Antibacterial Efficacy of Ethanol Extracts from Edible Rumex madaio Root and Application Potential for Eliminating Staphylococcus aureus and Vibrio cholerae in Aquatic Products for Green Food Preservation
Abstract
1. Introduction
2. Results and Discussion
2.1. Antibacterial Effects of RmEEs Extracted with Different Concentrations of Ethanol
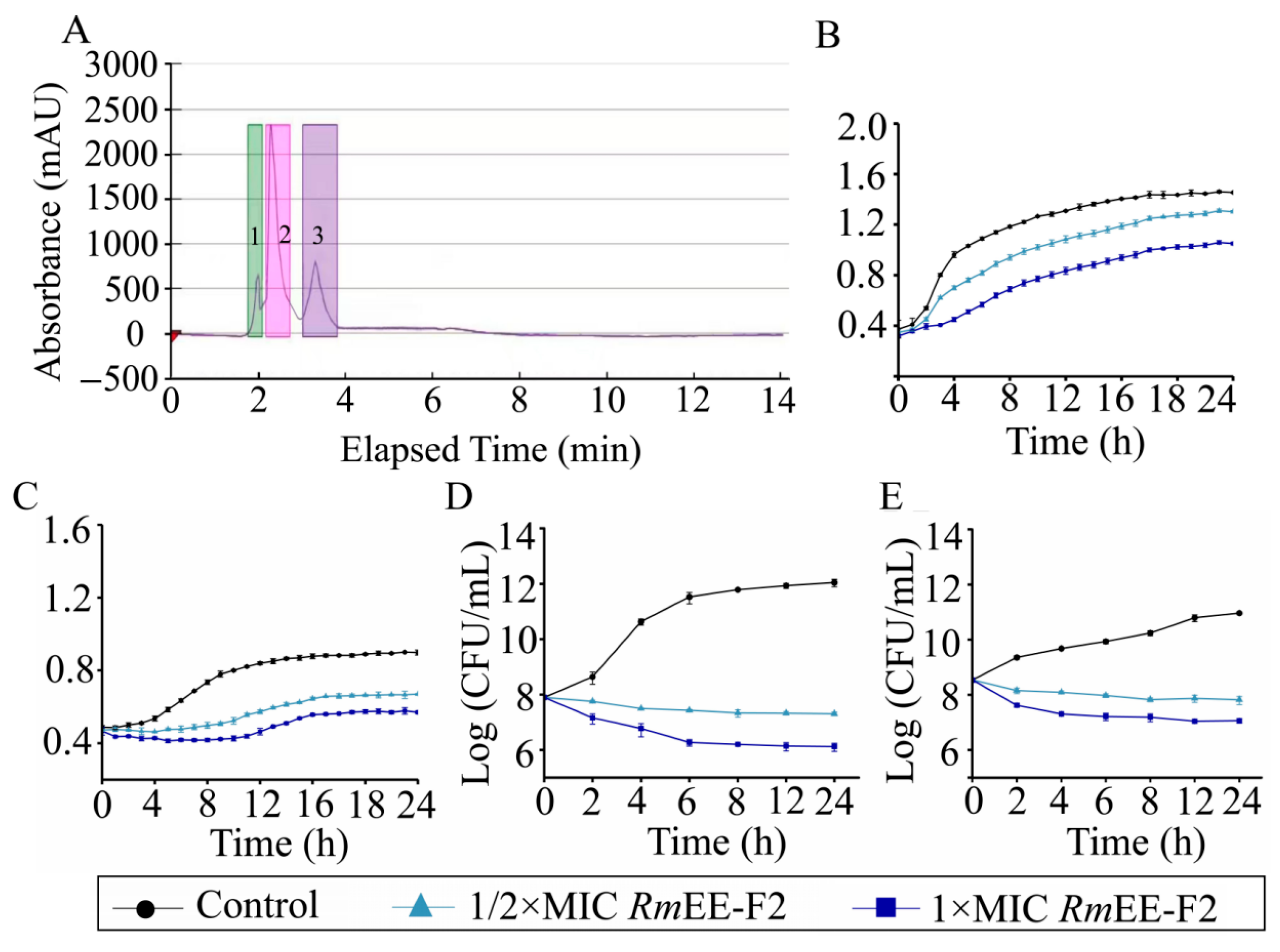
2.2. Purification of the RmEE (75% E)
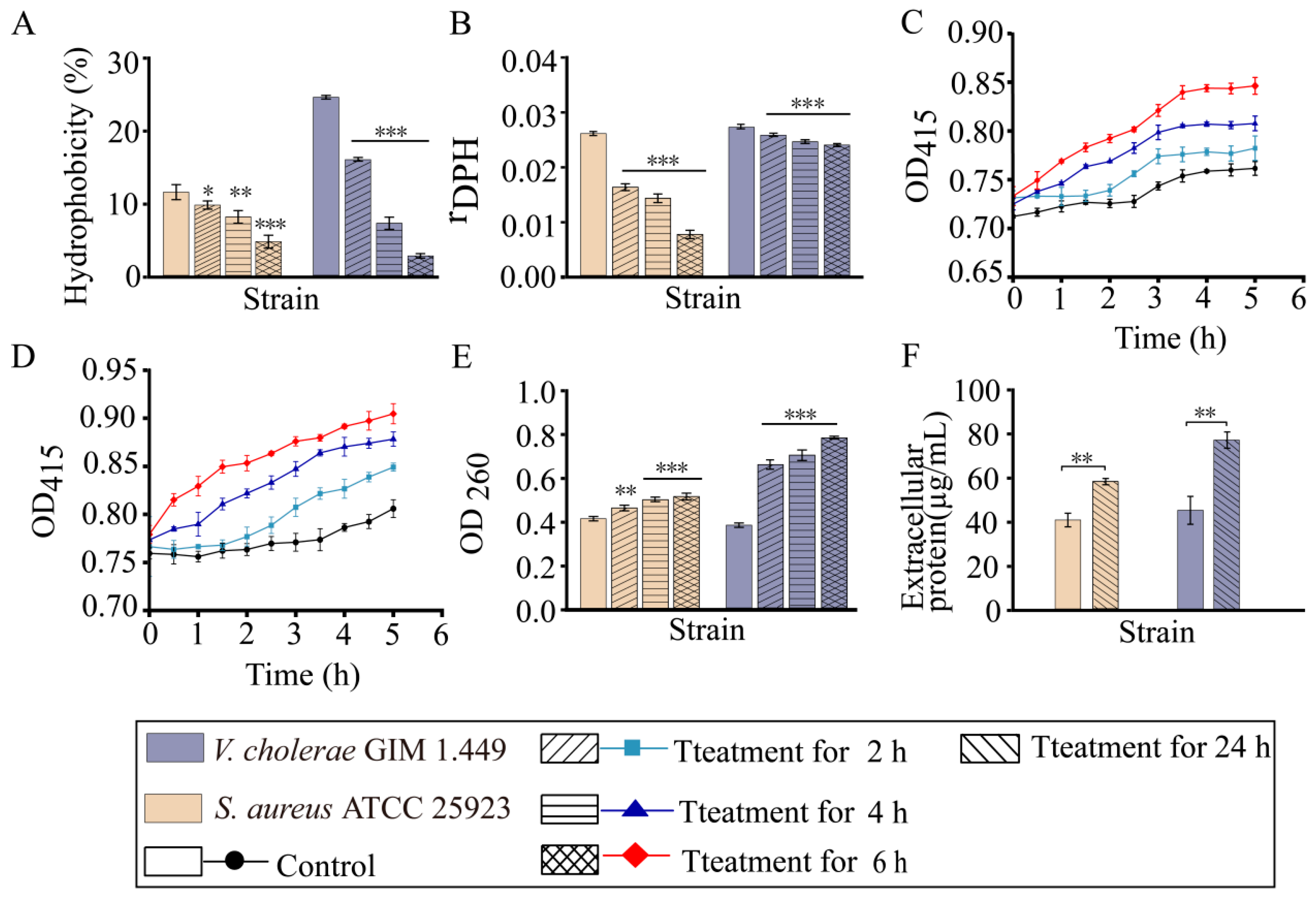
2.3. RmEE-F2 Inhibited Growth and Changed Cell Biophysical Parameters of the Tested Strains
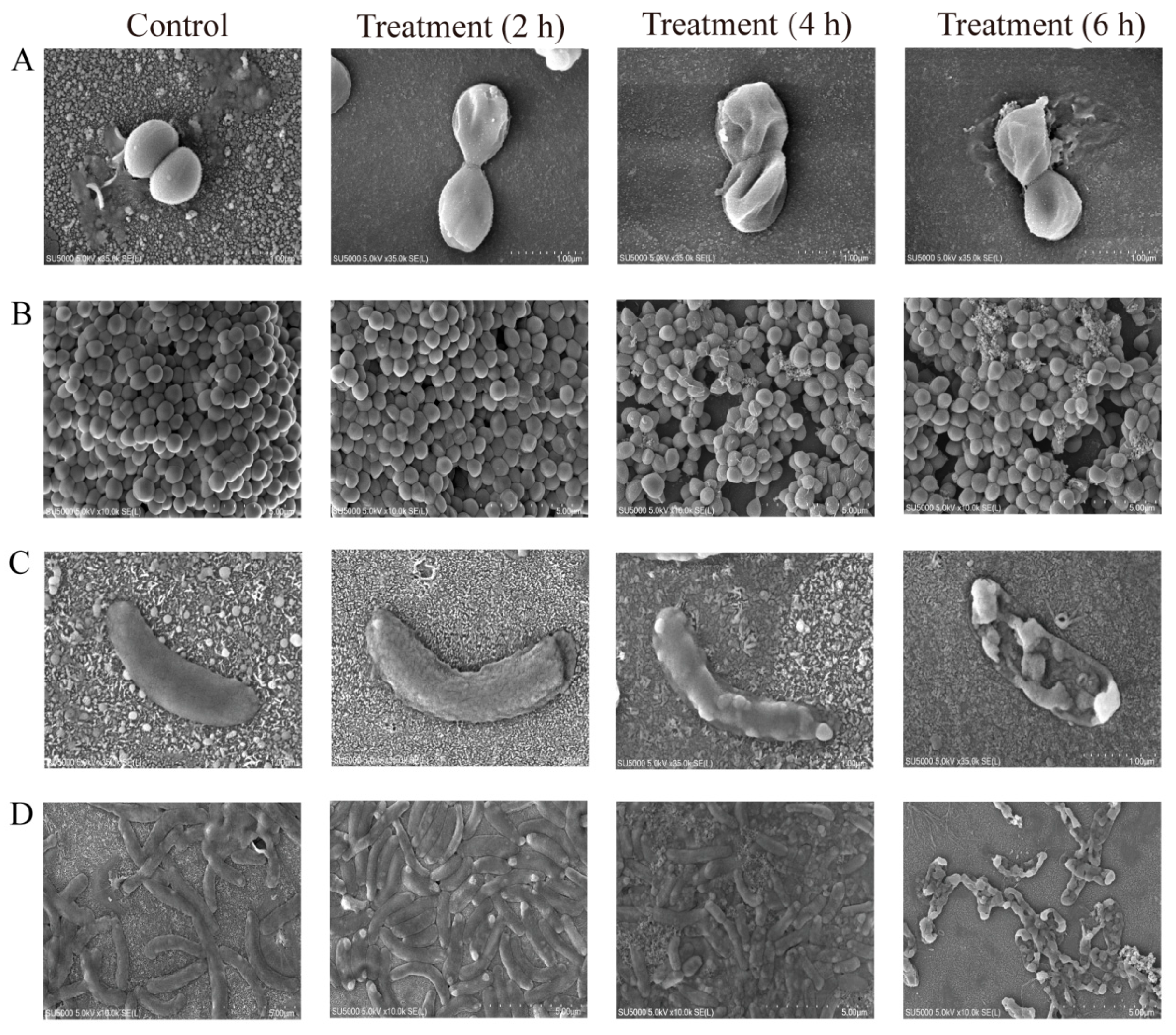
