda, D.; et al. Lemon Balm Extracts Prevent Breast Cancer Progression In Vitro and In Ovo on Chorioallantoic Membrane Assay. Evid.-Based Complement. Altern. Med. 2020, 2020, 6489159. [Google Scholar] [CrossRef]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane as an in Vivo Assay to Study Antiangiogenesis. Pharmaceuticals 2010, 3, 482–513. [Google Scholar] [CrossRef]
- Scheel, J.; Kleber, M.; Kreutz, J.; Lehringer, E.; Mehling, A.; Reisinger, K.; Steiling, W. Eye Irritation Potential: Usefulness of the HET-CAM under the Globally Harmonized System of Classification and Labeling of Chemicals (GHS). Regul. Toxicol. Pharmacol. 2011, 59, 471–492. [Google Scholar] [CrossRef]
- National Institute of Environmental Health Sciences. ICCVAM Test Method Evaluation Report: Current Validation Status of In Vitro Test Methods Proposed for Identifying Eye Injury Hazard Potential of Chemicals and Products; National Institute of Environmental Health Sciences: Rockville, MD, USA, 2010.
- Luepke, N.P. Hen’s Egg Chorioallantoic Membrane Test for Irritation Potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]
- Pan, F.; Li, Y.; Luo, X.; Wang, X.; Wang, C.; Wen, B.; Guan, X.; Xu, Y.; Liu, B. Effect of the Chemical Refining Process on Composition and Oxidative Stability of Evening Primrose Oil. J. Food Process. Preserv. 2020, 44, e14800. [Google Scholar] [CrossRef]
- Gotoh, N.; Wada, S. The Importance of Peroxide Value in Assessing Food Quality and Food Safety. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 437. [Google Scholar] [CrossRef]
- Kamkar, A.; Javan, A.J.; Asadi, F.; Kamalinejad, M. The Antioxidative Effect of Iranian Mentha Pulegium Extracts and Essential Oil in Sunflower Oil. Food Chem. Toxicol. 2010, 48, 1796–1800. [Google Scholar] [CrossRef] [PubMed]
- Ali Raza, S.; Rehman, A.; Adnan, A.; Qureshi, F. Comparison of Antioxidant Activity of Essential Oil of Centella Asiatica and Butylated Hydroxyanisole in Sunflower Oil at Ambient Conditions. Biharean Biol. 2009, 3, 71–75. [Google Scholar]
- Skiera, C.; Steliopoulos, P.; Kuballa, T.; Holzgrabe, U.; Diehl, B. 1H NMR Approach as an Alternative to the Classical P-Anisidine Value Method. Eur. Food Res. Technol. 2012, 235, 1101–1105. [Google Scholar] [CrossRef]
- Wang, D.; Meng, Y.; Zhao, X.; Fan, W.; Yi, T.; Wang, X. Sunflower Oil Flavored by Essential Oil from Punica Granatum Cv. Heyinshiliu Peels Improved Its Oxidative Stability and Sensory Properties. LWT 2019, 111, 55–61. [Google Scholar] [CrossRef]
- Wang, D.; Fan, W.; Guan, Y.; Huang, H.; Yi, T.; Ji, J. Oxidative Stability of Sunflower Oil Flavored by Essential Oil from Coriandrum sativum L. during Accelerated Storage. LWT—Food Sci. Technol. 2018, 98, 268–275. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Niklová, I.; Schmidt, Š.; Habalová, K.; Sekretár, S. Effect of Evening Primrose Extracts on Oxidative Stability of Sunflower and Rapeseed Oils. Eur. J. Lipid Sci. Technol. 2001, 103, 299–306. [Google Scholar] [CrossRef]
- Ermawati, D.; Cahyani, P.Z.; Shahnaz, I.; Juniarty, A.; Mahardhika, C.L.; Chasanah, U. Formulation of Serum Using a Combination of Tamanu Oil and Tea Tree Oil as Anti-Acne. J. Kefarmasian Indones. 2023, 13, 150–158. [Google Scholar]