2.4. RmEE-F2 Led to Nucleic Acid and Protein Exudation and Damaged Cell Structure of the Tested Strains
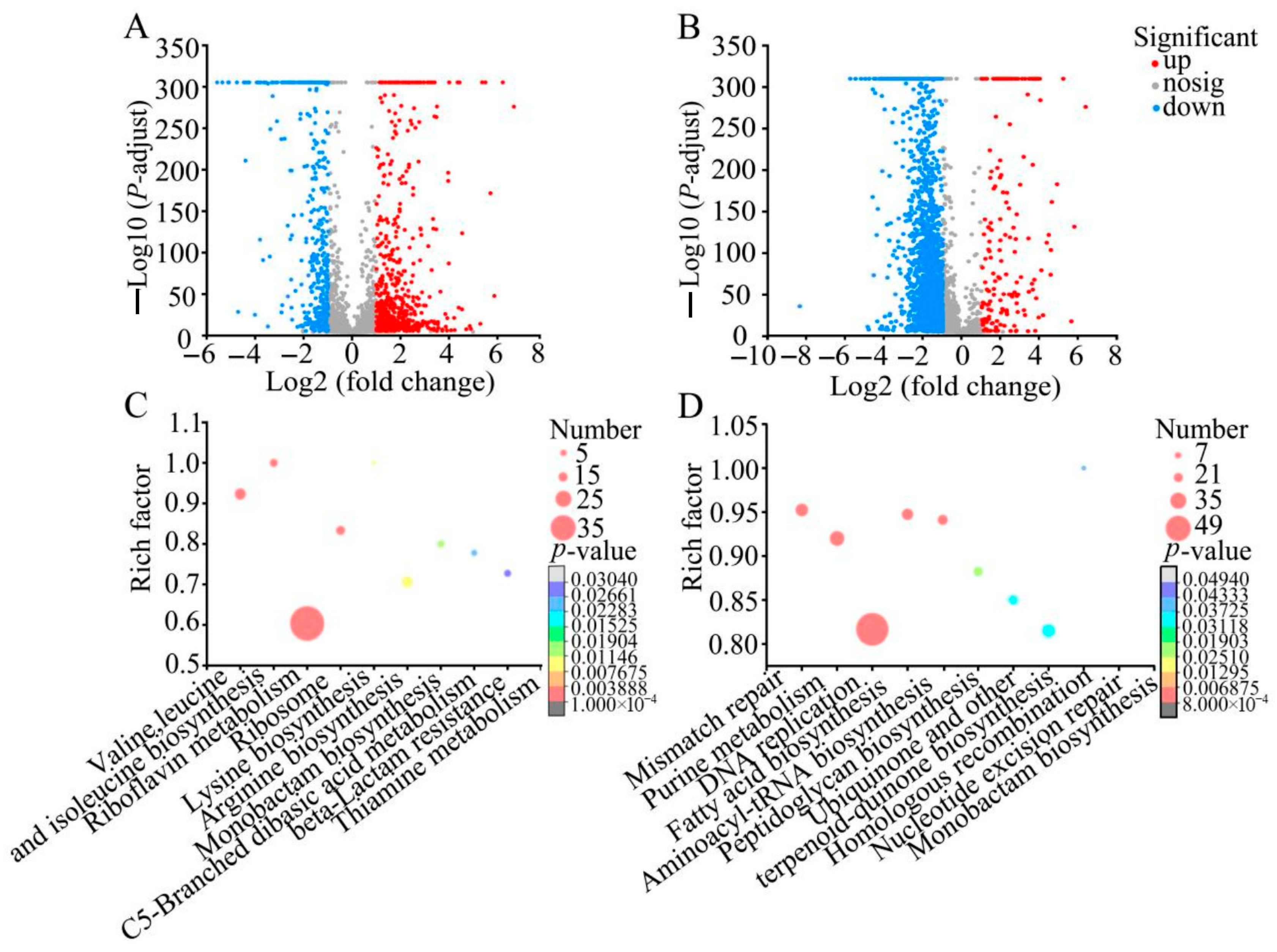
2.5. RmEE-F2 Disturbed Metabolic Pathways in the Tested Strains
2.5.1. Major Disturbed Metabolic Pathways in S. aureus ATCC 25923 Triggered by RmEE-F2
2.5.2. Major Disturbed Metabolic Pathways in V. cholerae GIM 1.449 Triggered by RmEE-F2
2.6. RmEE-F2 Effectively Killed the Tested Strains in the Spiked Fish and Shrimp During the Low-Temperature Preservation
2.6.1. RmEE-F2 Effectively Killed the Tested Strains in the C. auratus Sample
2.6.2. RmEE-F2 Effectively Killed the Tested Strains in the P. vannamei Sample
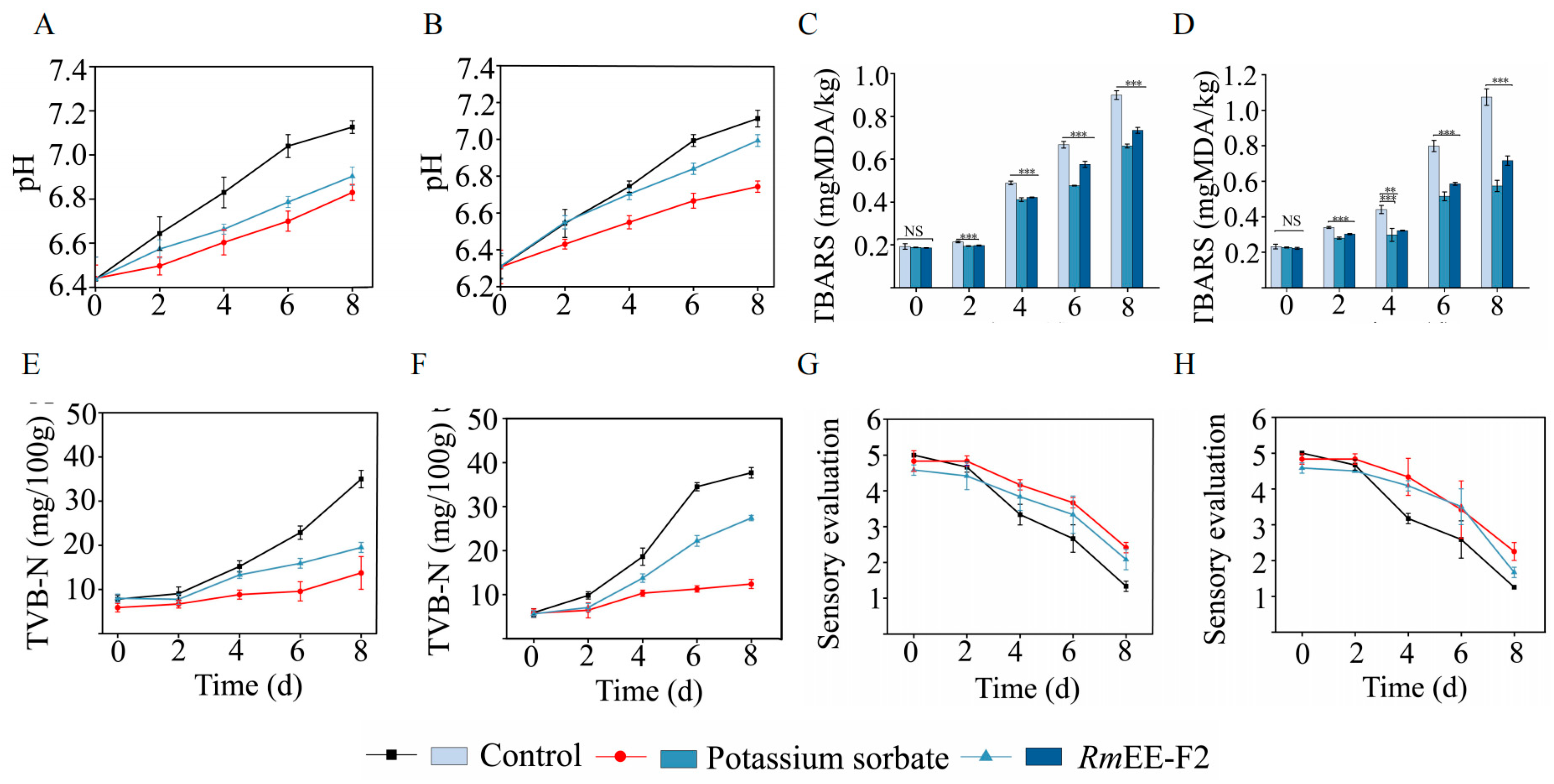
2.7. RmEE-F2 Effectively Preserved Quality and Sensory of the Fish and Shrimp Meat Samples at the Low-Temperature Storage
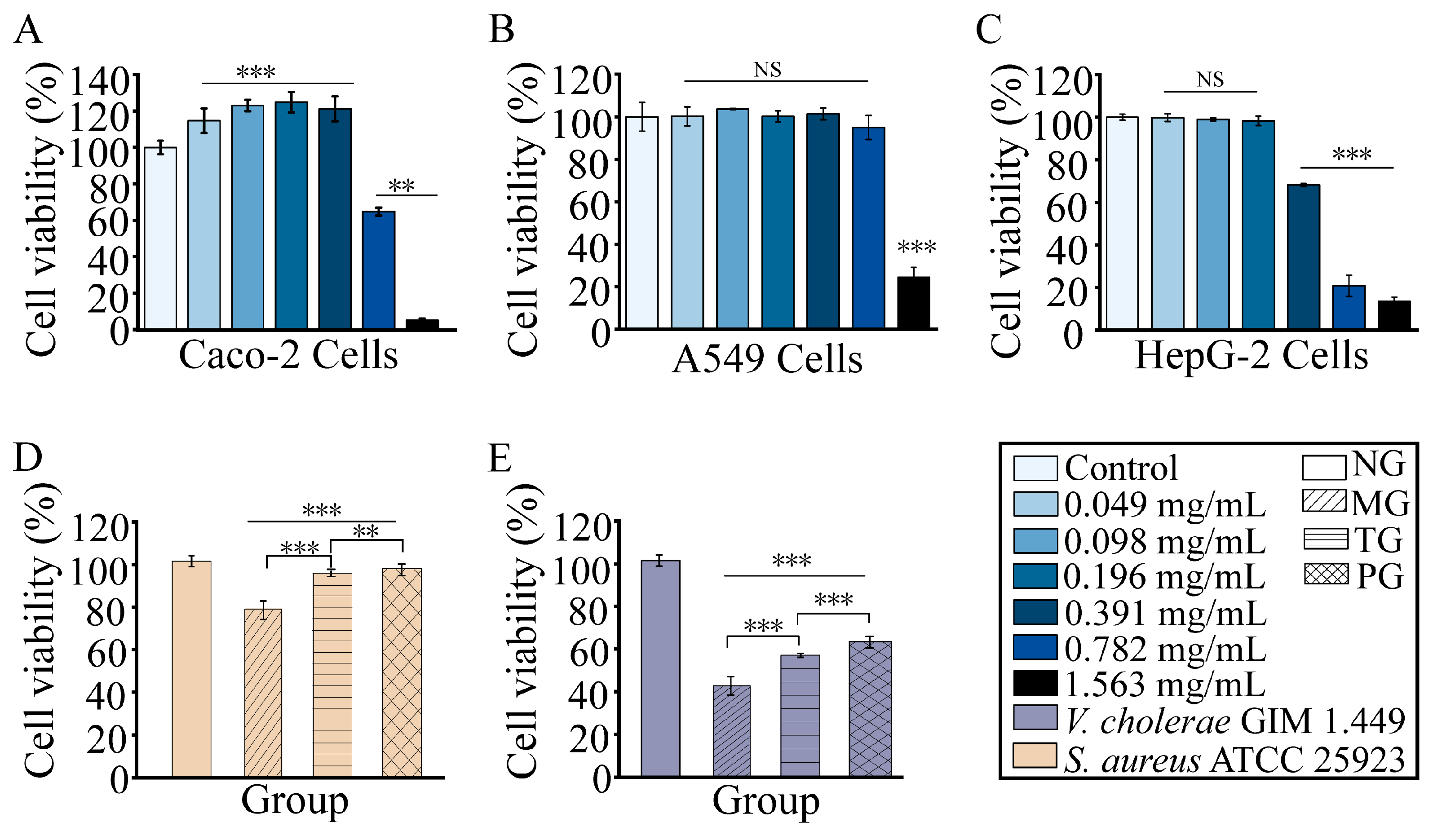
2.8. RmEE-F2 Showed No Cytotoxicity on Human Caco-2, HepG-2 and A549 Cells and Rescued Caco-2 cells Infested by the Tested Strains
2.9. Potential Antibacterial Compounds in RmEE-F2
3. Materials and Methods
3.1. Bacterial Strains and Culture Conditions
3.2. Ethanol Extraction of Bacteriostatic Substances from the Rhizome of R. madaio
3.3. Antibacterial Activity, Growth Curve, and Time-Kill Curve Analysis
3.4. Prep-HPLC Analysis
3.5. Bacterial Cell Surface Biophysical Parameter Assays
3.6. Nucleotide Acid and Protein Exudation and SEM Assays
3.7. Illumina RNA Sequencing and Analysis
3.8. Assessment of Antibacterial Effect of RmEE-F2 on Artificially Spiked Fish and Shrimp
3.9. Assessment of Quality and Sensory of the Fish and Shrimp Treated by RmEE-F2
3.10. Cytotoxicity Assay
3.11. Caco-2 Cell Infection Assay
3.12. Ultra HPLC-Mass Spectrometry (UHPLC-MS) Analysis
3.13. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- World Health Organization. Food Safety. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 24 April 2025).