- Bernardi, D.S.; Pereira, T.A.; Maciel, N.R.; Bortoloto, J.; Viera, G.S.; Oliveira, G.C.; Rocha-Filho, P.A. Formation and Stability of Oil-in-Water Nanoemulsions Containing Rice Bran Oil: In Vitro and in Vivo Assessments. J. Nanobiotechnol. 2011, 9, 44. [Google Scholar] [CrossRef]
- Kaniuk, Ł.; Podborska, A.; Stachewicz, U. Enhanced Mechanical Performance and Wettability of PHBV Fiber Blends with Evening Primrose Oil for Skin Patches Improving Hydration and Comfort. J. Mater. Chem. B 2022, 10, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, M.R. Prediction of Fatty Acid Composition of Sunflower Seeds by Near-Infrared Reflectance Spectroscopy. J. Food Sci. Technol. 2018, 55, 2318–2325. [Google Scholar] [CrossRef] [PubMed]
- Harun, M. Fatty Acid Composition of Sunflower in 31 Inbreed and 28 Hybrid. Biomed. J. Sci. Tech. Res. 2019, 16, 12032–12038. [Google Scholar] [CrossRef]
- Samson, F.P.; Patrick, A.T.; Fabunmi, T.E.; Yahaya, M.F.; Madu, J.; He, W.; Sripathi, S.R.; Tyndall, J.; Raji, H.; Jee, D.; et al. Oleic Acid, Cholesterol, and Linoleic Acid as Angiogenesis Initiators. ACS Omega 2020, 5, 20575–20585. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, N.; Matsuoka, T.; Yashiro, M.; Hirakawa, K.; Olden, K.; Roberts, J.D. Linoleic Acid Enhances Angiogenesis through Suppression of Angiostatin Induced by Plasminogen Activator Inhibitor 1. Br. J. Cancer 2011, 105, 1750–1758. [Google Scholar] [CrossRef]
- Bauer, A.K.; Dwyer-Nield, L.D.; Hankin, J.A.; Murphy, R.C.; Malkinson, A.M. The Lung Tumor Promoter, Butylated Hydroxytoluene (BHT), Causes Chronic Inflammation in Promotion-Sensitive BALB/CByJ Mice but Not in Promotion-Resistant CXB4 Mice. Toxicology 2001, 169, 1–15. [Google Scholar] [CrossRef]
- Sun, Z.; Gao, R.; Chen, X.; Liu, X.; Ding, Y.; Geng, Y.; Mu, X.; Liu, T.; Li, F.; Wang, Y.; et al. Exposure to Butylated Hydroxytoluene Compromises Endometrial Decidualization during Early Pregnancy. Environ. Sci. Pollut. Res. 2021, 28, 42024–42036. [Google Scholar] [CrossRef]
- Evening Primrose Oil. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Evening-Primrose-Oil (accessed on 16 January 2025).
- Andreoli Miyake, J.; Nascimento Gomes, R.; Colquhoun, A. Gamma-Linolenic Acid Alters Migration, Proliferation and Apoptosis in Human and Rat Glioblastoma Cells. Prostaglandins Other Lipid Mediat. 2020, 150, 106452. [Google Scholar] [CrossRef]
- Muggli, R. Systemic Evening Primrose Oil Improves the Biophysical Skin Parameters of Healthy Adults. Int. J. Cosmet. Sci. 2005, 27, 243–249. [Google Scholar] [CrossRef]
- Munir, R.; Semmar, N.; Farman, M.; Ahmad, N.S. An Updated Review on Pharmacological Activities and Phytochemical Constituents of Evening Primrose (Genus oenothera). Asian Pac. J. Trop. Biomed. 2017, 7, 1046–1054. [Google Scholar] [CrossRef]

| Sample | PV (meq O2/kg Oil) | ||||||
|---|---|---|---|---|---|---|---|
| Storage Period (Days) | |||||||
| Day 1 | Day 5 | Day 10 | Day 15 | Day 20 | Day 25 | Day 30 | |
| SFO | 1.125 ± 0.033 a,A | 4.228 ± 0.127 a,B | 7.705 ± 0.231 a,C | 9.846 ± 0.359 a,D | 12.442 ± 0.456 a,E | 15.224 ± 0.554 a,F | 16.458 ± 0.569 a,G |
| SFO–BHT | 1.050 ± 0.025 a,A | 3.345 ± 0.098 d,B | 5.568 ± 0.165 d,C | 8.058 ± 0.288 c,D | 9.228 ± 0.374 d,E | 10.861 ± 0.376 d,F | 12.246 ± 0.428 e,G |