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1496. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and bacterial resistance—A short story of an endless arms race. Int. J. Mol. Sci. 2023, 24, 5777. [Google Scholar] [CrossRef]
- Fahle, A.; Bereswill, S.; Heimesaat, M.M. Antibacterial effects of biologically active ingredients in hop provide promising options to fight infections by pathogens including multi-drug resistant bacteria. Eur. J. Microbiol. Immunol. 2022, 12, 22–30. [Google Scholar] [CrossRef]
- Xiao, S.-J.; Xu, X.-K.; Chen, W.; Xin, J.-Y.; Yuan, W.-L.; Zu, X.-P.; Shen, Y.-H. Traditional Chinese medicine Euodiae Fructus: Botany, traditional use, phytochemistry, pharmacology, toxicity and quality control. Nat. Prod. Bioprospecting 2023, 13, 6. [Google Scholar] [CrossRef]
- Kačániová, M.; Joanidis, P.; Lakatošová, J.; Kunová, S.; Benešová, L.; Ikromi, K.; Akhmedov, F.; Boboev, K.; Gulmahmad, M.; Niyatbekzoda, F.; et al. Effect of essential oils and dried herbs on the shelf life of fresh goat lump cheese. Foods 2024, 13, 2016. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.N.; Cadavez, V.; Caleja, C.; Pereira, E.; Calhelha, R.C.; Añibarro-Ortega, M.; Finimundy, T.; Kostić, M.; Soković, M.; Teixeira, J.A.; et al. Phytochemical composition and bioactive potential of Melissa officinalis L., Salvia officinalis L. and Mentha spicata L. extracts. Foods 2023, 12, 947. [Google Scholar] [CrossRef]
- Reyes, J.F.; Diez, A.M.; Melero, B.; Rovira, J.; Jaime, I. Antimicrobial effect of Simira ecuadorensis extracts and their impact on improving shelf life in chicken and fish products. Foods 2022, 11, 2352. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Li, Y.X.; Li, N.; Zhu, H.T.; Wang, D.; Zhang, Y.J. The genus Rumex (Polygonaceae): An ethnobotanical, phytochemical and pharmacological review. Nat. Prod. Bioprospecting 2022, 12, 21. [Google Scholar] [CrossRef]
- Berillo, D.; Kozhahmetova, M.; Lebedeva, L. Overview of the biological activity of anthraquinons and flavanoids of the plant Rumex species. Molecules 2022, 27, 1204. [Google Scholar] [CrossRef]
- Khaliq, T.; Akhter, S.; Sultan, P.; Hassan, Q.P. Critical review on Rumex dentatus L. a strong pharmacophore and the future medicine: Pharmacology, phytochemical analysis and traditional uses. Heliyon 2023, 9, e14159. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Liu, P.P.; Jin, Y.; Qin, S.; Chen, L. Identification of antibacterial components in the methanol-phase extract from edible herbaceous plant Rumex madaio Makino and their antibacterial action modes. Molecules 2022, 27, 660. [Google Scholar] [CrossRef] [PubMed]
- Kukhtenko, H.; Bevz, N.; Konechnyi, Y.; Kukhtenko, O.; Jasicka-Misiak, I. Spectrophotometric and chromatographic assessment of total polyphenol and flavonoid content in Rhododendron tomentosum extracts and their antioxidant and antimicrobial activity. Molecules 2024, 29, 1095. [Google Scholar] [CrossRef] [PubMed]
- Lourenço-Lopes, C.; Silva, A.; Garcia-Oliveira, P.; Soria-Lopez, A.; Echave, J.; Grosso, C.; Cassani, L.; Barroso, M.F.; Simal-Gandara, J.; Fraga-Corral, M.; et al. Kinetic extraction of fucoxanthin from Undaria pinnatifida using ethanol as a solvent. Mar. Drugs 2023, 21, 414. [Google Scholar] [CrossRef]
- Zhang, K.; Cao, F.; Zhao, Y.; Wang, H.; Chen, L. Antibacterial ingredients and modes of the methanol-phase extract from the fruit of Amomum villosum Lour. Plants 2024, 13, 834. [Google Scholar] [CrossRef]
- Danchik, C.; Casadevall, A. Role of cell surface hydrophobicity in the pathogenesis of medically-significant fungi. Front. Cell. Infect. Microbiol. 2020, 10, 594973. [Google Scholar] [CrossRef] [PubMed]
- Gohrbandt, M.; Lipski, A.; Grimshaw, J.W.; Buttress, J.A.; Baig, Z.; Herkenhoff, B.; Walter, S.; Kurre, R.; Deckers-Hebestreit, G.; Strahl, H. Low membrane fluidity triggers lipid phase separation and protein segregation in living bacteria. EMBO J. 2022, 41, e109800. [Google Scholar] [CrossRef]
- Fuerst-Wilmes, M.; Sahl, H.G. Determination of bacterial membrane impairment by antimicrobial agents. Methods Mol. Biol. 2023, 2601, 271–281. [Google Scholar] [CrossRef]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2009, 1794, 808–816. [Google Scholar] [CrossRef]
- Vind, A.C.; Genzor, A.V.; Bekker-Jensen, S. Ribosomal stress-surveillance: Three pathways is a magic number. Nucleic Acids Res. 2020, 48, 10648–10661. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z.; Wang, W.; Hou, S.; Cai, J.; Xia, L.; Lu, Y. Development of DNA vaccines encoding ribosomal proteins (RplL and RpsA) against Nocardia seriolae infection in fish. Fish Shellfish Immunol. 2020, 96, 201–212. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cai, D.; Lin, X.; Huang, X.; Yuan, Z.; Zhang, Y.; Lei, H.; Wang, P. Carrier-free binary self-assembled nanomedicines originated from traditional herb medicine with multifunction to accelerate MRSA-infected wound fealing by antibacterial, anti-inflammation and promoting angiogenesis. Int. J. Nanomed. 2023, 18, 4885–4906. [Google Scholar] [CrossRef]
- Amala, M.; Richard, M.; Saritha, P.; Prabhu, D.; Veerapandiyan, M.; Surekha, K.; Jeyakanthan, J. Molecular evolution, binding site interpretation and functional divergence of aspartate semialdehyde dehydrogenase. J. Biomol. Struct. Dyn. 2022, 40, 3223–3241. [Google Scholar] [CrossRef] [PubMed]
- Katayama, N.; Osanai, T. Arginine inhibition of the argininosuccinate lyases is conserved among three orders in cyanobacteria. Plant Mol. Biol. 2022, 110, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Antonoplis, A.; Zang, X.; Wegner, T.; Wender, P.A.; Cegelski, L. Vancomycin-arginine conjugate inhibits growth of carbapenem-resistant E. coli and targets cell-wall synthesis. ACS Chem. Biol. 2019, 14, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, N.; Gajera, G.; Mehta, D.; Kothari, V. Silversol® (a colloidal nanosilver formulation) inhibits growth of antibiotic-resistant Staphylococcus aureus by disrupting its physiology in multiple ways. Pharmaceutics 2024, 16, 726. [Google Scholar] [CrossRef]
- Mitchell, S.L.; Kearns, D.B.; Carlson, E.E. Penicillin-binding protein redundancy in Bacillus subtilis enables growth during alkaline shock. Appl. Environ. Microbiol. 2024, 90, e0054823. [Google Scholar] [CrossRef]
- Ramírez Muñoz, J.J.; Cuervo López, F.M.; Texier, A.C. Ampicillin biotransformation by a nitrifying consortium. World J. Microbiol. Biotechnol. 2020, 36, 21. [Google Scholar] [CrossRef]
- Sharma, A.D.; Kaur, I.; Chauhan, A. Compositional profiling and molecular docking studies of Eucalyptus polybrachtea essential oil against mucormycosis and aspergillosis. BioTechnologia 2023, 104, 233–245. [Google Scholar] [CrossRef]
- Jain, C.K.; Dasgupta, A.; Taneja, N.; Chaubey, S.; Gabrani, R.; Sharma, S.K.; Gupta, S. Putative drug targets in Rhizopus oryzae: In-silico insight. Int. J. Bioinform. Res. Appl. 2013, 9, 595–603. [Google Scholar] [CrossRef]
- Krasikova, Y.; Rechkunova, N.; Lavrik, O. Nucleotide excision repair: From molecular defects to neurological abnormalities. Int. J. Mol. Sci. 2021, 22, 6220. [Google Scholar] [CrossRef]
- Hao, Z.; Gowder, M.; Proshkin, S.; Bharati, B.K.; Epshtein, V.; Svetlov, V.; Shamovsky, I.; Nudler, E. RNA polymerase drives ribonucleotide excision DNA repair in E. coli. Cell 2023, 186, 2425–2437.e2421. [Google Scholar] [CrossRef]
- Olave, M.C.; Graham, R.P. Mismatch repair deficiency: The what, how and why it is important. Gene Chromosomes Cancer 2022, 61, 314–321. [Google Scholar] [CrossRef]
- Płaczkiewicz, J.; Adamczyk-Popławska, M.; Lasek, R.; Bącal, P.; Kwiatek, A. Inactivation of genes encoding MutL and MutS proteins influences adhesion and biofilm formation by Neisseria gonorrhoeae. Microorganisms 2019, 7, 647. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, A.; Łazowski, K.; Reijns, M.A.M.; Walsh, E.; McDonald, J.P.; Moreno, K.C.; Quiros, D.R.; Schmidt, M.; Kranz, H.; Yang, W.; et al. Novel Escherichia coli active site dnaE alleles with altered base and sugar selectivity. Mol. Microbiol. 2021, 116, 909–925. [Google Scholar] [CrossRef] [PubMed]
- Cross, E.M.; Adams, F.G.; Waters, J.K.; Aragão, D.; Eijkelkamp, B.A.; Forwood, J.K. Insights into Acinetobacter baumannii fatty acid synthesis 3-oxoacyl-ACP reductases. Sci. Rep. 2021, 11, 7050. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Y.; Cai, C.; Zhang, L.; Ye, F.; Zhang, L. The β-ketoacyl-ACP synthase FabF catalyzes carbon-carbon bond formation in a bimodal pattern for fatty acid biosynthesis. Angew. Chem. Int. Ed. 2024, 63, e202407921. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, Y.; Liu, Y.; An, H.; Deng, K.; Ashraf, M.A.; Zou, L.; Wang, J. Breaking down the cell wall: Still an attractive antibacterial strategy. Front. Microbiol. 2022, 13, 952633. [Google Scholar] [CrossRef]
- Green, D.W. The bacterial cell wall as a source of antibacterial targets. Expert Opin. Ther. Targets 2002, 6, 1–19. [Google Scholar] [CrossRef]
- Al-Dabbagh, B.; Henry, X.; El Ghachi, M.; Auger, G.; Blanot, D.; Parquet, C.; Mengin-Lecreulx, D.; Bouhss, A. Active site mapping of MraY, a member of the polyprenyl-phosphate N-acetylhexosamine 1-phosphate transferase superfamily, catalyzing the first membrane step of peptidoglycan biosynthesis. Biochemistry 2008, 47, 8919–8928. [Google Scholar] [CrossRef]
- Bertonha, A.F.; Silva, C.C.L.; Shirakawa, K.T.; Trindade, D.M.; Dessen, A. Penicillin-binding protein (PBP) inhibitor development: A 10-year chemical perspective. Exp. Biol. Med. 2023, 248, 1657–1670. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Xiao, X.; Li, G. Recent advances in sample preparation technologies for analysis of harmful substances in aquatic products. Chin. J. Chromatogr. 2021, 39, 34–45. [Google Scholar] [CrossRef]
- Ahmadi, N.; Mosleh, N.; Yeganeh, M.; Ahmadi, N.; Malakouti, S.; Shahsavari, S.; Shahraki, R.; Katebi, S.; Agapoor, M.; Sadeghi, S.; et al. Procedures to evaluate potential of plants as natural food preservatives: Phytochemical characterization, novel extraction technology, and safety evaluation-a comprehensive review. Food Sci. Nutr. 2024, 12, 6142–6156. [Google Scholar] [CrossRef]
- GB 2760–2024; National Food Safety Standard for Use of Food Additives. Narional Health Commission of the People’s Republic of China: Beijing, China, 2024.