| SFO–100 OBO | 1.122 ± 0.032 a,A | 4.118 ± 0.125 a,B | 7.221 ± 0.225 a,C | 9.507 ± 0.327 a,D | 12.105 ± 0.426 a,E | 14.804 ± 0.488 b,F | 15.551 ± 0.527 b,G |
| SFO–200 OBO | 1.118 ± 0.030 a,A | 3.872 ± 0.120 b,B | 6.824 ± 0.214 b,C | 9.016 ± 0.312 b,D | 11.755 ± 0.408 b,E | 14.169 ± 0.472 b,F | 15.024 ± 0.508 c,G |
| SFO–300 OBO | 1.113 ± 0.028 a,A | 3.567 ± 0.110 c,B | 6.229 ± 0.208 c,C | 8.721 ± 0.296 b,D | 11.207 ± 0.391 c,E | 13.275 ± 0.416 c,F | 14.473 ± 0.476 d,G |
| SFO–500 OBO | 1.111 ± 0.027 a,A | 3.389 ± 0.104 d,B | 5.705 ± 0.189 d,C | 8.119 ± 0.292 c,D | 9.633 ± 0.385 d,E | 11.132 ± 0.380 d,F | 12.583 ± 0.451 e,G |
| Sample | p-AV | ||||||
|---|---|---|---|---|---|---|---|
| Storage Period (Days) | |||||||
| Day 1 | Day 5 | Day 10 | Day 15 | Day 20 | Day 25 | Day 30 | |
| SFO | 0.225 ± 0.006 a,A | 6.742 ± 0.201 a,B | 10.074 ± 0.301 a,C | 13.423 ± 0.421 a,D | 16.627 ± 0.557 a,E | 18.297 ± 0.594 a,F | 21.183 ± 0.676 a,G |
| SFO–BHT | 0.159 ± 0.003 b,A | 1.669 ± 0.048 d,B | 3.341 ± 0.100 d,C | 4.547 ± 0.122 e,D | 5.824 ± 0.199 e,E | 6.993 ± 0.257 e,F | 9.725 ± 0.331 e,G |
| SFO–100 OBO | 0.221 ± 0.006 a,A | 6.529 ± 0.151 a,B | 9.943 ± 0.297 a,C | 11.826 ± 0.396 b,D | 13.309 ± 0.413 b,E | 17.246 ± 0.561 b,F | 19.552 ± 0.611 b,G |
| SFO–200 OBO | 0.212 ± 0.005 a,A | 4.884 ± 0.114 b,B | 7.182 ± 0.208 b,C | 8.734 ± 0.287 c,D | 10.776 ± 0.359 c,E | 15.055 ± 0.488 c,F | 17.506 ± 0.562 c,G |
| SFO–300 OBO | 0.204 ± 0.004 a,A | 2.546 ± 0.069 c,B | 4.930 ± 0.167 c,C | 6.525 ± 0.203 d,D | 8.228 ± 0.284 d,E | 10.119 ± 0.307 d,F | 13.892 ± 0.417 d,G |
| SFO–500 OBO | 0.168 ± 0.003 b,A | 1.840 ± 0.054 d,B | 3.441 ± 0.101 d,C | 4.669 ± 0.157 e,D | 5.931 ± 0.188 e,E | 7.197 ± 0.263 e,F | 9.932 ± 0.305 e,G |
| Samples | Irritation Score | Type of Effect |
|---|---|---|
| SLS 0.5% | 17.50 ± 0.21 | strong irritant |
| H2O dist. | 0 ± 0.0 | non-irritant |
| SFO | 0 ± 0.0 | non-irritant |
| SFO–BHT | 0 ± 0.0 | non-irritant |
| OBO | 0 ± 0.0 | non-irritant |
| SFO–500 OBO | 0 ± 0.0 | non-irritant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fecker, R.; Avram, Ș.; Cocan, I.; Alexa, E.; Bora, L.; Minda, D.; Magyari-Pavel, I.Z.; Dehelean, C.A.; Danciu, C. In Vitro and In Ovo Evaluation of Oenothera biennis L. Oil as an Alternative Preservative for Oil-Based Products. Foods 2025, 14, 332. https://doi.org/10.3390/foods14020332
Fecker R, Avram Ș, Cocan I, Alexa E, Bora L, Minda D, Magyari-Pavel IZ, Dehelean CA, Danciu C. In Vitro and In Ovo Evaluation of Oenothera biennis L. Oil as an Alternative Preservative for Oil-Based Products. Foods. 2025; 14(2):332. https://doi.org/10.3390/foods14020332
Chicago/Turabian StyleFecker, Ramona, Ștefana Avram, Ileana Cocan, Ersilia Alexa, Larisa Bora, Daliana Minda, Ioana Zinuca Magyari-Pavel, Cristina Adriana Dehelean, and Corina Danciu. 2025. "In Vitro and In Ovo Evaluation of Oenothera biennis L. Oil as an Alternative Preservative for Oil-Based Products" Foods 14, no. 2: 332. https://doi.org/10.3390/foods14020332
APA StyleFecker, R., Avram, Ș., Cocan, I., Alexa, E., Bora, L., Minda, D., Magyari-Pavel, I. Z., Dehelean, C. A., & Danciu, C. (2025). In Vitro and In Ovo Evaluation of Oenothera biennis L. Oil as an Alternative Preservative for Oil-Based Products. Foods, 14(2), 332. https://doi.org/10.3390/foods14020332