- Wijnker, J.J.; Weerts, E.A.; Breukink, E.J.; Houben, J.H.; Lipman, L.J. Reduction of Clostridium sporogenes spore outgrowth in natural sausage casings using nisin. Food Microbiol. 2011, 28, 974–979. [Google Scholar] [CrossRef]
- Djenane, D. Chemical profile, antibacterial and antioxidant activity of algerian citrus essential oils and their application in Sardina pilchardus. Foods 2015, 4, 208–228. [Google Scholar] [CrossRef]
- AhmIad, A.S.; Sae-Leaw, T.; Zhang, B.; Singh, P.; Kim, J.T.; Benjakul, S. Impact of ethanolic Thai indigenous leaf extracts on melanosis prevention and shelf-life extension of refrigerated Pacific white shrimp. Foods 2023, 12, 3649. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lan, W.; Xie, J. Characterization of active films based on chitosan/ polyvinyl alcohol integrated with ginger essential oil-loaded bacterial cellulose and application in sea bass (Lateolabrax japonicas) packaging. Food Chem. 2024, 441, 38343. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, H.; Xu, X.; Lu, X.; Song, X.; Zhou, G. Effects of nanoemulsion-based edible coatings with composite mixture of rosemary extract and ε-poly-L-lysine on the shelf life of ready-to-eat carbonado chicken. Food Hydrocoll. 2020, 102, 105576. [Google Scholar] [CrossRef]
- Xue, S.; Li, C.; Xiong, Z. Preparation of complex polysaccharide gels with zanthoxylum bungeanum essential oil and their application in C. auratus preservation. Gels 2024, 10, 533. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sui, K.; Qiu, F.; Zhu, Q.; Luo, J.; Yu, S. Application of ice temperature storage technology assisted by chlorine dioxide and chitosan for the preservation of fresh C. auratus slices. Food Sci. Nutr. 2025, 13, e70127. [Google Scholar] [CrossRef]
- Mahato, A.; Chatterjee, P.N.; Sarkar, S.; Sen, A.R.; Pal, A.; Roy, S.; Patra, A.K. Effects of chemically and green synthesized zinc oxide nanoparticles on shelf life and sensory quality of minced fish (Pangasius hypophthalmus). Foods 2024, 13, 2810. [Google Scholar] [CrossRef]
- GB 2733–2015; National Food Safety Standard for Fresh or Frozen Animal Aquatic Products. Standardization Administration of the People’s Republic of China: Beijing, China, 2015.
- ISO, 10993–5; Biological Evaluation of Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2009.
- Agius, C.; von Tucher, S.; Poppenberger, B.; Rozhon, W. Quantification of sugars and organic acids in tomato fruits. MethodsX 2018, 5, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Okabe, K.; Yoshida, M.; Nakabayashi, R.; Saito, K.; Kogure, N.; Takayama, H. New otonecine-type pyrrolizidine alkaloid from Petasites japonicus. J. Nat. Med. 2019, 73, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yu, S.; Liu, Q.; Zhang, T.; Jiang, B.; Mu, W. Enzymatic production of melibiose from raffinose by the levansucrase from Leuconostoc mesenteroides B-512 FMC. J. Agric. Food Chem. 2017, 65, 3910–3918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Z.; Chen, D.; Yu, Z.; Huang, L.; Yu, H.; Yao, W.; Xie, Y. Inactivation effect of Staphylococcus aureus and application on fresh-cut pineapples by plasma-activated tartaric acid. Food Biosci. 2023, 54, 102789. [Google Scholar] [CrossRef]
- Česonienė, L.; Labokas, J.; Jasutienė, I.; Šarkinas, A.; Kaškonienė, V.; Kaškonas, P.; Kazernavičiūtė, R.; Pažereckaitė, A.; Daubaras, R. Bioactive compounds, antioxidant, and antibacterial properties of Lonicera caerulea berries: Evaluation of 11 cultivars. Plants 2021, 10, 624. [Google Scholar] [CrossRef]
- Alshahrani, M.Y.; Ibrahim, E.H.; Asiri, M.; Kilany, M.; Alshehri, A.; Alkhathami, A.G.; Morsy, K.; Chandramoorthy, H.C. Inhibition realization of multidrug resistant bacterial and fungal isolates using Coccinia indica extracts. Saudi J. Biol. Sci. 2022, 29, 3207–3212. [Google Scholar] [CrossRef]
- Kang, Y.H.; Kim, K.K.; Kim, D.J.; Choe, M. Antiobesity effects of the water-soluble fraction of the ethanol extract of Smilax china L. leaf in 3T3-L1 adipocytes. Nutr. Res. Pr. 2015, 9, 606–612. [Google Scholar] [CrossRef]
- CLSI, M100–S23; Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Third Informational Supplement, Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- CLSI, M100–S18; Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard Tenth Edition, Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Ngene, A.; Ohaegbu, C.G.; Idu, E.G.; Odo, E.S. Time-kill kinetics and antibacterial activity of ethanolic extract of Allium sativum. Microbes Infect. Dis. 2024, 5, 389–397. [Google Scholar] [CrossRef]
- Xu, M.; Fu, H.; Chen, D.; Shao, Z.; Zhu, J.; Alali, W.Q.; Chen, L. Simple visualized detection method of virulence-associated genes of Vibrio cholerae by loop-mediated isothermal amplification. Front. Microbiol. 2019, 10, 2899. [Google Scholar] [CrossRef]
- Cao, F.; Liu, Y.; Tian, E.; Wang, Y.; Ren, S.; Wang, Y.; Zheng, H.; Chen, L. Elucidation of antibacterial efffcacy and mechanism of the extract from fruit of Phyllanthus emblica L. against Vibrio cholerae and Staphylococcus aureus. Food Control. 2025, 178, 111513. [Google Scholar] [CrossRef]
- Cheng, Y.; Ying, X.; Cai, X.; Chen, Y.; Xu, Y.; Song, R.; Gao, H. Characterization of γ-CD-MOF-stabilized thymol pickering emulsion films with enhanced preservation properties for Basa (Pangasius) C. auratus. Food Chem. 2025, 476, 143273. [Google Scholar] [CrossRef]
- Liu, H.; Ji, Z.; Liu, X.; Shi, C.; Yang, X. Non-destructive determination of chemical and microbial spoilage indicators of beef for freshness evaluation using front-face synchronous fluorescence spectroscopy. Food Chem. 2020, 321, 126628. [Google Scholar] [CrossRef] [PubMed]
- Jumilla-Lorenz, D.; Briones, T.; Parés, D. Physicochemical and sensory characterization of raw tuna muscle for plant-based fish analogs development purposes. Heliyon 2024, 10, e38749. [Google Scholar] [CrossRef] [PubMed]
- Mintsa, M.E.; Kumulungui, B.S.; Obiang, C.S.; Dussert, E.; Choque, E.; Herfurth, D.; Ravallec, R.; Ondo, J.P.; Mesnard, F. Cytotoxicity and identification of antibacterial compounds from Baillonella toxisperma bark using a LC-MS/MS and molecular networking approach. Metabolites 2023, 13, 599. [Google Scholar] [CrossRef]
- Yang, Q.; Xing, M.; Wang, K.; Wei, Q.; Zhao, J.; Wang, Y.; Ji, K.; Song, S. Application of fucoidan in Caco-2 model establishment. Pharmaceuticals 2022, 15, 418. [Google Scholar] [CrossRef]
- Sit, B.; Fakoya, B.; Waldor, M.K. Animal models for dissecting Vibrio cholerae intestinal pathogenesis and immunity. Curr. Opin. Microbiol. 2022, 65, 1–7. [Google Scholar] [CrossRef]
- Gómez-Lechón, M.J.; Tolosa, L.; Donato, M.T. Upgrading HepG2 cells with adenoviral vectors that encode drug-metabolizing enzymes: Application for drug hepatotoxicity testing. Expert Opin. Drug Metab. Toxicol. 2017, 13, 137–148. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Y.; Xu, W.; Geng, Y.; Su, Y.; Wang, Q.; Wang, J. Baicalin inhibits Salmonella typhimurium-induced inflammation and mediates autophagy through TLR4/MAPK/NF-κB signalling pathway. Basic Clin. Pharmacol. Toxicol. 2021, 128, 241–255. [Google Scholar] [CrossRef]

| Identified Compound | Compound Nature | Rt (min) | Formula | Exact Mass | Area (%) |
|---|---|---|---|---|---|
| Melibiose | Carbohydrates | 25.5 | C12H22O11 | 365.1040 | 9.86 |
| 3-(N, N-dimethylaminomethyl)indole | Alkaloids | 26.4 | C11H14N2 | 175.1183 | 7.12 |
| Citric Acid | Fatty acids | 27.5 | C6H8O7 | 191.0195 | 6.01 |
| 3-Methylbutanamine | Alkaloids | 356.5 | C5H13N | 88.1118 | 5.18 |
| Otonecine | Alkaloids | 51.2 | C9H15NO3 | 186.1118 | 4.42 |
| Pongamol | Flavonoids | 30.1 | C18H14O4 | 317.0830 | 4.29 |
| Ovalitenin B | Flavonoids | 30.8 | C19H18O4 | 309.1188 | 2.97 |
| Isorhapontin | Stilbenoids | 206.8 | C21H24O9 | 419.1342 | 2.93 |
| Sucrose | Carbohydrates | 26.0 | C12H22O11 | 341.1084 | 2.35 |
| Turanose | Carbohydrates | 26.0 | C12H22O11 | 341.1084 | 2.35 |
| Palatinose (hydrate) | Carbohydrates | 26.0 | C12H22O11 | 341.1084 | 2.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.; Liu, Y.; Tian, E.; Wang, Y.; Ren, S.; Li, B.; Zheng, H.; Chen, L. Antibacterial Efficacy of Ethanol Extracts from Edible Rumex madaio Root and Application Potential for Eliminating Staphylococcus aureus and Vibrio cholerae in Aquatic Products for Green Food Preservation. Foods 2025, 14, 3479. https://doi.org/10.3390/foods14203479
Fan H, Liu Y, Tian E, Wang Y, Ren S, Li B, Zheng H, Chen L. Antibacterial Efficacy of Ethanol Extracts from Edible Rumex madaio Root and Application Potential for Eliminating Staphylococcus aureus and Vibrio cholerae in Aquatic Products for Green Food Preservation. Foods. 2025; 14(20):3479. https://doi.org/10.3390/foods14203479
Chicago/Turabian StyleFan, Huanhuan, Yue Liu, Enyu Tian, Yaping Wang, Shunlin Ren, Bailin Li, Huajun Zheng, and Lanming Chen. 2025. "Antibacterial Efficacy of Ethanol Extracts from Edible Rumex madaio Root and Application Potential for Eliminating Staphylococcus aureus and Vibrio cholerae in Aquatic Products for Green Food Preservation" Foods 14, no. 20: 3479. https://doi.org/10.3390/foods14203479
APA StyleFan, H., Liu, Y., Tian, E., Wang, Y., Ren, S., Li, B., Zheng, H., & Chen, L. (2025). Antibacterial Efficacy of Ethanol Extracts from Edible Rumex madaio Root and Application Potential for Eliminating Staphylococcus aureus and Vibrio cholerae in Aquatic Products for Green Food Preservation. Foods, 14(20), 3479. https://doi.org/10.3390/foods14203479






